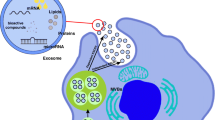
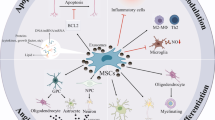
Abstract
Neurologic complications are commonly regarded as irreversible impairments that stem from limited potential of regeneration of the central nervous system (CNS). On the other side, the regenerative potential of stem cells has been evaluated in basic research, as well as in preclinical studies. Mesenchymal stem cells (MSCs) have been regarded as candidate cell sources for therapeutic purposes of various neurological disorders, because of their self-renewal ability, plasticity in differentiation, neurotrophic characteristics, and immunomodulatory properties. Exosomes are extracellular vesicles which can deliver biological information over long distances and thereby influencing normal and abnormal processes in cells and tissues. The therapeutic capacity of exosomes relies on the type of cell, as well as on the physiological condition of a given cell. Therefore, based on tissue type and physiological condition of CNS, exosomes may function as contributors or suppressors of pathological conditions in this tissue. When it comes to the therapeutic viewpoint, the most promising cellular source of exosomes is considered to be MSCs. The aim of this review article is to discuss the current knowledge around the potential of stem cells and MSC-derived exosomes in the treatment of neurodegenerative diseases.

Similar content being viewed by others
References
Przedborski S, Vila M, Jackson-Lewis V (2003) Series Introduction: Neurodegeneration: What is it and where are we? J Clin Invest 111(1):3–10
Skovronsky DM, Lee VM-Y, Trojanowski JQ (2006) Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol Mech Dis 1:151–170
Barchet TM, Amiji MM (2009) Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin Drug Deliv 6(3):211–225
Banks WA (2016) From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 15(4):275
Andaloussi SE, Mäger I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12(5):347
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383
Aryani A, Denecke B (2016) Exosomes as a nanodelivery system: a key to the future of neuromedicine? Mol Neurobiol 53(2):818–834
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341
Bongso A, Fong CY, Gauthaman K (2008) Taking stem cells to the clinic: major challenges. J Cell Biochem 105(6):1352–1360
Raff M (2003) Adult stem cell plasticity: fact or artifact? Annu Rev Cell Dev Biol 19(1):1–22
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. science 318(5858):1917–1920
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Menon S, Shailendra S, Renda A, Longaker M, Quarto N (2016) An overview of direct somatic reprogramming: the ins and outs of iPSCs. Int J Mol Sci 17(1):141
Lopez-Verrilli M, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M (2016) Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 320:129–139
Salgado AJ, Sousa JC, Costa BM, Pires AO, Mateus-Pinheiro A, Teixeira F, Pinto L, Sousa N (2015) Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities. Front Cell Neurosci 9:249
Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G (2010) Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry 15(12):1164
Levy Y, Bahat-Stroomza M, Levy Y, Bahat-Stroomza M, Barzilay R, Burshtein A, Bulvik S, Barhum Y et al (2008) Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson’s disease. Cytotherapy 10(4):340–352
Somoza R, Juri C, Baes M, Wyneken U, Rubio FJ (2010) Intranigral transplantation of epigenetically induced BDNF-secreting human mesenchymal stem cells: implications for cell-based therapies in Parkinson’s disease. Biol Blood Marrow Transplant 16(11):1530–1540
Zhu J, Liu Q, Jiang Y, Wu L, Xu G, Liu X (2015) Enhanced angiogenesis promoted by human umbilical mesenchymal stem cell transplantation in stroked mouse is Notch1 signaling associated. Neuroscience 290:288–299
Marconi S, Bonaconsa M, Scambi I, Squintani G, Rui W, Turano E, Ungaro D, D’Agostino S et al (2013) Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience 248:333–343
Baez-Jurado E, Hidalgo-Lanussa O, Guio-Vega G, Ashraf GM, Echeverria V, Aliev G, Barreto GE (2018) Conditioned medium of human adipose mesenchymal stem cells increases wound closure and protects human astrocytes following scratch assay in vitro. Mol Neurobiol 55(6):5377–5392. https://doi.org/10.1007/s12035-017-0771-4
Torrente D, Avila MF, Cabezas R, Morales L, Gonzalez J, Samudio I, Barreto GE (2014) Paracrine factors of human mesenchymal stem cells increase wound closure and reduce reactive oxygen species production in a traumatic brain injury in vitro model. Hum Exp Toxicol 33(7):673–684. https://doi.org/10.1177/0960327113509659
Cabezas R, Baez-Jurado E, Hidalgo-Lanussa O, Echeverria V, Ashrad GM, Sahebkar A, Barreto GE (2018) Growth factors and neuroglobin in astrocyte protection against neurodegeneration and oxidative stress. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1203-9
Baez E, Echeverria V, Cabezas R, Avila-Rodriguez M, Garcia-Segura LM, Barreto GE (2016) Protection by neuroglobin expression in brain pathologies. Front Neurol 7:146. https://doi.org/10.3389/fneur.2016.00146
Baez-Jurado E, Vega GG, Aliev G, Tarasov VV, Esquinas P, Echeverria V, Barreto GE (2018) Blockade of neuroglobin reduces protection of conditioned medium from human mesenchymal stem cells in human astrocyte model (T98G) under a scratch assay. Mol Neurobiol 55(3):2285–2300. https://doi.org/10.1007/s12035-017-0481-y
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW et al (1999) Multilineage potential of adult human mesenchymal stem cells. science 284(5411):143–147
Bianco P (2014) “Mesenchymal” stem cells. Annu Rev Cell Dev Biol 30:677–704
Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang C-Y (2013) The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19(1):35
Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, Robey PG (1997) Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res 12(9):1335–1347
Batouli S, Miura M, Brahim J, Tsutsui T, Fisher L, Gronthos S, Robey PG, Shi S (2003) Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 82(12):976–981
Cocucci E, Meldolesi J (2015) Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25(6):364–372
Cocucci E, Racchetti G, Meldolesi J (2009) Shedding microvesicles: artefacts no more. Trends Cell Biol 19(2):43–51
Wideman JG, Leung KF, Field MC, Dacks JB (2014) The cell biology of the endocytic system from an evolutionary perspective. Cold Spring Harb Perspect Biol 6(4):a016998
Lopez-Verrilli MA (2013) Exosomes: mediators of communication in eukaryotes. Biol Res 46(1):5–11
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Pan B-T, Teng K, Wu C, Adam M, Johnstone RM (1985) Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101(3):942–948
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97(2):329–339
Hanson PI, Cashikar A (2012) Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 28:337–362
Savina A, Furlán M, Vidal M, Colombo MI (2003) Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem 278(22):20083–90
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319(5867):1244–1247
Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T (2013) Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 288(15):10849–59
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F et al (2012) Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14(7):677
Hurley JH, Odorizzi G (2012) Get on the exosome bus with ALIX. Nat Cell Biol 14(7):654
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A et al (2013) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 4:2980
Simons M, Raposo G (2009) Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol 21(4):575–581
Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE (2007) Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol 67(13):1815–1829
Chaput N, Théry C (2011) Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 33(5):419–40
Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E et al (2011) Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20(1):131–139
Pols MS, Klumperman J (2009) Trafficking and function of the tetraspanin CD63. Exp Cell Res 315(9):1584–1592
Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ (1998) Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 273(32):20121–20127
Théry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 30(1):3.22.21–23.22.29
Strauss K, Goebel C, Runz H, Möbius W, Weiss S, Feussner I, Simons M, Schneider A (2010) Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem 285(34):26279–88
Grapp M, Wrede A, Schweizer M, Hüwel S, Galla H-J, Snaidero N, Simons M, Bückers J et al (2013) Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 4:2123
Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV et al (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014; 3:https://doi.org/10.3402/jev.v3.26913
Kowal J, Tkach M, Thery C (2014) Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29:116–125
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA et al (2012) Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119(3):756–766
Christianson HC, Svensson KJ, van Kuppevelt TH, Li J-P, Belting M (2013) Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci 110(43):17380–5
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654
Tian T, Zhu Y-L, Zhou Y-Y, Liang G-F, Wang Y-Y, Hu F-H, Xiao Z-D (2014) Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 289(32):22258–67
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF (2010) Cellular internalization of exosomes occurs through phagocytosis. Traffic 11(5):675–687
Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch U-K et al (2011) Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 124(3):447–458
Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Morgelin M, Belting M (2013) Exosome uptake depends on ERK1/2-heat shock protein 27 signalling and lipid raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem 288(24):17713-24
Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD (2013) Dynamics of exosome internalization and trafficking. J Cell Physiol 228(7):1487–1495
Mulcahy LA, Pink RC, Carter DRF (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3(1):24641
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E et al (2009) Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 284(49):34211-22
Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 11(9):1143
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief C, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183(3):1161–1172
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G et al (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med 4(5):594
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J et al (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19(10):1769–1779
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A et al (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30
Pusic AD, Pusic KM, Clayton BL, Kraig RP (2014) IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol 266(1–2):12–23
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 81(10):1171–1182
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A et al (2014) Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25(4):501–515
Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H et al (2015) Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat Commun 6:6716
Pivoraitė U, Jarmalavičiūtė A, Tunaitis V, Ramanauskaitė G, Vaitkuvienė A, Kašėta V, Biziulevičienė G, Venalis A et al (2015) Exosomes from human dental pulp stem cells suppress carrageenan-induced acute inflammation in mice. Inflammation 38(5):1933–1941
S-i O, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T et al (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21(1):185–191
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, Jiang X, Hou D et al (2015) Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep 5:17543
Lee C, Hu J, Ralls S, Kitamura T, Loh YP, Yang Y, Mukouyama Y-s, Ahn S (2012) The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS One 7(11):e50501
Chever O, Pannasch U, Ezan P, Rouach N (2014) Astroglial connexin 43 sustains glutamatergic synaptic efficacy. Philos Trans R Soc B 369(1654):20130596
Mercier F, Hatton GI (2001) Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? J Comp Neurol 431(1):88–104
Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, Ferreira JV, Catarino S, Pinho MJ, Zuzarte M, Anjo SI et al (2015) Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep 5:13243
Evans WH, Leybaert L (2007) Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun Adhes 14(6):265–273
Evans W, Boitano S (2001) Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans 2001;29(Pt 4):606-12
Garcion E, Halilagic A, Faissner A (2004) Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 131(14):3423–3432
Chiquet-Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS (2014) Tenascins in stem cell niches. Matrix Biol 37:112–123
Ferhat L, Chevassus-Au-Louis N, Khrestchatisky M, Ben-Ari Y, Represa A (1996) Seizures induce tenascin-C mRNA expression in neurons. J Neurocytol 25(1):535–546
Kim MY, Kim OR, Choi YS, Lee H, Park K, Lee C-T, Kang KW, Jeong S (2012) Selection and characterization of tenascin C targeting peptide. Mol Cell 33(1):71–77
Schwarz JM (2015) Using fluorescence activated cell sorting to examine cell-type-specific gene expression in rat brain tissue. J Vis Exp 99:e52537
Okaty BW, Sugino K, Nelson SB (2011) A quantitative comparison of cell-type-specific microarray gene expression profiling methods in the mouse brain. PLoS One 6(1):e16493
Pastrana E, Cheng L-C, Doetsch F (2009) Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci 106(15):6387–6392
Bonaguidi MA, Peng C-Y, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA (2008) Noggin expands neural stem cells in the adult hippocampus. J Neurosci 28(37):9194–9204
Heng YHE, Zhou B, Harris L, Harvey T, Smith A, Horne E, Martynoga B, Andersen J et al (2014) NFIX regulates proliferation and migration within the murine SVZ neurogenic niche. Cereb Cortex 25(10):3758–3778
Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, Alvarez-Buylla A (2015) Embryonic origin of postnatal neural stem cells. Cell 161(7):1644–1655
Alvarez-Buylla A, García-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2(4):287
Shen Q, Temple S (2009) Fine control: microRNA regulation of adult neurogenesis. Nat Neurosci 12(4):369
Zheng K, Li H, Zhu Y, Zhu Q, Qiu M (2010) MicroRNAs are essential for the developmental switch from neurogenesis to gliogenesis in the developing spinal cord. J Neurosci 30(24):8245–8250
Zhang Z, Yan R, Zhang Q, Li J, Kang X, Wang H, Huan L, Zhang L et al (2014) Hes1, a Notch signaling downstream target, regulates adult hippocampal neurogenesis following traumatic brain injury. Brain Res 1583:65–78
Coolen M, Katz S, Bally-Cuif L (2013) miR-9: a versatile regulator of neurogenesis. Front Cell Neurosci 7:220
Wang C, Yao N, Lu C-L, Li D, Ma X (2010) Mouse microRNA-124 regulates the expression of Hes1 in P19 cells. Front Biosci (Elite Ed) 2:127–132
Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L et al (2011) MicroRNA profiling in subventricular zone after stroke: miR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One 6(8):e23461
Patterson M, Gaeta X, Loo K, Edwards M, Smale S, Cinkornpumin J, Xie Y, Listgarten J et al (2014) let-7 miRNAs can act through notch to regulate human gliogenesis. Stem Cell Rep 3(5):758–773
Rafalski VA, Ho PP, Brett JO, Ucar D, Dugas JC, Pollina EA, Chow LM, Ibrahim A et al (2013) Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nat Cell Biol 15(6):614
Zhang L, Wang X, Chen P (2013) MiR-204 down regulates SIRT1 and reverts SIRT1-induced epithelial-mesenchymal transition, anoikis resistance and invasion in gastric cancer cells. BMC Cancer 13(1):290
Lee HK, Finniss S, Cazacu S, Xiang C, Brodie C (2014) Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expression. Stem Cells Dev 23(23):2851–2861
Huang G-J, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88(9):792–806
Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L et al (2014) Glial origin of mesenchymal stem cells in a tooth model system. Nature 513(7519):551
Jarmalavičiūtė A, Tunaitis V, Strainienė E, Aldonytė R, Ramanavičius A, Venalis A, Magnusson K-E, Pivoriūnas A (2013) A new experimental model for neuronal and glial differentiation using stem cells derived from human exfoliated deciduous teeth. J Mol Neurosci 51(2):307–317
Kiraly M, Porcsalmy B, Pataki A, Kadar K, Jelitai M, Molnar B, Hermann P, Gera I et al (2009) Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int 55(5):323–332
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S (2008) Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 26(7):1787–1795
Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R et al (2012) Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 122(1):80–90
Nosrat IV, Widenfalk J, Olson L, Nosrat CA (2001) Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol 238(1):120–132
Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A (2015) Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy 17(7):932–939
Mazzio EA, Reams RR, Soliman KF (2004) The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro. Brain Res 1004(1–2):29–44
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M et al (2013) Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4(2):34
Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR (2010) αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One 5(10):e12578
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y et al (2013) Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3:1197
Miners JS, Barua N, Kehoe PG, Gill S, Love S (2011) Aβ-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol 70(11):944–959
Farinazzo A, Turano E, Marconi S, Bistaffa E, Bazzoli E, Bonetti B (2015) Murine adipose-derived mesenchymal stromal cell vesicles: in vitro clues for neuroprotective and neuroregenerative approaches. Cytotherapy 17(5):571–578
Bonafede R, Scambi I, Peroni D, Potrich V, Boschi F, Benati D, Bonetti B, Mariotti R (2016) Exosome derived from murine adipose-derived stromal cells: neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res 340(1):150–158
Kordelas L, Rebmann V, Ludwig A, Radtke S, Ruesing J, Doeppner T, Epple M, Horn P et al (2014) MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28(4):970
Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig A-K, Radtke S, de Miroschedji K, Horn PA et al (2015) Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med 4(10):1131–1143
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33(11):1711–1715
Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y (2015) Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg 122(4):856–867
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG et al (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7):1556–1564
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A (2007) A microRNA feedback circuit in midbrain dopamine neurons. Science 317(5842):1220–1224
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y et al (2012) Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 22(6):845–854
Bernardo ME, Fibbe WE (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13(4):392–402
Author information
Authors and Affiliations
Contributions
Designed and conceived the idea: AMG, NK, HAT, AS
Wrote the manuscript: AMG, NK, GEB, HAT, AS
All authors approved the final manuscript and submission.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gorabi, A.M., Kiaie, N., Barreto, G.E. et al. The Therapeutic Potential of Mesenchymal Stem Cell–Derived Exosomes in Treatment of Neurodegenerative Diseases. Mol Neurobiol 56, 8157–8167 (2019). https://doi.org/10.1007/s12035-019-01663-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01663-0