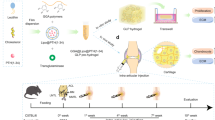
Abstract
Background
Osteoarthritis (OA) is a progressive chronic inflammatory disease that leads to cartilage degradation in the joints. OA is the topmost cause of disability worldwide, and its management poses a huge clinical challenge due to the dense, avascular, and occluded structure of the tissues. Current medications provide temporary relief, resorting to joint replacement as the last option in advanced OA. The existing treatments using therapeutic drugs suffer from systematic toxicity, low retention time, and low bioavailability. Stable and biocompatible hydrogels and emulsions have individually been utilized for delivering OA drugs to the target site, circumventing some of the challenges.
Discussion
Emulsion gels made up of hydrogel and oil-based emulsions have the potential to improve drug delivery efficacy and bioavailability as demonstrated in several studies for delivering nutrients and drugs. Given the efficacy of the hybrid emulsion gels, the prospect of synergistic therapy for OA treatment is discussed here. Moreover, stimuli-responsive emulsion-gel systems can help tune the release kinetics of therapeutics, opening up new possibilities for effective OA treatment.
Conclusion
In this perspective, we systematically present the current diagnosis methods, treatment options, and the current needs for therapeutic interventions in OA, discussing the prospect of combination drug delivery using emulsion gels.
Lay summary
The prevalence of osteoarthritis (OA) worldwide calls for urgent treatment strategies to be developed for OA treatment. In this perspective, we discussed the utility and limitations of existing materials or systems employed in OA treatment. Emulsion gels, consisting of oil droplets inside hydrogels, can be used as a dual drug delivery scaffold for OA treatment. The hydrogel in the system would increase the biocompatibility of the emulsion gel and prevent the degradation of drugs encapsulated within the gel as well as the oil droplets. Hydrophobic drugs would go into the oil part whereas the hydrophilic ones will reside in the hydrogel. In the future, intelligent stimuli-responsive emulsion gel materials for chondrocytes regeneration will respond to endogenous stimuli produced from the OA-affected joints to facilitate tissue growth, healing, and recovery.



Adapted from ref. [20]. Copyright © 2015, American Chemical Society

Reproduced from Ref. [65] with permission from the Royal Society of Chemistry

Reproduced from Ref. [21] with permission from the Royal Society of Chemistry


Similar content being viewed by others
References
Osteoarthritis — Level 3 cause | Institute for Health Metrics and Evaluation. https://www.healthdata.org/results/gbd_summaries/2019/osteoarthritis-level-3-cause. Accessed 15 March 2022.
Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet. 2020;396:1711–2.
United Nations. World Population to 2300. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2002_world_population_to_2300.pdf. Accessed 18 March 2022.
Priority diseases and reasons for inclusion. https://www.who.int/medicines/areas/priority_medicines/Ch6_12Osteo.pdf. Accessed 18 March 2022.
Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, et al. Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2013;43:303–13.
Xie F, Kovic B, Jin X, He X, Wang M, Silvestre C. Economic and humanistic burden of osteoarthritis: a systematic review of large sample studies. Pharmacoeconomics. 2016;34:1087–100.
Akkiraju H, Nohe A, Dettman RW, Wessels A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol. 2015;3:177–92.
Kou L, Xiao S, Sun R, Bao S, Yao Q, Chen R. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Deliv. 2019;26:870–85.
Rahmati M, Nalesso G, Mobasheri A, Mozafari M. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30.
van den Berg WB. Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthr Cartil. 2011;19:338–41.
Peng Z, Sun H, Bunpetch V, Koh Y, Wen Y, Wu D, et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268: 120555.
Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis Nat Rev Dis Prim. 2016;2:1–18.
Wang Z, Wang S, Wang K, Wu X, Tu C, Gao C. Stimuli-sensitive nanotherapies for the treatment of osteoarthritis. Macromol Biosci. 2021;21:2100280.
He Y, Li Z, Alexander PG, Ocasio-Nieves BD, Yocum L, Lin H, et al. Pathogenesis of osteoarthritis: risk factors, regulatory pathways in chondrocytes, and experimental models. Biology (Basel). 2020;9:194.
da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-33.
Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA. 2019;322:1360–70.
Conaghan PG, Bowes MA, Kingsbury SR, Brett A, Guillard G, Rizoska B, et al. Disease-modifying effects of a novel cathepsin k inhibitor in osteoarthritis: a randomized controlled trial. Ann Intern Med. 2020;172:86–95.
Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil. 2013;21:16–21.
He Z, Wang B, Hu C, Zhao J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloids Surfaces B Biointerfaces. 2017;154:33–9.
Chung MF, Chia WT, Wan WL, Lin YJ, Sung HW. Controlled release of an anti-inflammatory drug using an ultrasensitive ROS-responsive gas-generating carrier for localized inflammation inhibition. J Am Chem Soc. 2015;137:12462–5.
Saeedi T, Prokopovich P. Poly beta amino ester coated emulsions of NSAIDs for cartilage treatment. J Mater Chem B. 2021;9:5837–47.
Skou ST, Koes BW, Grønne DT, Young J, Roos EM. Comparison of three sets of clinical classification criteria for knee osteoarthritis: a cross-sectional study of 13,459 patients treated in primary care. Osteoarthr Cartil. 2020;28:167–72.
Zhang W, Doherty M, Peat G, Bierma-Zeinstra SMA, Arden NK, Bresnihan B, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–9.
Cibulka MT, Threlkeld J. The early clinical diagnosis of osteoarthritis of the hip. J Orthop Sport Phys Ther. 2004;34:461–573.
Runhaar J, Özbulut Ö, Kloppenburg M, Boers M, Bijlsma JWJ, Bierma-Zeinstra SMA. Diagnostic criteria for early hip osteoarthritis: first steps, based on the CHECK study. Rheumatology. 2021;60:5158–64.
Altman R, Alarcón G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–14.
Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33:1601–10.
Haugen IK, Felson DT, Abhishek A, Berenbaum F, Bierma-Zeinstra S, Borgen T, et al. Development of classification criteria for hand osteoarthritis: comparative analyses of persons with and without hand osteoarthritis. RMD Open. 2020;6: e001265.
Lawson TB, Mäkelä JTA, Klein T, Snyder BD, Grinstaff MW. Nanotechnology and osteoarthritis. Part 1: Clinical landscape and opportunities for advanced diagnostics. J Orthop Res. 2021;39:465–72.
Mao L, Wu W, Wang M, Guo J, Li H, Zhang S, et al. Targeted treatment for osteoarthritis: drugs and delivery system. Drug Deliv. 2021;28:1861–76.
Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, et al. Exercise as a prescription for patients with various diseases. J Sport Health Sci. 2019;8:422–41.
Goff AJ, De Oliveira SD, Merolli M, Bell EC, Crossley KM, Barton CJ. Patient education improves pain and function in people with knee osteoarthritis with better effects when combined with exercise therapy: a systematic review. J Physiother. 2021;67:177–89.
Savvidou O, Milonaki M, Goumenos S, Flevas D, Papagelopoulos P, Moutsatsou P. Glucocorticoid signaling and osteoarthritis. Mol Cell Endocrinol. 2019;480:153–66.
Bert J, Kenney J, Sgaglione NA, McClelland S, Brophy R, Toth J, et al. Viscosupplementation for osteoarthritis of the knee: a key opinion leader panel discussion. J Manag Care Spec Pharm. 2018;24:S2-7.
Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr Cartil. 2008;16:137–62.
Stevens RM, Ervin J, Nezzer J, Nieves Y, Guedes K, Burges R, et al. Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthritis Rheumatol. 2019;71:1524–33.
Gregori D, Giacovelli G, Minto C, Barbetta B, Gualtieri F, Azzolina D, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564–79.
Zhang K, Lin S, Feng Q, Dong C, Yang Y, Li G, et al. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017;64:389–400.
Chevalier X, Giraudeau B, Conrozier T, Marliere J, Kiefer P, Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005;32:1317–23.
Xuan H, Hu H, Geng C, Song J, Shen Y, Lei D, et al. Biofunctionalized chondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. Acta Biomater. 2020;105:97–110.
Maudens P, Meyer S, Seemayer CA, Jordan O, Allémann E. Self-assembled thermoresponsive nanostructures of hyaluronic acid conjugates for osteoarthritis therapy. Nanoscale. 2018;10:1845–54.
Jeong SH, Kim M, Kim TY, Kim H, Ju JH, Hahn SK. Supramolecular injectable hyaluronate hydrogels for cartilage tissue regeneration. ACS Appl Bio Mater. 2020;3:5040–7.
Janssen M, Timur UT, Woike N, Welting TJM, Draaisma G, Gijbels M, et al. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J Control Release. 2016;244:30–40.
Zhao Y, Wei C, Chen X, Liu J, Yu Q, Liu Y, et al. Drug delivery system based on near-infrared light-responsive molybdenum disulfide nanosheets controls the high-efficiency release of dexamethasone to inhibit inflammation and treat osteoarthritis. ACS Appl Mater Interfaces. 2019;11:11587–601.
Yang L, Sun L, Zhang H, Bian F, Zhao Y. Ice-inspired lubricated drug delivery particles from microfluidic electrospray for osteoarthritis treatment. ACS Nano. 2021;15:20600–6.
Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM. Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints. J Orthop Res. 2014;32:1044–51.
DeJulius CR, Gulati S, Hasty KA, Crofford LJ, Duvall CL. Recent advances in clinical translation of intra-articular osteoarthritis drug delivery systems. Adv Ther. 2021;4:2000088.
Spitzer AI, Richmond JC, Kraus VB, Gomoll A, Jones DG, Huffman KM, et al. Safety and efficacy of repeat administration of triamcinolone acetonide extended-release in osteoarthritis of the knee: a phase 3b, open-label study. Rheumatol Ther. 2019;6:109–24.
Malone A, Price J, Price N, Peck V, Getgood A, Petrella R, et al. Safety and pharmacokinetics of EP-104IAR (sustained-release fluticasone propionate) in knee osteoarthritis: a randomized, double-blind, placebo-controlled phase 1 trial. Osteoarthr Cartil Open. 2021;3: 100213.
Hunter DJ, Chang CC, Wei JCC, Lin HY, Brown C, Tai TT, et al. TLC599 in patients with osteoarthritis of the knee: a phase IIa, randomized, placebo-controlled, dose-finding study. Arthritis Res Ther. 2022;24:1–11.
Alan IS, Alan B. Side effects of glucocorticoids. Pharmacokinet Advers Eff Drugs - Mech Risks Factors. IntechOpen. 2018. https://doi.org/10.5772/intechopen.72019.
Kavanaugh TE, Werfel TA, Cho H, Hasty KA, Duvall CL. Particle-based technologies for osteoarthritis detection and therapy. Drug Deliv Transl Res. 2015;6:132–47.
Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis - an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:1–22.
He Y, Lei L, Cao J, Yang X, Cai S, Tong F, et al. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting. Sci Adv. 2021;7:eaba0776.
Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:1–19.
Araújo F, Shrestha N, Gomes MJ, Herranz-Blanco B, Liu D, Hirvonen JJ, et al. In vivo dual-delivery of glucagon like peptide-1 (GLP-1) and dipeptidyl peptidase-4 (DPP4) inhibitor through composites prepared by microfluidics for diabetes therapy. Nanoscale. 2016;8:10706–13.
Kharaghani D, Gitigard P, Ohtani H, Kim KO, Ullah S, Saito Y, et al. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci Reports. 2019;9:1–11.
Rani NNIM, Chen XY, Al-Zubaidi ZM, Azhari H, Khaitir TMN, Ng PY, et al. Surface-engineered liposomes for dual-drug delivery targeting strategy against methicillin-resistant Staphylococcus aureus (MRSA). Asian J Pharm Sci. 2022;17:102–19.
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211.
Wan X, Beaudoin JJ, Vinod N, Min Y, Makita N, Bludau H, et al. Co-delivery of paclitaxel and cisplatin in poly(2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials. 2019;192:1–14.
Patel R, Evitt L, Mariolis I, Di Giambenedetto S, d’Arminio Monforte A, Casado J, et al. HIV treatment with the two-drug regimen dolutegravir plus lamivudine in real-world clinical practice: a systematic literature review. Infect Dis Ther. 2021;10:2051–70.
Kang ML, Kim JE, Im GIL. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016;39:65–78.
Xue S, Zhou X, Sang W, Wang C, Lu H, Xu Y, et al. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact Mater. 2021;6:2372–89.
Zhang K, Yang J, Sun Y, He M, Liang J, Luo J, et al. Thermo-sensitive dual-functional nanospheres with enhanced lubrication and drug delivery for the treatment of osteoarthritis. Chem A Eur J. 2020;26:10564–74.
Yang L, Liu Y, Shou X, Ni D, Kong T, Zhao Y. Bio-inspired lubricant drug delivery particles for the treatment of osteoarthritis. Nanoscale. 2020;12:17093–102.
Geiger BC, Wang S, Padera RF, Grodzinsky AJ, Hammond PT. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Transl Med. 2018;10:eaat8800.
McClements DJ, Decker EA, Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72:R109–24.
Marin E, Briceño MI, Caballero-George C. Critical evaluation of biodegradable polymers used in nanodrugs. Int J Nanomedicine. 2013;8:3071–91.
Geiger BC, Grodzinsky AJ, Hammond PT. Designing drug delivery systems for articular Joints. Chem Eng Prog. 2018;114:46–51. https://www.webofscience.com/wos/woscc/full-record/WOS:000432231600020?SID=EUW1ED0C98RSJdDsMycKvWrjdmL2t; http://hammondlab-archive.mit.edu/sites/default/files/images/Geiger%20et%20al-2018-CEP%20May2018%20Reprint.pdf.
Lin X, Tsao CT, Kyomoto M, Zhang M. Injectable natural polymer hydrogels for treatment of knee osteoarthritis. Adv Healthc Mater. 2022;11:2101479. https://doi.org/10.1002/adhm.202101479; https://onlinelibrary.wiley.com/action/showCitFormats?doi=10.1002%2Fadhm.202101479.
Nayak A, Olatunji O, Bhusan Das D, Vladisavljević G. Pharmaceutical applications of natural polymers. In: Olatunji O, editor. Natural polymers. Cham: Springer; 2016. https://doi.org/10.1007/978-3-319-26414-1_9; https://link.springer.com/chapter/10.1007/978-3-319-26414-1_9#citeas.
Ajazuddin, Alexander A, Khichariya A, Gupta S, Patel RJ, Giri TK, et al. Recent expansions in an emergent novel drug delivery technology: Emulgel. J Control Release. 2013;171:122–32.
Hiranphinyophat S, Otaka A, Asaumi Y, Fujii S, Iwasaki Y. Particle-stabilized oil-in-water emulsions as a platform for topical lipophilic drug delivery. Colloids Surfaces B Biointerfaces. 2021;197: 111423.
Zembyla M, Murray BS, Sarkar A. Water-in-oil emulsions stabilized by surfactants, biopolymers and/or particles: a review. Trends Food Sci Technol. 2020;104:49–59.
Gundogdu E, Alvarez IG, Karasulu E. Improvement of effect of water-in-oil microemulsion as an oral delivery system for fexofenadine: in vitro and in vivo studies. Int J Nanomedicine. 2011;6:1631–40.
Sawant A, Kamath S, Kg H, Kulyadi GP. Solid-in-oil-in-water emulsion: an innovative paradigm to improve drug stability and biological activity. AAPS PharmSciTech. 2021;22:1–14.
Farjami T, Madadlou A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci Technol. 2019;86:85–94.
Tan C, McClements DJ. Application of advanced emulsion technology in the food industry: a review and critical evaluation. Foods. 2021;10:812.
Yaakov N, Ananth Mani K, Felfbaum R, Lahat M, Da Costa N, Belausov E, et al. Single cell encapsulation via Pickering emulsion for biopesticide applications. ACS Omega. 2018;3:14294–301.
Yang H, Fu L, Wei L, Liang J, Binks BP. Compartmentalization of incompatible reagents within Pickering emulsion droplets for one-pot cascade reactions. J Am Chem Soc. 2015;137:1362–71.
Paik J, Duggan ST, Keam SJ. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79:455–62.
Li C, Chen X, Luo X, Wang H, Zhu Y, Du G, et al. Nanoemulsions target to ectopic lymphoids in inflamed joints to restore immune tolerance in rheumatoid arthritis. Nano Lett. 2021;21:2551–61.
Mohammadifar M, Aarabi MH, Aghighi F, Kazemi M, Vakili Z, Memarzadeh MR, et al. Anti-osteoarthritis potential of peppermint and rosemary essential oils in a nanoemulsion form: behavioral, biochemical, and histopathological evidence. BMC Complement Med Ther. 2021;21:1–12.
Goindi S, Narula M, Kalra A. Microemulsion-based topical hydrogels of tenoxicam for treatment of arthritis. AAPS PharmSciTech. 2016;17:597–606.
Gokhale JP, Mahajan HS, Surana SS. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: in vivo and in vitro studies. Biomed Pharmacother. 2019;112: 108622.
Lu G, Shen X, Xiao D, Rong L, Mao Z, Wang B, et al. Antibacterial thyme oil-loaded zwitterionic emulsion hydrogels. J Mater Chem B. 2022;10:2691–8.
Edwards SHR. Intra-articular drug delivery: the challenge to extend drug residence time within the joint. Vet J. 2011;190:15–21.
Maltais A, Remondetto GE, Subirade M. Tabletted soy protein cold-set hydrogels as carriers of nutraceutical substances. Food Hydrocoll. 2010;24:518–24.
Lee MC, Tan C, Ravanfar R, Abbaspourrad A. Ultrastable water-in-oil high internal phase emulsions featuring interfacial and biphasic network stabilization. ACS Appl Mater Interfaces. 2019;11:26433–41.
Mwangi AN, Njogu PM, Maru SM, Njuguna NM, Njaria PM, Kiriiri GK, et al. Meloxicam emulgels for topical management of rheumatism: formulation development, in vitro and in vivo characterization. Saudi Pharm J. 2021;29:351–60.
Shingel KI, Faure MP, Azoulay L, Roberge C, Deckelbaum RJ. Solid emulsion gel as a vehicle for delivery of polyunsaturated fatty acids: implications for tissue repair, dermal angiogenesis and wound healing. J Tissue Eng Regen Med. 2008;2:383–93.
Liang L, Leung Sok Line V, Remondetto GE, Subirade M. In vitro release of α-tocopherol from emulsion-loaded β-lactoglobulin gels. Int Dairy J. 2010;20:176–81.
Freire M, Cofrades S, Pérez-Jiménez J, Gómez-Estaca J, Jiménez-Colmenero F, Bou R. Emulsion gels containing n-3 fatty acids and condensed tannins designed as functional fat replacers. Food Res Int. 2018;113:465–73.
Perioli L, Ambrogi V, Venezia L, Giovagnoli S, Pagano C, Rossi C. Formulation studies of benzydamine mucoadhesive formulations for vaginal administration. Drug Dev Ind Pharm. 2009;35:769–79.
Huerta RR, Silva EK, El-Bialy T, Saldaña MDA. Clove essential oil emulsion-filled cellulose nanofiber hydrogel produced by high-intensity ultrasound technology for tissue engineering applications. Ultrason Sonochem. 2020;64: 104845.
Lu Y, Mao L, Hou Z, Miao S, Gao Y. Development of emulsion gels for the delivery of functional food ingredients: from structure to functionality. Food Eng Rev. 2019;11:245–58.
Mou D, Yu Q, Zhang J, Zhou J, Li X, Zhuang W, et al. Intra-articular injection of chitosan-based supramolecular hydrogel for osteoarthritis treatment. Tissue Eng Regen Med. 2021;18:113–25.
Vigata M, Meinert C, Hutmacher DW, Bock N. Hydrogels as drug delivery systems: a review of current characterization and evaluation techniques. Pharmaceutics. 2020;12:1188.
Duan R, Xie H, Liu ZZ. The role of autophagy in osteoarthritis. Front Cell Dev Biol. 2020;8:1437.
Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65.
Yu SP, Hunter DJ. Intra-articular therapies for osteoarthritis. Expert Opin Pharmacother. 2016;17:2057–71.
Tang R, Kim CS, Solfiell DJ, Rana S, Mout R, Velázquez-Delgado EM, et al. Direct delivery of functional proteins and enzymes to the cytosol using nanoparticle-stabilized nanocapsules. ACS Nano. 2013;7:6667–73.
Van Hoeck J, Van de Vyver T, Harizaj A, Goetgeluk G, Merckx P, Liu J, et al. Hydrogel-induced cell membrane disruptions enable direct cytosolic delivery of membrane-impermeable cargo. Adv Mater. 2021;33:2008054.
Xiao YP, Zhang J, Liu YH, Huang Z, Yu XQ. Fluorinated polymer emulsion systems: construction and application in delivering genes and proteins. Eur J Med Chem. 2020;207: 112799.
Hsieh FY, Han HW, Chen XR, Yang CS, Wei Y, Hsu SH. Non-viral delivery of an optogenetic tool into cells with self-healing hydrogel. Biomaterials. 2018;174:31–40.
Chen K, Li S, Yuan F, Sun P, Zhang Y. GEL-MAN hydrogel loaded with triamcinolone acetonide for the treatment of osteoarthritis. Front Bioeng Biotechnol. 2020;0:872.
Xie XQ, Zhang Y, Liang Y, Wang M, Cui Y, Li J, et al. Programmable transient supramolecular chiral G-quadruplex hydrogels by a chemically fueled non-equilibrium self-assembly strategy. Angew Chemie Int Ed. 2022;61: e202114471.
Zahan OM, Serban O, Gherman C, Fodor D. The evaluation of oxidative stress in osteoarthritis. Med Pharm Reports. 2020;93:12–22.
Acknowledgements
S.R. acknowledges Science and Engineering Research Board (ECR/2018/00255) for financial support. M.S. is thankful to UGC for the doctoral research fellowship. Financial support from the DBT-RA Program in Biotechnology and Life Sciences (Award Letter No. DBT-RA/2021/January/NE/231) is gratefully acknowledged by S.D.
Author information
Authors and Affiliations
Contributions
SD and MS: conceptualization, drafting, and literature survey.
SR: conceptualization, drafting, literature survey, editing, and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, S., Solra, M. & Rana, S. Emulsion Gel: a Dual Drug Delivery Platform for Osteoarthritis Treatment. Regen. Eng. Transl. Med. 9, 279–294 (2023). https://doi.org/10.1007/s40883-022-00282-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00282-x