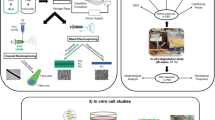
Abstract
Purpose
Cell therapy is a new and evolving treatment for large-scale bone defects. Here, we describe a novel approach that combines hydrogel-based cell therapy with low-intensity pulsed ultrasound (LIPUS), an FDA-approved treatment for fracture repair.
Methods
Bone marrow–derived stromal cells (BMSCs) were encapsulated in RGD-peptide-coupled alginate hydrogels and mechanically stimulated using LIPUS-derived acoustic radiation force (ARF).
Results
Mechanical analysis of alginate hydrogels revealed a dependence on either alginate concentration or cross-linker concentration, revealing different ways to adjust the mechanical properties of the hydrogel. An optimal alginate and cross-linker concentration were found that allowed both robust cell proliferation and hydrogel mechanical stability. Cell proliferation was inversely proportional to hydrogel stiffness, suggesting that stiffer hydrogels were preventing full cell spreading and migration within the hydrogel. Cells encapsulated in hydrogels of optimal stiffness responded immediately after the onset of ARF by upregulating calcium, and shortly after upregulating cyclooxygenase-2 and prostaglandin E2, and later forming mineralized tissue in culture. Interestingly, cells seeded on top of hydrogels did not demonstrate the same calcium flux as encapsulated cells, suggesting that encapsulating the cells provided an additional mechanical stimulus. COX-2 and PGE2 data from cells encapsulated in hydrogels with no ARF showed a similar response to encapsulation alone.
Conclusion
These studies support the idea of combining cell therapy with LIPUS-derived ARF to enhance mineralized tissue formation.
Lay Summary
Ultrasound has been a tool available to clinicians for a long time, and for a wide range of applications, including bone repair. Here, we show that it can stimulate stem cells that have been encapsulated in hydrogels to differentiate into bone cells through mechanical stimulation. This work would allow a clinician to implant stem cells into a large bone defect and stimulate them using ultrasound to differentiate into bone cells and help these large defects heal more quickly. This stimulation would be done transdermally and non-invasively, allowing the defect to heal with minimal intervention.








Similar content being viewed by others
References
Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32(1):112–22.
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408.
Murphy MP, Quarto N, Longaker MT, Wan DC. (*) Calvarial defects: cell-based reconstructive strategies in the murine model. Tissue Eng Part C Methods. 2017;23(12):971–81.
Stewart S, Bryant SJ, Ahn J, Hankenson KD. Chapter 24 - Bone regeneration. In: Atala A, Allickson JG, editors. Translational Regenerative Medicine. Boston: Academic Press; 2015. p. 313–33.
Zimmermann G, Moghaddam A. Allograft bone matrix versus synthetic bone graft substitutes. Injury. 2011;42(Suppl 2):S16–21.
Parikh SN. Bone graft substitutes: past, present, future. J Postgrad Med. 2002;48(2):142–8.
Ibrahim A. 13 - 3D bioprinting bone. In: Thomas DJ, Jessop ZM, Whitaker IS, editors. 3D Bioprinting for Reconstructive Surgery. Woodhead Publishing; 2018. p. 245–75.
He R, Chen J, Jiang J, Liu B, Liang D, Zhou W, Chen W, Wang Y. Synergies of accelerating differentiation of bone marrow mesenchymal stem cells induced by low intensity pulsed ultrasound, osteogenic and endothelial inductive agent, Artif. Cells, Nanomed. Biotechnol. 2019;47(1):673–83.
Wang Y, Peng W, Liu X, Zhu M, Sun T, Peng Q, Zeng Y, Feng B, Zhi W, Weng J, Wang J. Study of bilineage differentiation of human-bone-marrow-derived mesenchymal stem cells in oxidized sodium alginate/N-succinyl chitosan hydrogels and synergistic effects of RGD modification and low-intensity pulsed ultrasound. Acta Biomater. 2014;10(6):2518–28.
Nguyen B-NB, Moriarty RA, Kamalitdinov T, Etheridge JM, Fisher JP. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J Biomed Mater Res A. 2017;105(4):1123–31.
Zhou X, Castro NJ, Zhu W, Cui H, Aliabouzar M, Sarkar K, Zhang LG. Improved human bone marrow mesenchymal stem cell osteogenesis in 3D bioprinted tissue scaffolds with low intensity pulsed ultrasound stimulation. Sci Rep. 2016;6(1):32876.
Maisani M, Ziane S, Ehret C, Levesque L, Siadous R, Le Meins J-F, Chevallier P, Barthélémy P, De Oliveira H, Amédée J, Mantovani D, Chassande O. A new composite hydrogel combining the biological properties of collagen with the mechanical properties of a supramolecular scaffold for bone tissue engineering. J Tissue Eng Regen Med. 2018;12(3):e1489–500.
Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, Grigolo B. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C. 2017;78:1246–62.
Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, Li S, Deng Y, He N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Research. 2017;5(1):17014.
Arrighi N. 3 - Stem cells at the core of cell therapy. In: Arrighi N, editor. Stem Cells. Elsevier; 2018. p. 73–100.
Carson CT, Emre N, McIntyre C, Fong TC. 3.36 - Cellular therapies. In: Moo-Young M, editor. Comprehensive Biotechnology (Third Edition). Oxford: Pergamon; 2011. p. 446–59.
Rothrauff BB, Pirosa A, Lin H, Sohn J, Langhans MT, Tuan RS. Chapter 54 - Stem cell therapy for musculoskeletal diseases. In: Atala A, Lanza R, Mikos AG, Nerem R, editors. Principles of Regenerative Medicine (Third Edition). Boston: Academic Press; 2019. p. 953–70.
Veronick J, Assanah F, Nair LS, Vyas V, Huey B, Khan Y. The effect of acoustic radiation force on osteoblasts in cell/hydrogel constructs for bone repair. Exp Biol Med. 2016;241(10):1149–56.
Veronick JA, Assanah F, Piscopo N, Kutes Y, Vyas V, Nair LS, Huey BD, Khan Y. Mechanically loading cell/hydrogel constructs with low-intensity pulsed ultrasound for bone repair. Tissue Eng Part A. 2018;24(3-4):254–63.
Assanah F, Khan Y. Cell responses to physical forces, and how they inform the design of tissue-engineered constructs for bone repair: a review. J Mater Sci. 2018;53(8):5618–40.
Lee DA, Knight MM, Campbell JJ, Bader DL. Stem cell mechanobiology. J Cell Biochem. 2011;112(1):1–9.
MacQueen L, Sun Y, Simmons CA. Mesenchymal stem cell mechanobiology and emerging experimental platforms. J R Soc Interface. 2013;10(84):20130179.
Huang CH, Chen MH, Young TH, Jeng JH, Chen YJ. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem. 2009;108(6):1263–73.
Klein-Nulend J, Burger EH, Semeins CM, Raisz LG, Pilbeam CC. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res. 1997;12(1):45–51.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89.
Sun M, Chi G, Li P, Lv S, Xu J, Xu Z, Xia Y, Tan Y, Xu J, Li L, Li Y. Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. Int J Med Sci. 2018;15(3):257–68.
Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y, Li Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res Ther. 2015;6(1):103.
Pal P, Nguyen QC, Benton AH, Marquart ME, Janorkar AV. Drug-loaded elastin-like polypeptide–collagen hydrogels with high modulus for bone tissue engineering. Macromol Biosci. 2019;19(9):1900142.
Assanah F, Grassie K, Anderson H, Xin X, Rowe D, Khan Y. Ultrasound-derived mechanical stimulation of cell-laden collagen hydrogels for bone repair. J Biomed Mater Res A. 2023;111(8):1200–15.
Duan P, Kandemir N, Wang J, Chen J. Rheological characterization of alginate based hydrogels for tissue engineering. MRS Advances. 2017;2(24):1309–14.
Campbell JJ, Bader DL, Lee DA. Mechanical loading modulates intracellular calcium signaling in human mesenchymal stem cells. J Appl Biomater Biomech. 2008;6(1):9–15.
Guo G, Ma Y, Guo Y, Zhang C, Guo X, Tu J, Yu ACH, Wu J, Zhang D. Enhanced porosity and permeability of three-dimensional alginate scaffolds via acoustic microstreaming induced by low-intensity pulsed ultrasound. Ultrason Sonochem. 2017;37:279–85.
Guo G, Lu L, Ji H, Ma Y, Dong R, Tu J, Guo X, Qiu Y, Wu J, Zhang D. Low intensity pulse ultrasound stimulate chondrocytes growth in a 3-D alginate scaffold through improved porosity and permeability. Ultrasonics. 2015;58:43–52.
Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci. 2003;100(9):5413–8.
Wells R. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–400.
Bott K, Upton Z, Schrobback K, Ehrbar M, Hubbell J, Lutolf M, Rizzi S. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 2010;31:8454–64.
Anamizu M, Tabata Y. Design of injectable hydrogels of gelatin and alginate with ferric ions for cell transplantation. Acta Biomater. 2019;100:184–90.
Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53.
Sarker B, Rompf J, Silva R, Lang N, Detsch R, Kaschta J, Fabry B, Boccaccini AR. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int J Biol Macromol. 2015;78:72–8.
You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–86.
Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10(1):11–20.
Chen T, Buckley M, Cohen I, Bonassar L, Awad HA. Insights into interstitial flow, shear stress, and mass transport effects on ECM heterogeneity in bioreactor-cultivated engineered cartilage hydrogels. Biomech Model Mechanobiol. 2012;11(5):689–702.
Hung CT, Allen FD, Pollack SR, Brighton CT. Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J Biomech. 1996;29(11):1411–7.
Yoon CW, Jung H, Goo K, Moon S, Koo KM, Lee NS, Weitz AC, Shung KK. Low-intensity ultrasound modulates Ca(2+) dynamics in human mesenchymal stem cells via connexin 43 hemichannel. Ann Biomed Eng. 2018;46(1):48–59.
Zhang S, Cheng J, Qin Y-X. Mechanobiological modulation of cytoskeleton and calcium influx in osteoblastic cells by short-term focused acoustic radiation force. PloS One. 2012;7(6):e38343.
Li YJ, Batra NN, You L, Meier SC, Coe IA, Yellowley CE, Jacobs CR. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22(6):1283–9.
Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31(11):969–76.
Wenfu Z, Xie Y, Zhang W, Wang D, Wanshun M, Wang Z, Jiang X. Fluid flow stress induced contraction and re-spread of mesenchymal stem cells: a microfluidic study. Integr Biol. 2012;4:1102–11.
Reich K, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol. 1991;261:C428–32.
Nauman E, Satcher R, Keaveny T, Halloran B, Bikle D. Osteoblasts respond to pulsatile fluid flow with short-term increases in PGE2 but no change in mineralization. J Appl Physiol. 1985;90(2001):1849–54.
Donahue T, Haut T, Yellowley CE, Donahue H, Jacobs C. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech. 2003;36:1363–71.
Mullender M, El Haj AJ, Yang Y, van Duin MA, Burger EH, Klein-Nulend J. Mechanotransduction of bone cellsin vitro: mechanobiology of bone tissue. Med Biol Eng Comput. 2004;42(1):14–21.
Kokubu T, Matsui N, Fujioka H, Tsunoda M, Mizuno K. Low intensity pulsed ultrasound exposure increases prostaglandin E2 production via the induction of cyclooxygenase-2 mRNA in mouse osteoblasts. Biochem Biophys Res Commun. 1999;256(2):284–7.
Li JG, Chang WH, Lin JC, Sun JS. Optimum intensities of ultrasound for PGE(2) secretion and growth of osteoblasts. Ultrasound Med Biol. 2002;28(5):683–90.
Li J, Chang W, Lin C-A, Sun J-S. Optimum intensities of ultrasound for PGE2 secretion and growth of osteoblasts. Ultrasound Med Biol. 2002;28:683–90.
Tsai CL, Chang WH, Liu TK, Song GM. Ultrasound can affect bone healing both locally and systemically. Chin J Physiol. 1991;34(2):213–22.
Harle J, Salih V, Mayia F, Knowles JC, Olsen I. Effects of ultrasound on the growth and function of bone and periodontal ligament cells in vitro. Ultrasound Med Biol. 2001;27(4):579–86.
Yang K-H, Parvizi J, Wang S-J, Lewallen DG, Kinnick RR, Greenleaf JF, Bolander ME. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res. 1996;14(5):802–9.
Nagai M, Suzuki Y, Ota M. Systematic assessment of bone resorption, collagen synthesis, and calcification in chick embryonic calvaria in vitro: effects of prostaglandin E2. Bone. 1993;14(4):655–9.
Reher P, Harris M, Whiteman M, Hai HK, Meghji S. Ultrasound stimulates nitric oxide and prostaglandin e2 production by human osteoblasts. Bone. 2002;31(1):236–41.
Reher P, Elbeshir E-NI, Harvey W, Meghji S, Harris M. The stimulation of bone formation in vitro by therapeutic ultrasound. Ultrasound Med Biol. 1997;23(8):1251–8.
Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86(1):617–28.
Delaine-Smith RM, Reilly GC. Mesenchymal stem cell responses to mechanical stimuli. Muscles, Ligaments Tendons J. 2012;2(3):169–80.
Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7(3):211–24.
Zhao F, Chella R, Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: experiments and hydrodynamic modeling. Biotechnol Bioeng. 2007;96(3):584–95.
McCoy RJ, O'Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Eng Part B Rev. 2010;16(6):587–601.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
Research reported in this publication was wholly supported by the National Science Foundation under award #1752915 and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR073206.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y. K., F. A. Experimental design: Y. K., F. A., H. A., K. G., L. N. Experimental work: F. A., H. A., K. G. Manuscript preparation, writing, and editing: Y. K., F. A., L. N. Final approval of manuscript: F. A., Y. K., K. G., H. A., L. N.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
Supplementary Figure I: The COX-2 and the PGE2 quantification of 0.25 % and 0.5 % alginate hydrogels were exposed to a one-time ultrasound exposure of 300 mW/cm2 spatial intensity. (A) COX-2 gene expression was quantified through RT-PCR. Ultrasound treatment of BMSCs encapsulated in 0.5 % alginate hydrogel enhanced COX-2 expression over the control, although not significantly. (B) Normalized PGE2 expression per 100,000 cells quantified through ELISA. PGE2 expression was significantly upregulated in 0.5 % hydrogels when treated with ultrasound compared to the control groups. As the matrix stiffness increased from 0.25 % to the 0.5 % alginate hydrogel, both COX-2 and PGE2 levels were increased. Error is reported in bar graphs as the standard deviation of the mean. Statistical significance noted (*P < 0.05), (n = 3). (DOCX 440 kb)
ESM 2
Supplementary Figure II: (A) Rheology data showing the storage and loss modulus of 2 % alginate with varying concentrations of CaCl2. Both storage and loss modulus increase with increasing concentration of CaCl2 showing that increased ionic cross-linking within the alginate solution makes stiffer hydrogels. (B) Storage and loss modulus of varying alginate solution with 10 mM CaCl2. Both the storage and loss modulus increase with an increase in the concentration of alginate solution. (DOCX 274 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Assanah, F., Anderson, H., Grassie, K. et al. Ultrasound-Derived Mechanical Stimulation of Alginate Hydrogels for Bone Repair: an In Vitro Study. Regen. Eng. Transl. Med. (2023). https://doi.org/10.1007/s40883-023-00312-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40883-023-00312-2