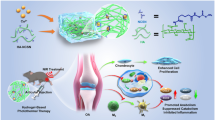
Abstract
Purpose
Osteoarthritis (OA) is a highly prevalent joint disease for which common therapies may provide symptomatic relief but have failed to provide a curative solution. There has been a growing interest in disease-modifying therapies, such as the use of stem cells and bioactive factors in treating OA. The synthetic artificial stem cell (SASC) system has previously been introduced as one such disease-modifying therapy: designed to mimic the paracrine effect of biological stem cells but with the added ability to engineer the composition and tailor it to different tissue types.
Methods
In the present study, the efficacy of a single intra-articular injection was evaluated across three doses (1, 3, or 5 million) of SASC cells in a collagenase-induced OA rat model. Inflammation was monitored by joint swelling through the 63 day treatment period and structural and functional cartilage regeneration outcomes as well as local immune and inflammatory outcomes were evaluated by histology, nano-indentation of the gross cartilage, and immunohistochemistry. Further, in vitro SASC modulation of the NF-κβ pathway was observed by monitoring various upstream and downstream gene expression profiles along the pathway.
Results
A single administration of 3 million and 5 million SASC cells reduced knee swelling attenuates cartilage degeneration as observed by Safranin O staining, compared to the OA control. Further, major genes along the NF-κβ pathway were significantly modulated by SASC to a similar extent compared to ADSC control.
Conclusion
The present study demonstrated that SASC Cells have a dose-dependent response in attenuating collagenase-induced OA progression and effectively modulates the major inflammatory pathway involved in the pathogenesis of OA.
Lay Summary
In this study, we investigated the efficacy of synthetic artificial stem cells (SASC) in treating osteoarthritis (OA). Different doses of SASC cells were injected into rats with OA. Higher doses of SASC cells reduced knee swelling, protected against cartilage degeneration, and modulated the inflammatory NF-κβ pathway. This work highlights potential of SASC cells as a curative treatment for OA.









Similar content being viewed by others
Data Availability
Published data will be open-access and available publicly.
References
Bhattacharjee M, et al. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc Natl Acad Sci USA. 2022;119(4):e2120968119. https://doi.org/10.1073/pnas.2120968119.
Shah S, Otsuka T, Bhattacharjee M, Laurencin CT. Minimally Invasive Cellular Therapies for Osteoarthritis Treatment. Regen Eng Transl Med. 2021;7(1):76–90. https://doi.org/10.1007/s40883-020-00184-w.
Daneshmandi L, et al. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020;38(12):1373–84. https://doi.org/10.1016/j.tibtech.2020.04.013.
Richardson SM, et al. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69–80. https://doi.org/10.1016/j.ymeth.2015.09.015.
Mei L, et al. Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS ONE. 2017;12(4):e0176107. https://doi.org/10.1371/journal.pone.0176107.
Katagiri W, Sakaguchi K, Kawai T, Wakayama Y, Osugi M, Hibi H. A defined mix of cytokines mimics conditioned medium from cultures of bone marrow-derived mesenchymal stem cells and elicits bone regeneration. Cell Prolif. 2017;50(3):e12333. https://doi.org/10.1111/cpr.12333.
Shah S, Esdaille CJ, Bhattacharjee M, Kan H-M, Laurencin CT. The synthetic artificial stem cell (SASC): Shifting the paradigm of cell therapy in regenerative engineering. Proc Natl Acad Sci USA. 2022;119(2):e2116865118. https://doi.org/10.1073/pnas.2116865118.
Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil. 2013;21(1):16–21. https://doi.org/10.1016/j.joca.2012.11.012.
Lawrence T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651–a001651. https://doi.org/10.1101/cshperspect.a001651.
Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Sig Transduct Target Ther. 2017;2(1):17023. https://doi.org/10.1038/sigtrans.2017.23.
Man GS, Mologhianu G. Osteoarthritis pathogenesis - a complex process that involves the entire joint. J Med Life. 2014;7(1):37–41.
ter Huurne M, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64(11):3604–13. https://doi.org/10.1002/art.34626.
Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–74. https://doi.org/10.1002/art.11365.
Ude CC, et al. Cartilage Regeneration by Chondrogenic Induced Adult Stem Cells in Osteoarthritic Sheep Model. PLoS ONE. 2014;9(6):e98770. https://doi.org/10.1371/journal.pone.0098770.
Saito T, Tanaka S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-κB. Arthritis Res Ther. 2017;19(1):94. https://doi.org/10.1186/s13075-017-1296-y.
Mariani E, Pulsatelli L, Facchini A. Signaling Pathways in Cartilage Repair. IJMS. 2014;15(5):8667–98. https://doi.org/10.3390/ijms15058667.
Marcu KB, Otero M, Olivotto E, Maria Borzi R, Goldring MB. NF-κB Signaling: Multiple Angles to Target OA. CDT. 2010;11(5):599–613. https://doi.org/10.2174/138945010791011938.
Burguera EF, Vela-Anero Á, Magalhães J, Meijide-Faílde R, Blanco FJ. Effect of hydrogen sulfide sources on inflammation and catabolic markers on interleukin 1β-stimulated human articular chondrocytes. Osteoarthr Cartil. 2014;22(7):1026–35. https://doi.org/10.1016/j.joca.2014.04.031.
Kobayashi M, et al. Role of interleukin-1 and tumor necrosis factor ? in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52(1):128–35. https://doi.org/10.1002/art.20776.
Kojima F, Naraba H, Miyamoto S, Beppu M, Aoki H, Kawai S. Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res Ther. 2004;6(4):R355. https://doi.org/10.1186/ar1195.
Olivotto E, Otero M, Marcu KB, Goldring MB. Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open. 2015;1(Suppl 1):e000061. https://doi.org/10.1136/rmdopen-2015-000061.
Montaseri A, et al. IGF-1 and PDGF-bb Suppress IL-1β-Induced Cartilage Degradation through Down-Regulation of NF-κB Signaling: Involvement of Src/PI-3K/AKT Pathway. PLoS ONE. 2011;6(12):e28663. https://doi.org/10.1371/journal.pone.0028663.
Choi Jo. Park, Kang, and Park, “NF-B Signaling Pathways in Osteoarthritic Cartilage Destruction.” Cells. 2019;8(7):734. https://doi.org/10.3390/cells8070734.
Li T, et al. TGF-β type 2 receptor–mediated modulation of the IL-36 family can be therapeutically targeted in osteoarthritis. Sci Transl Med. 2019;11(491):eaan2585. https://doi.org/10.1126/scitranslmed.aan2585.
Li X-G, et al. Fibroblast growth factor 18 alleviates hyperoxia-induced lung injury in mice by adjusting oxidative stress and inflammation. Eur Rev Med Pharmacol Sci. 2021;25(3):1485–94. https://doi.org/10.26355/eurrev_202102_24856.
Bhattacharjee M, et al. Preparation and characterization of amnion hydrogel and its synergistic effect with adipose derived stem cells towards IL1β activated chondrocytes. Sci Rep. 2020;10(1):18751. https://doi.org/10.1038/s41598-020-75921-w.
Sun Z, Nair LS, Laurencin CT. The Paracrine Effect of Adipose-Derived Stem Cells Inhibits IL-1β-induced Inflammation in Chondrogenic Cells through the Wnt/β-Catenin Signaling Pathway. Regen Eng Transl Med. 2018;4(1):35–41. https://doi.org/10.1007/s40883-018-0047-1.
Yang W-T, et al. Stromal-vascular fraction and adipose-derived stem cell therapies improve cartilage regeneration in osteoarthritis-induced rats. Sci Rep. 2022;12(1):2828. https://doi.org/10.1038/s41598-022-06892-3.
Wang RM, et al. Humanized mouse model for assessing the human immune response to xenogeneic and allogeneic decellularized biomaterials. Biomaterials. 2017;129:98–110. https://doi.org/10.1016/j.biomaterials.2017.03.016.
Jenei-Lanzl Z, Meurer A, Zaucke F. Interleukin-1β signaling in osteoarthritis – chondrocytes in focus. Cell Signal. 2019;53:212–23. https://doi.org/10.1016/j.cellsig.2018.10.005.
Ahmad N, Ansari MY, Haqqi TM. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J Cell Physiol. 2020;235(10):6366–76. https://doi.org/10.1002/jcp.29607.
Paik J, Duggan ST, Keam SJ. Triamcinolone Acetonide Extended-Release: A Review in Osteoarthritis Pain of the Knee. Drugs. 2019;79(4):455–62. https://doi.org/10.1007/s40265-019-01083-3.
Zhang Z, Huang G. Intra-articular lornoxicam loaded PLGA microspheres: enhanced therapeutic efficiency and decreased systemic toxicity in the treatment of osteoarthritis. Drug Delivery. 2012;19(5):255–63. https://doi.org/10.3109/10717544.2012.700962.
Woods PS et al. Automated Indentation Demonstrates Structural Stiffness of Femoral Articular Cartilage and Temporomandibular Joint Mandibular Condylar Cartilage Is Altered in FgF2KO Mice. CARTILAGE. 2020; 194760352096256. https://doi.org/10.1177/1947603520962565
Acknowledgements
This work was supported by NIH Grant T32 AR079114. Support from the Raymond and Beverly Sackler Center is gratefully acknowledged.
The authors gratefully thank the Center for Comparative Medicine at University of Connecticut Health Center. We also thank Dr. Chia-Ling Kuo of the Connecticut Convergence Institute for Translation in Regenerative Engineering for her help with statistical analysis.
Funding
National Institutes of Health grant DP1AR068147 (CTL)
National Institutes of Health grant T32 AR079114 (CTL)
Author information
Authors and Affiliations
Contributions
Conceptualization: SS, LSN, CTL.
Methodology: SS, TAS, CTL
Investigation: SS, MB, HMK
Analysis: SS
Funding acquisition: CTL
Writing: SS, MB, HMK, RLM, TAS, LSN, CTL
Corresponding author
Ethics declarations
Competing Interests
University of Connecticut has filed a patent application on behalf of the inventors (S.S., H.M.K., L.S.N., C.T.L) entitled The Synthetic Artificial Stem Cell. L.S.N has the following competing financial interest: Soft tissue regeneration/Biorez. C.T.L. has the following competing financial interests: Mimedx, Alkermes Company, Biobind, Soft tissue regeneration/Biorez, Healing Orthopedic Technologies-Bone.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, S., Bhattacharjee, M., Kan, HM. et al. A Single Administration of Synthetic Artificial Stem Cells (SASC) Attenuates Osteoarthritis Progression. Regen. Eng. Transl. Med. 10, 78–92 (2024). https://doi.org/10.1007/s40883-023-00307-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-023-00307-z