Abstract
Purpose
We focused on the composition and architecture of the prostate extracellular matrix (ECM) and interactions with its cellular constituents. We also discussed the role of ECM remodeling on cellular behaviors in vivo.
Methods
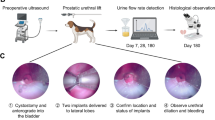
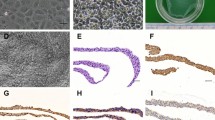
Nine natural, BPH, and malignant human prostate tissues were decellularized using a modified technique. Then, the scaffolds were implanted adjacent to the prostate in nine male GFP-positive rats which were randomly divided into group A (n = 3, natural), group B (n = 3, BPH), and group C (n = 3, malignant tissue). Biopsies were taken after six months of implantation to assess the quality of recellularization by histological evaluations. We also evaluated the impact of malignant and BPH prostatic ECM on cell proliferation, differentiation, and expression of ECM components in follow-up.
Results
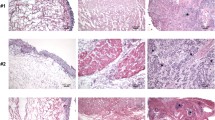
Cell removal with preservation of ECM structure was confirmed in all decellularized prostates. Histological examinations after the decellularization process illustrated down-regulated expression of laminin and vimentin in normal, BPH, and malignant tissues. Furthermore, malignant samples revealed simply lower levels in type IV Collagen content of the ECM. All of the implanted samples also contained an abundance of newly formed vessels suggestive of prominent angiogenesis. Immunostaining of all scaffolds after implantation was negative for AMACR marker revealing the absence of cancerous cells in the recellularized tissues in follow-up. The samples in group C demonstrated the highest decrease in the ECM content after recellularization.
Conclusion
The outcomes could bring us one step closer to understanding the complex dynamics of ECM–cell interactions in prostate adenocarcinoma. We concluded that the expression of some markers may change in malignant human prostatic tissues after the decellularization process.
Lay summary
Previously, we used recellularization of testicular feminization testis in C57bl6 as a natural bio-reactor for the creation of cellularized seminiferous tubules. In this study, we try to evaluate the composition and architecture of the prostate ECM and its interactions with its cellular constituents. We also aim to assess the role of ECM remodeling on cellular behaviors in vivo.
Future works
We will emphasize on assessing in vitro and in vivo characteristics of bio-scaffolds from diverse benign and malignant tissues. We will also examine the role of ECM behavior on the development of prostate cancer in our long-term follow-ups.










Similar content being viewed by others
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
T. Tarver, Cancer facts & Figures 2012. American cancer society (ACS) Atlanta, GA: American Cancer Society, 2012. 66 p., pdf. Available from, Taylor & Francis, 2012.
Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164(1):101–5.
Antonarakis ES, Shaukat F, Velho PI, Kaur H, Shenderov E, Pardoll DM, Lotan TL. Clinical features and therapeutic outcomes in men with advanced prostate cancer and DNA mismatch repair gene mutations. Eur Urol. 2019;75(3):378–82.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2016;40(2):244–52.
Tosco L, Briganti A, D’amico AV, Eastham J, Eisenberger M, Gleave M, Haustermans K, Logothetis CJ, Saad F, Sweeney C. Systematic review of systemic therapies and therapeutic combinations with local treatments for high-risk localized prostate cancer. Eur Urol. 2019;75(1):44–60.
Leach DA, Need EF, Toivanen R, Trotta AP, Palenthorpe HM, Tamblyn DJ, Kopsaftis T, England GM, Smith E, Drew PA. Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome. Oncotarget. 2015;6(18):16135.
Clark AK, Taubenberger AV, Taylor RA, Niranjan B, Chea ZY, Zotenko E, Sieh S, Pedersen JS, Norden S, Frydenberg M. A bioengineered microenvironment to quantitatively measure the tumorigenic properties of cancer-associated fibroblasts in human prostate cancer. Biomater. 2013;34(20):4777–85.
Čunderlíková B, Filová B, Kajo K, Vallová M, Balázsiová Z, Trnka M, Mateašík A. Extracellular matrix affects different aspects of cell behaviour potentially involved in response to aminolevulinic acid-based photoinactivation. J Photochemist Ph7otobiol B: Biol. 2018;189:283–91.
Sabetkish S, Kajbafzadeh A-M, Sabetkish N. Recellularization of testicular feminization testis in C57bl6 as a natural bioreactor for creation of cellularized seminiferous tubules: an experimental study. Cell Tissue Bank. 2021;22(2):287–95.
Nguyen-Ngoc K-V, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, Yaswen P, Werb Z, Ewald AJ. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proceed Nat Acad Sci. 2012;109(39):E2595–604.
Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243–53.
Walker C, Mojares E, del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19(10):3028.
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhibit Med Cchemist. 2016;31(sup1):177–83.
S Aghlara-Fotovat, A Nash, B Kim, R Krencik, O Veiseh. Targeting the extracellular matrix for immunomodulation: applications in drug delivery and cell therapies. Drug Deliv Transl Res. 2021; 1–20.
Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Models Mech. 2011;4(2):165–78.
Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol. 2012;226(2):185–99.
Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221–32.
Josefsson A, Adamo H, Hammarsten P, Granfors T, Stattin P, Egevad L, Laurent AE, Wikström P, Bergh A. Prostate cancer increases hyaluronan in surrounding nonmalignant stroma, and this response is associated with tumor growth and an unfavorable outcome. Am Pathol. 2011;179(4):1961–8.
K Girigoswami, D Saini, A Girigoswami. Extracellular matrix remodeling and development of cancer. Stem Cell Rev Rep. 2020; 1–9.
Lü W-D, Zhang L, Wu C-L, Liu Z-G, Lei G-Y, Liu J, Gao W, Hu Y-R. Development of an acellular tumor extracellular matrix as a three-dimensional scaffold for tumor engineering. PLoS One. 2014;9(7):e103672.
Worthington P, Pochan DJ, Langhans SA. Peptide hydrogels–versatile matrices for 3D cell culture in cancer medicine. Front Oncol. 2015;5:92.
HJ Lee, S Mun, DM Pham, P Kim. Extracellular matrix-based hydrogels to tailoring tumor organoids. ACS Biomater Sci Eng. 2021.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89.
Genovese L, Zawada L, Tosoni A, Ferri A, Zerbi P, Allevi R, Nebuloni M, Alfano M. Cellular localization, invasion, and turnover are differently influenced by healthy and tumor-derived extracellular matrix. Tissue Eng A. 2014;20(13–14):2005–18.
Byrne CE, Decombe J-B, Bingham GC, Remont J, Miller LG, Khalif L, King CT, Hamel K, Bunnell BA, Burow ME. Evaluation of extracellular matrix composition to improve breast cancer modeling. Tissue Eng A. 2021;27(7–8):500–11.
S Josson, S Sharp, S-Y Sung, PA Johnstone, R Aneja, R Wang, M Gururajan, T Turner, LW Chung, C Yates. Tumor-stromal interactions influence radiation sensitivity in epithelial-versus mesenchymal-like prostate cancer cells. J Oncol 2010 (2010).
Begemann D, Anastos H, Kyprianou N. Cell death under epithelial–mesenchymal transition control in prostate cancer therapeutic response. Int J Urol. 2018;25(4):318–26.
Damodarasamy M, Vernon RB, Chan CK, Plymate SR, Wight TN, Reed MJ. Hyaluronan in aged collagen matrix increases prostate epithelial cell proliferation. In Vitro Cell Dev Biol-Animal. 2015;51(1):50–8.
Cazzaniga W, Nebuloni M, Longhi E, Locatelli I, Allevi R, Lucianò R, Senatore G, Ventimiglia E, Cucchiara V, Genovese L. Human prostate tissue-derived extracellular matrix as a model of prostate microenvironment. Eur Urol Focus. 2016;2(4):400–8.
Taylor DA, Frazier OH, Elgalad A, Hochman-Mendez C, Sampaio LC. Building a total bioartificial heart: harnessing nature to overcome the current hurdles. Artif Organ. 2018;42(10):970–82.
Roacho-Pérez JA, Garza-Treviño EN, Moncada-Saucedo NK, Carriquiry-Chequer PA, Valencia-Gómez LE, Matthews ER, Gómez-Flores V, Simental-Mendía M, Delgado-Gonzalez P, Delgado-Gallegos JL. Artificial scaffolds in cardiac tissue eng. Life. 2022;12(8):1117.
Funding
This study was funded by the Tehran University of Medical Sciences (grant number: 35794).
Author information
Authors and Affiliations
Contributions
Concept/design: Abdol-Mohammad Kajbafzadeh. Data analysis/interpretation: Fahimeh Jafarnezhad-Ansariha, Seyed Hossein Hosseini Sharifi, Shabnam Sabetkish, Mahmoud Parvin, Shahin Tabatabaei, Kiarad Fendereski, Aram Akbarzadeh, Seyedeh-Sanam Ladi-Seyedian, Ahad Mohammadnejad, Behnam Nabavizadeh, Amirnader Emami Razavi, and Reza Esmaeili-Pour. Drafting article: Shabnam Sabetkish, Fahimeh Jafarnezhad-Ansariha, and Seyed Hossein Hosseini Sharifi. Critical revision of article and approval of article: Abdol-Mohammad Kajbafzadeh.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kajbafzadeh, AM., Jafarnezhad-Ansariha, F., Sharifi, S.H.H. et al. Decellularization and Recellularization of Natural, Benign Prostatic Hyperplasia and Malignant Human Prostatic Tissues: Role of Extracellular Matrix Behavior on Development of Prostate Cancer. Regen. Eng. Transl. Med. 9, 533–546 (2023). https://doi.org/10.1007/s40883-023-00299-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-023-00299-w