Abstract
Purpose
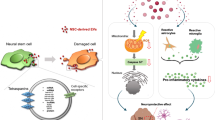
The underlying mechanism of today’s neurodegenerative diseases is disordered due to neuronal damage and very limited neuronal regeneration. Formulations that can be developed against the factors that cause damage must have a neuroprotective effect. Particularly, formulations that can be developed in nanoscales may play a key role in central nervous system diseases. This article aimed to investigate the neuroprotective activity of dopamine-loaded exosomes in which exosomes derived from Wharton jelly stem cells (WJ-MSCs) are used as carrier systems in 2D and 3D cell cultures induced in cell damage.
Materials and Methods
This study created 2D and 3D cell cultures with a bioprinter in the dopaminergic neuroblastoma cell line (SH-SY5Y). Cell lines were damaged with 6-OHDA. The neuroprotective effect of dopamine-loaded exosomes was investigated by Live&Dead analysis and immunostaining.
Results
The findings showed that dopamine-loaded exosomes protected against 6-OHDA-induced neurotoxicity in both cultures. The increase in the number of viable cells in the 6-OHDA administered groups proved the neuroprotective effect.
Conclusion
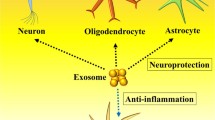
We predict that the carrier systems developed with exosomes obtained from Wharton jelly stem cells will be a light of hope in the treatment of neurodegenerative diseases, considering both their size and their contribution to regeneration.
Lay Summary
Especially in neurodegenerative diseases, it is very important to be able to protect and regenerate neurons. Wharton jelly stem cell-based exosomes are known to have many properties, including other wound healing activities. In addition, considering both the structure and size of exosomes, its development as a drug delivery system is promising.






Similar content being viewed by others
References
Shamim IA, Neurodegenerative diseases,” Advance in experiment medicine and biology, New York: Springer; 2012th Edition
Popa-Wagner A, Dumitrascu DI, Capitanescu B, Petcu EB, Surugiu R, Fang WH, Dumbrava DA. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen Res. 2020;15(3):394–400.
Maitre L, de Bont J, Casas M, Robinson O, Aasvang GM, Agier L, Andrušaitytė S, Ballester F, Basagaña X, Borràs E, Brochot C, Bustamante M, Carracedo C, de Castro M, Dedele A, Donaire-Gonzalez D, Estivill X, Evandt L, Fossati S, et al. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open 8(9):e021311
Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. |Annu. Rev Neurosci. 2011;34:131–52.
Di Giovanni S. Molecular targets for axon regeneration: focus on the intrinsic pathways. Expert Opin Ther Targets. 2009;13:1387–98.
Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol. 2014;27:53–60.
Tedeschi A, Bradke F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr Opin Neurobiol. 2017;42:118–27.
Kovacs GG. Molecular pathological classification of neurodegenerative diseases: turning towards precision medicine. Int J Mol Sci. 2016;17(2):189. https://doi.org/10.3390/ijms17020189.
Rao SS, Hofmann LA, Shakil SS. Parkinson’s disease: diagnosis and treatment. Am Fam Physician. 2006;15(74):2046–54.
Peira E, Marzola P, Podio V, Aime S, Sbarbati A, Gasco MR. In vitro and in vivo study of solid lipid nanoparticles loaded with superparamagnetic iron oxide. J Drug Target. 2003;11(1):19–24.
Ahmad Z, Shah A, Siddiq M, Kraatz HB. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4(33):17028–38.
Karthivashan G, Ganesan P, Parkı SY, Kim JS, Dong-Kug Choi S. Therapeutic strategies and nano-drug delivery applications in the management of aging Alzheimer’s disease. Drug Deliv. 2018;25(1):307–20.
Zhang Y, Liu Y, Liu H, Tang WH, Exosomes: biogenesis, biologic function, and clinical potential. Cell Biosci. 2019;9(1):1–18.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20.
Ingato D, Lee JU, Sim SL, Kwon YL. Good things come in small packages: overcoming challenges to harness extracellular vesicles for therapeutic delivery. J Control Release. 2016;241:174–85.
Lin J, Li J, Huang B, Liu J, Chen X, Wang X. Exosomes: novel biomarkers for clinical diagnosis. Scientific World Journal. 2015;2015:657086. https://doi.org/10.1155/2015/657086
Saeedi S, Israel S, Nag C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019;9(1):122.
Jan AT, et al. Perspective insights of exosomes in neurodegenerative diseases: a critical appraisal. Front Aging Neurosci. 2017;9:317.
Cao XY, Lu MJ, Zhao ZQ, Li MC, Lu T, An XS, Xue LJ. MicroRNA biomarkers of Parkinson’s disease in serum exo-some-like microvesicles. Neurosci Lett. 2017;644:94–9.
de Rivero Vaccari JP, Brand F, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, Bullock MR, Dietrich WD, Keane WR. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136:39–48.
Han D, Wu C, Xiong Q, Zhou L, Tian Y. Anti-inflammatory mechanism of bone marrow mesenchymal stem cell transplantation in a rat model of spinal cord injury. Cell Biochem Biophys. 2015;71:1341–7.
Vilaça-Faria H, Salgado AJ, Teixeira FG. Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for Parkinson’s disease? Cells. 2019;8(2):118. https://doi.org/10.3390/cells8020118. PMID: 30717429; PMCID: PMC6406999
Chen HX, Liang FC, Gu P, Xu BU, Xu HX, Wang WT, Hou JY, Xie DX, Chai X, An SJ, Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020;11:(288). https://doi.org/10.1038/s41419-020-2473-5.
Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci. 2014;107(1-2):1–7.
Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection, and therapeutics. Mol Neurobiol. 2014;49(1):590–600.
Ran N, Gao X, Dong X, Li J, Lin C, Geng M, Yin H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of MDX mice. Biomaterials. 2020;236:119826.
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–14.
Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. 2010;9(3):425–74.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikov EG, He Z, Patel T, Piroyona A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30.
Yavuz B, Darici H, Zorba Yildiz AP, Abamor ES, Topuzoğullari M, Bağirova M, Allahverdiyev A, Karaoz E. Formulating and characterizing an exosome-based dopamine carrier system. J Vis Exp. 2022. https://doi.org/10.3791/63624.
Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(24):4195–200.
Schmidt JJ, Rowley J, Kong HJ. Hydrogels used for cell-based drug delivery. J Biomed Mater Res A. 2008;87:1113–22.
Kelava I, Lancaster MA. Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev Biol. 2016;420:199–209.
Völkner M, Zschätzsch M, Rostovskaya M, Overall RW, Busskamp V, Anastassiadis K, Karl MO. Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Reports. 2016;6:525–38.
Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–17.
Kim Y, Ju JH. Generation of 3D skin organoid from cord blood-derived induced pluripotent stem cells. JoVE. 2019;146:e59297.
Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 2018;23:869–881.e868.
Arganda-Carreras I, Kaynig V, Rueden C, Eliceiri KW, Schindelin J, Cardona A, et al. Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics. 2017;33:2424–6. pmid:28369169.
Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, Chang RC. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30:127–35.
Zorba Yildiz AP, Darici H, Yavuz B, Abamor ES, Ozdemir C, Yasin ME, Bagirova M, Allahverdiyev A, Karaoz E. Preparation and characterization of graphene-based 3D biohybrid hydrogel bioink for peripheral neuroengineering. J Visualized Exp 2022:e63622.
Guo S, Bezard E, Zhao B. Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS–NO pathway. Free Radic Biol Med. 2005;39(5):682–95.
Sabnis RW. Handbook of biological dyes and stains: synthesis and industrial applications. Wiley, ISBN:9780470586242; 2010.
Abeliovich A, Beal FM. Parkinsonism genes: culprits and clue. J Neurochem. 2006;99:1062–72.
Avwenagha O, Campbell G, Bird MM. Distribution of GAP-43, beta-III tubulin and F-actin in developing and regenerating axons and their growth cones in vitro, following neurotrophin treatment. J Neurocytol. 2003;32:1077–89.
Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability—a point of convergence in Parkinsonian neurodegeneration? J Neurochem. 2009;110:1514–22.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641.
Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7(1):1525–41.
Yuan Z, Kolluri KK, Gowers KH, Janes SM. Trail delivery by MKH-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles. 2017;6(1):1265291.
Qing L, Chen H, Tang J, Jia X. Exosomes and their microRNA cargo: new players in peripheral nerve regeneration. Neurorehabil Neural Repair. 2018;32(9):765–76.
Darici H, Sun E, Koyuncu-Irmak D, Karaöz E, Mesenchymal stem cells for the treatment of COVID-19: why and when they should be used? In Khan M. (eds), Human mesenchymal stem cells, Journal of Stem Cells, 4 (15),159-181; 2021.
Koyuncu-Irmak D, Darici H, Karaoz E. Stem cell-based therapy option in COVID-19: is it promising? Aging Dis. 2020;11(5):1174.
Othman SA, Soon CF, Ma F, Tee KS, Lim GP, Morsin M, Ahmad MK, Abdulmaged AI, Cheong SC. Alginate-gelatin bioink for bioprinting of HeLa spheroids in alginate-gelatin hexagon-shaped scaffolds. Polym Bull. 2021;78:6115-6135.
Achilli TM, McCalla S, Tripathi A, Morgan JR. Quantification of the kinetics and extent of self-sorting in three-dimensional spheroids. Tissue Eng Part C Methods. 2012;4(18):302–9.
Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L, Sun X. Dopamine-loaded blood exosomes targeted to the brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–66.
Imaninezhad M, Hill L, Kolar G, Vogt K, Zustiak SP. Templated macroporous polyethylene glycol hydrogels for spheroid and aggregate cell culture. Bioconjug Chem. 2019;30(1):34–46.
Funding
This work was supported by the Yildiz Technical University Scientific Research Projects Coordination Department (YTU BAP, Project Number: TSA-2021-4713), respectively.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by BY, APZY, ESA, HD, and AA. The first draft of the manuscript was written by BY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yavuz, B., Yildiz, A.P.Z., Abamor, E.S. et al. Neuroprotective Effects of Wharton Jelly Stem Cell-Derived Exosomes Developed as Nano-Drug Delivery System in 6-OHDA-Induced Neurotoxicity in 2D and 3D Neuronal Cell Line. Regen. Eng. Transl. Med. (2023). https://doi.org/10.1007/s40883-023-00322-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40883-023-00322-0