Abstract
Several hybrid Forward osmosis (FO) systems for seawater and brackish water desalination have been developed in recent years. This study investigates two approaches of integrated systems comprising brackish water reverse osmosis desalination (BWRO) and forward osmosis (FO) systems. These approaches aim to minimize the environmental effect of inland reject brine disposal while enhancing total water recovery. Experiments were performed using different draw solution (DS) concentrations of KCl and MgCl2.6H2O (0.5, 1, 1.5 and 2 Molar) to reach maximum recovery. In scheme (A), feed water is directed to BWRO with recovery 45% and 97% rejection, while the brine water is directed to the FO system as FS stream. In scheme (B), the feed water is divided into two streams, one of which is directed to the BWRO with recovery 75% and 98% rejection and the other stream is sent to the FO unit as FS stream. KCl DS displayed higher flux rate of an average 28.5 L/(m2.h) using 2 M concentration, while MgCl2.6H2O 2M solution shows an average flux of 23.5 L/(m2.h) during the first 100 min of operation. Scheme A shows 81% total system recovery, while scheme B shows 78% total system recovery. The cost indicators for desalination system with small capacity of 10 m3/d showed that scheme (A) has lower capital cost 55,732$ for scheme (A), while total capital cost was 59,400 $ for scheme (B).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ever-increasing and exponential expansion of the world's population has created serious worries about the water resources sustainability and energy supplies. As a result, fulfilling the growing water demand with a low energy consumption technology is one of the century’s major problems (Okello et al. 2015; Phebe et al. 2016). Improvement of low-energy desalination methods for safe domestic water production is becoming so critical, and it has attracted growing attention in recent decades. Membrane technologies are now the most extensively utilized ways for producing safe domestic water, and reverse osmosis (RO) is the most viable technique of membrane separation procedure for desalination (Mahmoud and Saffa 2014).
Forward osmosis (FO) technology received much attention in the recent years. FO desalination’s power consumption was reported to be from 0.25–1 kWh/m3 (Feria-Díaz et al. 2021), which is many orders of magnitude less than RO’s power consumption, which normally ranges from 2–6 kWh/m3 (Haidar et al. 2021).
In recent years, numerous hybrid/integrated membrane systems including FO have been utilized for various treatments such as seawater or brackish water desalination, in addition to wastewater reclamation and treatment (Şahin Deniz 2021; Wei et al. 2021; Ibrar et al. 2022). Other uses include fertigation (Nasr and Sewilam 2015a, b), protein concentration (Wang et al. 2011), RO concentrate (El Zayat et al. 2021) and dewatering (Nguyen et al. 2013; Blandin et al. 2020; Hau et al. 2014). The various hybrid systems include a FO unit into an existing or newly constructed process, either to replace the traditional pretreatment methods or as a post-treatment stage to minimize the final amount of industrial effluent.
Integrated systems addressed by researchers including FO in combination with Nanofiltration (NF) (Tan and Ng 2010; Wang et al. 2021) in different applications including seawater desalination. Zhao et al. (2012) investigated the integrated NF/FO system for brackish water desalination. The research revealed that this integrated system has numerous benefits over standard individual RO processes, including better produced water quality, where it was observed that the salt rejection reaches 97.7 percent while the TDS levels reaches 10 mg/L. Less fouling tendency with better flux recovery following membrane cleaning (deprived of the use of chemicals), and lower operating pressure of less than 10 bar compared to 30 bar in RO system was also reported.
Yangali et al. (2011), evaluated the effectiveness of coupling LPRO with FO for Red Sea water desalination. Their findings revealed that the power consumption related to the proposed LPRO/FO system varies from 1.3 to 1.5 kWh/m3, representing less than half of the energy consumption in SWRO system (2.5 to 4 kWh/m3) due to the application of high-pressure pumps in these systems. As a result, from an energy-saving standpoint, the proposed integrated LPRO/FO system is appealing.
In a wastewater treatment system comprising RO-FO, the upstream FO opposes the wastewater while the diluted draw solution (DS) is subsequently used as the RO feed to achieve better water recovery. When compared to the standard RO process, the integration reduces fouling of the RO membrane and produces much improved overall energy efficiency (Hafiz et al. 2021; Goh et al. 2019).
For inland brackish water desalination, Altaee and Hilal (2015) presented an integrated trihybrid system comprising NF/FO/BWRO, where brackish water of varying concentrations are supplied into an NF system after pretreatment, and the brine generated is delivered to a FO system for additional concentration. The BWRO permeate is either used directly or combined with the NF permeate to change the salinity of the produced water, and the concentrated brine produced from the same unit is used as a DS in the FO system. The simulation results revealed that depending on the supply water quality, the tri-hybrid system can reach a system recovery exceeding 90%. Khanzada et al. (2017) investigated the implementation of integrated trihybrid UF/RO/FO for inland desalination. The results showed an overall process rejection of 98%, while by adding a cartridge filter before UF the overall rejection reduced to 95%.
The draw solution selection is a significant characteristic for the effectiveness of the FO procedures especially in hybrid and integrated systems. Water flow and process behavior are known to be influenced by the type and nature of solutions used (organic or inorganic/single or multiple solute). Generally, a suitable draw solution must retain higher osmotic pressure than the feed solution (FS) and can be recovered easily. Currently, a new group of DS has been developed, such as magnetic nanoparticles (MNPs) (Tayel et al. 2020), or polymer hydrogels (Wang et al. 2020; Fan et al. 2016; Li et al. 2013), and they have demonstrated excellent performance for future applications in desalination (Li et al. 2013; Amir et al. 2013; Jangizehi and Seiffert 2021).
NaCl, KCl, and CaCl2 are the most often utilized inorganic salts due to their availability, low cost, and simplicity of recovery. Different DS solutions have been studied in order to enhance membrane performance and productivity (Chekli et al. 2012; Ahmed et al. 2018).
KCl (muriate of potash) is widely utilized as potassium (K) fertilizer due to its inexpensive cost and higher nutritional value (59–61 percent K2O) than most other sources. Because of its high osmotic potential, KCl is an excellent draw solute of osmotic pressure 92.27 bar at 2 Molar concentration. As a result, it has the ability to generate higher water flux than other inorganic salts. (Phuntsho et al. 2011; Ahmed et al. 2018).
This study investigates two approaches of integrated systems comprising brackish water reverse osmosis desalination (BWRO) and forward osmosis (FO) systems. These approaches aim to minimize the environmental effect of inland reject brine disposal while enhancing total water recovery. The schemes used include a BWRO and FO system with two draw solutions (KCl and MgCl2.6H2O) and a DS recovery stage. A techno-economic investigation was conducted between the two desalination schemes for brackish water desalination. The indicators used are obtained by evaluating and assessing global efforts as well as experimental outcomes reported in various publications.
Materials and methods
Experimental membrane setup
The experimental investigations in this research were carried out by means of a FO bench-scale FO flux-meter unit (fluxometer). The membrane in the FO unit, as shown in Fig. 1, has a total area of approximately 1.257 × 10–3 m2, with an average flow rate of 200 mL/min. Both of the membrane sheet and the flux-meter were developed by Porifera Inc., USA. Membrane characteristics are presented in Table 1. The operation mode adopted in all experiments is FO mode of operation, where the draw solution was facing the support. To maintain both solutions at a constant temperature of 25 ℃; a temperature controller (Polyscience temperature controller) was used.
Digital precision scales that gave real-time measurements are used to compute the resulted flux, by documenting the mass changes in both solutions at 2-min interval. The readings of the balances were recorded using a software (LabVIEW 2010, National Instruments). The flux was then calculated in terms the mass/volume change for both the DS and the FS.
Chemicals
The experimental work was done using sodium chloride salt (analytical grade) as well as KCl and MgCl2.6H2O were acquired from ADWIC company—Egypt. To confirm that all the salt was totally dissolved, adequate mixing at 200 to 300 rpm with a magnetic stirrer for both FS and DS solutions was performed. All tests were conducted at room temperature. Table 2 shows the standard specifications for reagent-grade NaCl.
Methods
All experimental investigations were performed at constant temperature of 25 ℃. The change in feed solution volume was used to determine the water flux across the membrane. The water flux “Jw” calculated using Equation (in L/m2/h), as seen in Eq. (1). The online digital mass scale connected to the membrane setup, collecting and recording all the data concerning any water volume change (repetitively) at 2-min time periods is shown as follows:
where ∆V: total volume to the DS from FS
A: membrane area,
T: time
The occurrence of reverse salt permeation is unfavorable to the FO process because it not only disrupts feed water concentration and reduces the net osmotic driving force, but also enhances the FS fouling proclivity by the formation of complexes with the feed ions. The reverse solute flow for each fertilizer type varies greatly depending on its properties. Taking into account that greater hydrated diameter ions in DS have lower reverse permeability than smaller hydrated diameter ions in DS (Achilli et al. 2010; Nasr and Sewilam 2015b). Because of the concentration difference, reverse diffusion or reverse permeation of the solute from the DS side to the FS side is predicted.
whereVi: FS primary volume
ΔV: water volume transferred from FS to the DS
Cs: concentration of the draw solute in the FS after experiment
Process description of integrated BWRO/FO system
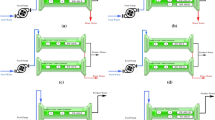
The proposed integrated systems have an output capacity of 10 m3/day for BWRO unit, Figure 2 presents the process design for each scheme and the recovery from different streams based on material balance. The process comprises BWRO, FO, and DS recovery system. In scheme (A), feed water is directed to BWRO with recovery 45% and 97% rejection and capacity of 10 m3/day. The brine water is then split into two streams, one stream is supplied to the FO system with capacity 10 m3/day as FS stream, while the second stream is directed as the DS stream where it is mixed with either KCl or MgCl2.6H2O.
In scheme (B), the feed water line is divided into two streams, one of which is directed to the BWRO with recovery 75% and 98% rejection and capacity of 10 m3/day. The other stream is sent to the FO unit as FS stream with 10 m3/day. The FO DS stream is the reject brine produced from the BWRO unit mixed with different concentrations of either KCl or MgCl2.6H2O.
Results and discussion
Experimental investigation
Characterization of draw solution and baseline investigations
The baseline studies were carried out to evaluate the fundamental performance of the membrane and to give baseline measurements for the generated flux. To evaluate the flow rate of the membrane process for varied DS concentrations of KCl and MgCl2.6H2O, two baseline experiment groups were done (0.5, 1, 1.5 and 2 Molar). The first group had KCl as the DS and DI as the FS. The second group has MgCl2.6H2O as the DS and DI as the FS. Total Dissolved Solids (TDS) were measured before and after each experiment using an Orion Thermo Scientific portable TDS meter. After each experiment, the membrane was cleaned using inline DI water cleaning. All studies were conducted in AL-FS operation mode.
The first baseline experiment determined the highest concentration of KCl and MgCl2.6H2O in DI that would generates the highest flux rate. For each experiment TDS readings, and changes in FS masses were registered. The findings of the experiment as shown in Fig. 3 reveal that KCl shows the highest water flux of 38 L/(m2.h), while for the same DS concentration using MgCl2.6H2O it shows 23 L/(m2.h).
Draw solution performance investigation
The proposed integrated system was investigated using the bench scale FO unit and synthesized brackish water. Figure 4 presents the experimental matrix developed. Two FS concentrations were investigated in this study: the first was 9200 mg/L, DS concentration values for scheme A are presented in Figs. 2, 5 and 6. For scheme A, using KCl of 2 M solution displayed the higher flux of an average value of 26 L/(m2.h) in the initial operation for 150 min, while DS concentration value of 0.5 Molar showed a fixed flux rate of 3.3 L/(m2.h) along the cycle of operation.
For DS using MgCl2.6H2O of 0.5 M showed a similar pattern as 1 M after 100 min of operations of 14 L/(m2.h), while 2 M concentrations start with 23 L/(m2.h) and a rise in flux to 25.72 L/(m2.h) after 114 min of operation.
The second FS concentration investigated in this study was scheme B where different DS concentration values were used as presented in Figs. 2, 4, 7 and 8. KCl DS showed higher flux rate of an average 28.5 L/(m2.h) using 2 Molar concentration. MgCl2.6H2O 2 Molar solution shows a flux rate of 23.5 L/(m2.h) during the initial operation for 100 min, and a decline in flux reaches 19 L/(m2.h) afterwards as presented in Fig. 8. Flux decline is induced by a decrease in net osmotic potential and the increasing DS dilution caused by water transport. After 200 min of operation, the water flux was reduced to almost 18 percent of the starting flux (Dutta and Nath 2019).
It is clear that the FO flux reduced considerably as the DS's molecular weight increased (KCl shows higher fluxes as of its lower molecular weight) as shown in Table 2. The lower diffusivity of the large DS molecules caused severe internal concentration polarization, which hindered the flow (Yasukawa et al. 2015).
Figure 7 displays a water flow average of 16.5 L/(m2.h) for KCl 0.5 M concentration, whereas Fig. 8 shows a water flux average of 17 L/(m2.h) for MgCl2.6H2O. Unlike KCl different DS concentration, MgCl2.6H2O shows flux decline pattern as presented in Fig. 8. DS of 1 M concentration shoes a flux decline after 124 min of operation from 23 to 19 L/(m2.h), while 1.5 M concentration face the flux decline after 164 min of operation from 23 to 18 L/(m2.h). Concentrative ECP and dilutive ICP are the key resistances in AL-FS orientation. Because pure water contains no solute, the impact of concentrative ECP is negligible, and dilutive ICP is solely responsible for a decrease in water flow. ICP occurs inside the membrane’s support layer as a result of water migration from FS to DS, leading in dilution of the draw solution that is difficult to eliminate. Because the membrane employed includes extensive support layers, the water flow fell to almost 17–21 percent of the original flux after 125–165 min of operation due to the dilutive ICP phenomena.
By comparing the results with Devia et al. (2015), where the flux ranges from 7.55 to 15 L/(m2.h) for different types of membranes. This shows that the reduced salt flux was caused by the FO membrane’s increased steric barrier and electrostatic repulsion of MgCl2.6H2O.
Figures 9, 10, 11, and 12 show the results of the evaluation of membrane selectivity Js/Jw for varied FS and DS concentrations. Because greater DS concentrations result in a more severe ICP impact, the computed Js/Jw ranges between 0.8 and 1 for varied DS concentrations (Imane et al. 2022). Since ions with greater diameters have lower permeability, KCl has a higher specific reverse flow than MgCl2.6H2O due to its smaller diameter, as seen in Figs. 9, 10, 11, and 12.
The results shown in Figs. 9, 10, 11, and 12 clearly indicate that flux increases as feed concentration decreases. Such trend can only be explained by reduced ECP caused by lowering the mass transfer coefficient with the FS concentration. In comparison to the published data, the reported specific reverse solute fluxes of commonly used typical DS such as NaCl (0.7 g/L) or MgCl2 and CaCl2 (0.6 and 2.5 g/L) (Achilli et al. 2010; Tai-Shung et al. 2012; Ray et al. 2018) (Table 3).
It was observed that utilizing KCl as a DS resulted in the greatest FS volume reduction for both Schemes A and B. The largest FS volume reduction was reached for Scheme B by utilizing KCl at a concentration of 2 M, which resulted in a 27 percent volume reduction.
The diluted KCl solution (fertilizer solution) produced by the FO unit has a significant nutrient level and may be used directly for fertigation or in hydroponics. Post treatment by NF achieves two basic goals: reducing nutrient level to a suitable concentration needed for fertigation (as in the permeate stream) and recycling surplus nutrient (as presented in retentate stream) back to the FO unit. Another recovery method may be used, which is an evaporator/crystallizer unit. The recovery unit will achieve 90 percent recovery, and increase the overall water recovery of the system rather than NF unit.
Integrated BWRO/FO system
Industrial scale production financial indicators
The adopted hybrid schemes comprise BWRO and FO as well as DS recovery system are presented in Fig. 2. Techno-economic investigation has been conducted. BWRO system indicators have been calculated using “WT Cost II” software (Al Bazedi et al. 2016; Nikolay Voutchkov 2018), other cost indicators are accomplished by assessment and analysis of efforts in addition to the experimental results presented in literature. All costs are updated using the current Engineering News-Record 2023 (https://www.enr.com).
Two integrated schemes in this investigation, A and B, previously assigned to the first and second configurations were explored. The plant capacity was considered to be 10 m3/h for both configurations. The cost estimate used a 6% interest rate amortized over a 30-year period. The energy cost was expected to be 0.08 USD/kWh. RO desalting system Capital and operating costs were projected using “WT Cost II” software. The cost price of the FO module was assumed to be 100 USD/m2 (Porifera Inc 2021). The capital cost has been updated to 2022 using ENR-CCI cost index. All the expenses including the capital and operating costs of the feed water delivery system are not factored into the financial analysis (Tables 4 and 5).
Table 6 shows the capital as well as operating and maintenance costs for the designed integrated FO/BWRO facility. The cost indicators show that the total capital cost was 55,732$ for scheme A, and 59,400$ for scheme B. The cost of the DS recovery system (evaporator/crystallizer unit) is based on USBR report (2006) and updated using ENR cost index for 2022, showing 27,332 $ and the operating cost is 0.7$/m3. The cost of KCl is 37 $/kg, while for MgCl2.6H2O, it is 28 $/kg (Achilli et al. 2010). The unit cost for water produced by scheme A is $1.85/m3, while for scheme B, it is $1.93/m3.
Conclusion
Because the draw solution employed in the FO process has a direct influence on the recovery mechanism, several DS solutions including KCl and MgCl2.6H2O were studied. The performance of KCl and MgCl2.6H2O at different concentrations was evaluated. Js/Jw membrane selectivity was estimated for various feed and DS concentrations. The proposed method might yield fertilizer solution suited for fertigation or hydroponics systems. The computed Js/Jw fluctuates between 0.8 and 1 for different DS concentrations, owing to the fact that higher DS concentrations result in a more severe ICP impact.
Two integrated FO/BWRO systems have been developed to investigate the maximum overall system recovery and cost estimates. Both schemes showed volume reduction of 27% after 3 h of operation when using a 2 M concentration of KCl, whereas Scheme B showed a higher volume reduction of 26% when using a 2 M concentration of MgCl2.6H2O. For both Schemes A and B, it was discovered that using KCl as a DS resulted in the highest FS volume decrease. The cost indicators showed that the total capital cost was 55,732$ for scheme A, and 59,000 $ for scheme B. Scheme A shows unit production cost of 1.8 $/m3, while for scheme B the unit cost is 1.93 $/m3.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Achilli A, Cath TY, Childress AE (2010) Selection of inorganic-based draw solutions for forward osmosis applications. J Membr Sci 364(1–2):233–241. https://doi.org/10.1016/j.memsci.2010.08.010
Ahmed M, Kumar R, Al-Wazzan Y et al (2018) Assessment of performance of inorganic draw solutions tested in forward osmosis process for desalinating Arabian Gulf seawater. Arab J Sci Eng 43:6171–6180. https://doi.org/10.1007/s13369-018-3394-9
Al Bazedi Gh A, El-Sayed M, Abdel-Fatah M (2016) A, Comparison between reverse osmosis desalination cost estimation trends. J Sci Eng Res 3(5):56–62
Altaee A, Hilal N (2015) High recovery rate NF–FO–RO hybrid system for inland brackish water treatment. Desalination 363:19–25
Amir R, George P, Simon HW (2013) Effect of particle size on the performance of forward osmosis desalination by stimuli-responsive polymer hydrogels as a draw agent. Chem Eng J 215–216:913–920. https://doi.org/10.1016/j.cej.2012.11.088
Blandin G, Federico F, Geoffroy L, Pierre L, Marc H, Xavier M (2020) Forward osmosis as concentration process: review of opportunities and challenges. Membranes 10(10):284. https://doi.org/10.3390/membranes10100284
Chekli L, Phuntsho S, Shon HK, Vigneswaran S, Kandasamy J, Chanan A (2012) A review of draw solutes in forward osmosis process and their use in modern applications. Desalination Water Treat 43(1–3):167–184. https://doi.org/10.1080/19443994.2012.672168
Devia Y, Imai T, Higuchi T, Kanno A, Yamamoto K, Sekine M, Le T (2015) Potential of magnesium chloride for nutrient rejection in forward osmosis. J Water Resour Prot 7:730–740. https://doi.org/10.4236/jwarp.2015.79060
Dutta S, Nath K (2019) Dewatering of brackish water and wastewater by an integrated forward osmosis and nanofiltration system for direct fertigation. Arab J Sci Eng. https://doi.org/10.1007/s13369-019-04102-3
El Zayat H, Nasr P, Sewilam H (2021) Investigating sustainable management of desalination brine through concentration using forward osmosis. Environ Sci Pollut Res 28(29):39938–39951
Fan X, Liu H, Gao Y, Zou Z, Craig VS, Zhang G, Liu G (2016) Forward-osmosis desalination with poly(ionic liquid) hydrogels as smart draw agents. Adv Mater 28:4156–4161
Feria-Díaz JJ, Correa-Mahecha F, López-Méndez MC, Rodríguez-Miranda JP, Barrera-Rojas J (2021) Recent desalination technologies by hybridization and integration with reverse osmosis: a review. Water 13:1369. https://doi.org/10.3390/w13101369
Goh PS, Ismail AF, Ng BC, Abdullah MS (2019) Recent progresses of forward osmosis membranes formulation and design for wastewater treatment. Water 11:2043. https://doi.org/10.3390/w11102043
Hafiz M, Alfahel R, Hawari AH, Hassan MK, Altaee A (2021) A hybrid NF-FO-RO process for the supply of irrigation water from treated wastewater: simulation study. Membranes 11:191. https://doi.org/10.3390/membranes11030191
Haidar ZA, Al-Saud M, Orfi J, Al-Ansary H (2021) Reverse osmosis desalination plants energy consumption management and optimization for improving power systems voltage stability with PV generation resources. Energies 14:7739. https://doi.org/10.3390/en14227739
Hau NT, Chen S-S et al (2014) Exploration of EDTA sodium salt as novel draw solution in forward osmosis process for dewatering of high nutrient sludge. J Membr Sci 455:305–311
Ibrar I, Sudesh Y, Osamah N, Adnan A, Noreddine Gh, Sébastien D, Senthilmurugan S, Ali A (2022) Development in forward osmosis-membrane distillation hybrid system for wastewater treatment. Sep Purif Technol 286:120498. https://doi.org/10.1016/j.seppur.2022.120498
Imane C, Issa N, Jaouad E, Souad A, Sébastien V, Tijani B (2022) Experimental and theoretical parametric study of forward osmosis system using NH4HCO3 and NaCl draw solutes. Chem Eng Res Des 180:402–413. https://doi.org/10.1016/j.cherd.2022.01.006
Jangizehi A, Seiffert S (2021) Salt partitioning in ionized, thermo-responsive hydrogels: perspective to water desalination. J Chem Phys 154(14):144902. https://doi.org/10.1063/5.0044376
Khanzada NK, Khan SJ, Davies PA (2017) Performance evaluation of reverse osmosis (RO) pre-treatment technologies for in-land brackish water treatment. Desalination 406:44–50. https://doi.org/10.1016/j.desal.2016.06.030
LabVIEW (2010) https://www.ni.com/pdf/manuals/lv2010SP1.html
Li D, Zhang X, Simon GP, Wang H (2013) Forward osmosis desalination using polymer hydrogels as a draw agent: influence of draw agent, feed solution and membrane on process performance. Water Res 47(1):209–215. https://doi.org/10.1016/j.watres.2012.09.049
Mahmoud S, Saffa B (2014) Water desalination technologies utilizing conventional and renewable energy sources. Int J Low-Carbon Technol 9(1):1–19. https://doi.org/10.1093/ijlct/cts025
Nasr P, Sewilam H (2015a) Investigating the performance of ammonium sulphate draw solution in fertilizer drawn forward osmosis process. Clean Technol Environ Policy 18(3):717–727
Nasr P, Sewilam H (2015b) The potential of groundwater desalination using forward osmosis for irrigation in Egypt. Clean Technol Environ Policy 17(7):1883–1895
Nguyen NC, Chen S-S et al (2013) Application of forward osmosis on dewatering of high nutrient sludge. Bioresour Technol 132:224–229
Okello C, Bruno T, Nicolas G, Nina W, Marco A (2015) Impact of population growth and climate change on the freshwater resources of Lamu Island, Kenya. Water 7(3):1264–1290. https://doi.org/10.3390/w7031264
Phebe Asantewaa O, Samuel Asumadu-S, Shashi Dubey (Reviewing Editor) (2016) A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng 3:1. DOI: https://doi.org/10.1080/23311916.2016.1167990
Phuntsho S, Shon HK, Hong S, Lee S, Vigneswaran S (2011) A novel low energy fertilizer driven forward osmosis desalination for direct fertigation: evaluating the performance of fertilizer draw solutions. J Membr Sci 375:172–181. https://doi.org/10.1016/j.memsci.2011.03.038
Porifera Inc (2016) Fluxometer user manual, ver 2.1. Porifera
Porifera Inc (2021) Membrane modules. http://www.porifera.com/modules. Accessed Dec 2021
Ray SS, Chen SS, Nguyen NC, Nguyen HT, Dan NP, Thanh BX, Trang LT (2018) Exploration of polyelectrolyte incorporated with Triton-X 114 surfactant based osmotic agent for forward osmosis desalination. J Environ Manag 209:346–353. https://doi.org/10.1016/j.jenvman.2017.12.086
Şahin D (2021) Forward osmosis membrane technology in wastewater treatment. In: Ince, Kaplan (eds) Osmotically driven membrane processes. IntechOpen, Doi: https://doi.org/10.5772/intechopen.97483
Tai-Shung C, Sui Z, Kai YuW, Jincai S, Ming L (2012) Forward osmosis processes: yesterday, today and tomorrow. Desalination 287:78–81. https://doi.org/10.1016/j.desal.2010.12.019
Tan CH, Ng HY (2010) A novel hybrid forward osmosis—nanofiltration (FO-NF) process for seawater desalination: draw solution selection and system configuration. Desalination and Water Treat 13(1–3):356–361. https://doi.org/10.5004/dwt.2010.1733
Tayel A, Nasr P, Sewilam H (2020) Enhanced water flux using uncoated magnetic nanoparticles as a draw solution in forward osmosis desalination. Desalination Water Treat 193:169–176. https://doi.org/10.5004/dwt.2020.25827
United States Bureau of Reclamation (USBR) (2006) Zero discharge seawater desalination; integrating the production of freshwater, salt, magnesium and bromine, Report No. 111.
Voutchkov N (2018) Desalination project cost estimating and management. CRC Press
Wang KY, Teoh MM, Nugroho A, Chung T-S (2011) Integrated forward osmosis–membrane distillation (FO–MD) hybrid system for the concentration of protein solutions. Chem Eng Sci 66(11):2421–2430. https://doi.org/10.1016/j.ces.2011.03.001
Wang J, Gao S, Tian J, Cui F, Shi W (2020) Recent developments and future challenges of hydrogels as draw solutes in forward osmosis process. Water 12(3):692. https://doi.org/10.3390/w12030692
Wang H, Gao Y, Gao B, Guo K, Shon HK, Yue Q, Wang Z (2021) Comprehensive analysis of a hybrid FO-NF-RO process for seawater desalination: with an NF-like FO membrane. Desalination 515:115203. https://doi.org/10.1016/j.desal.2021.115203
Wei Jie L, Pei Sean G, Ahmad F (2021) Chapter 7—forward osmosis-based hybrid processes for water and wastewater treatment. In: Abdul Wahab Mohammad, Wei Lun Ang (eds) Integrated and hybrid process technology for water and wastewater treatment. Elsevier, p 121–144 https://doi.org/10.1016/B978-0-12-823031-2.00011-2.
Yangali-Quintanilla V, Li Z, Valladares R, Li Q, Amy G (2011) Indirect desalination of Red Sea water with forward osmosis and low pressure reverse osmosis for water reuse. Desalination 280(1–3):160–166. https://doi.org/10.1016/j.desal.2011.06.066
Yasukawa M, Tanaka Y, Takahashi T, Shibuya M, Mishima S, Matsuyama H (2015) Effect of molecular weight of draw solute on water permeation in forward osmosis process. Ind Eng Chem Res 54(33):8239–8246. https://doi.org/10.1021/acs.iecr.5b01960
Zhao S, Zou L et al (2012) Brackish water desalination by a hybrid forward osmosis–nanofiltration system using divalent draw solute. Desalination 284:175–181
Acknowledgements
The American University in Cairo’s Center for Applied Research on the Environment and Sustainability (CARES), School of Science and Engineering, provided funding for this research.
Funding
Open Access funding enabled and organized by Projekt DEAL. Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sewilam, H., Al Bazedi, G. Hybrid BWRO/FO system for high recovery in-land brackish water desalination: techno-economic assessment. Sustain. Water Resour. Manag. 9, 136 (2023). https://doi.org/10.1007/s40899-023-00912-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-023-00912-4