Abstract
Sweet potato is one of the most important staple food crops consumed in Kenya and throughout Africa but yields are greatly reduced by plant parasitic nematodes (PPN). The aim of this study was to determine the prevalence of PPN in Kenyan sweet potato fields and their relationship with soil and climatic variables. Soil samples were collected from sweet potato fields in Busia, Teso, Kisii, Embu and Makueni counties. Thirteen nematode genera were identified across the five counties with Meloidogyne, Pratylenchus and Rotylenchus being the most prevalent. There was a significant (P <0.05) relationship between PPN abundance and sodium, calcium and iron. Canonical correspondence analysis of climatic variables revealed that the relationship between rainfall and nematode genera was significant (P <0.05) while maximum and minimum temperatures were not significant. This description of PPN assemblages associated with sweet potato in Kenya and their relationship with environmental variables provides a starting point from which appropriate nematode management strategies can be implemented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant parasitic nematodes (PPN) cause substantial yield losses of agricultural crops (Strange and Scott 2005) with a resultant global cost of > $120 billion p.a. (Chitwood 2011). The nematodes, Meloidogyne spp., Heterodera spp., Globodera spp., Radopholus similis, Ditylenchus dispaci, Bursaphelenchus xylophilus, Rotylenchulus reniformis, Xiphinema index, Naccobus aberrans and Aphelenchoides bessseyi are considered as the top 10 plant parasitic by nematologists (Jones et al. 2013). Due to its capability of rapidly spreading to and colonizing new localities (Bebber et al. 2014)) and wide host range, root knot nematode (RKN, Meloidogyne spp), is ranked as the most economically damaging (Jones et al. 2013). Twenty Meloidogyne species occur in Africa with M. incognita, M. javanica and M. arenaria being the most prevalent (Onkendi et al. 2014).
Plant parasitic nematodes are widely distributed in Kenyan agro-ecosystems (Waceke 2007; Maina et al. 2011; Mwangi et al. 2014; Nzesya et al. 2014). In Kenya, sweet potato is an important subsistence crop that is grown by smallholder farmers in most agro-ecological zones (AEZ) (Mwololo et al. 2007). Sweet potato is a preferred staple crop in developing countries due to its low cost of production and its ability to grow in a range of environments (Woolfe 2002). However, sweet potato yield and quality are reduced by PPN infection (Coyne et al. 2003; Olabiyi 2007). The presence of PPN in sweet potato growing AEZ in Kenya may be an important production constraint and the situation may deteriorate due to an increase in nematode abundance and a shift in distribution as a result of changes in temperature and moisture. Population trends of PPN and the relationship between nematodes and plants are affected by environmental conditions (Griffin et al. 1996). At both global and continental scales, nematode community structure is influenced by temperature and rainfall (Bakonyi et al. 2007; Nielsen et al. 2014). Temperature and moisture may directly or indirectly affect nematode abundance and distribution. Temperature directly affects different nematode processes such as rate of feeding (Boag 1980), and root penetration (Roberts et al. 1979), while nematode infection rate is directly impacted by moisture (Colagiero and Ciancio 2011). A risk analysis of climate change on coffee nematodes in Brazil, suggested that high temperature would result in an increase in the area infested with Meloidogyne spp. by 2060 as a result of increased nematode reproduction rates (Ghini et al. 2008). An increase in temperature has also been correlated with increased rate of development and reproduction of Meloidogyne (Bird 1972) and Pratylenchus (Duyck et al. 2012). The objective of this study was to therefore determine the current environmental drivers and distribution of parasitic nematodes associated with sweet potato in Busia, Teso, Kisii, Embu and Makueni counties in Kenya.
Materials and methods
Soil sampling sites
In April 2015, rhizosphere soil was collected from sweet potato fields in five counties that represent the main agro-ecological zones where sweet potato is grown in Kenya (Table 1; Fig. 1). Busia, Teso, Kisii, Embu and Makueni counties are located at altitudes of 1220 m, 1140 m, 1700 m, 1300 m and 1260 m respectively. The sampled areas were fields that were exclusively cultivated with sweet potato for 5 years or more. Classification of the Kenyan agro-ecological zones is as described by Jaetzold and Schmidt (1983) and it is based on the probability of major crops achieving their water and temperature requirements. For each location, samples were collected from 10 sweet potato fields at a depth of 25 cm using a 3.5 cm diameter soil auger. In each field, soil samples were collected along three separate W shaped “sample walks” (25 kg of soil from each “sample walk”) with 30 sampling points where the distance between two points was 10 m. Subsequently three 200 g sub-samples from each walk were used for nematode extraction (Wiesel et al. 2015).
Nematode extraction and identification
Nematodes were extracted from 200 g of soil for 48 h using the modified Baermann technique (Hooper 1990). Nematodes were fixed in formalin-acetic fixative before identification (Stamps and Linit 1998). The nematodes were enumerated and identified to genus level using a Leica compound microscope. Physical and chemical properties of soil (pH, clay, sand, silt, calcium, copper, iron, magnesium, manganese, phosphorous, potassium, sodium, zinc, total nitrogen and organic carbon) were evaluated at Kenya Agricultural and Livestock Research Organization (KALRO), Nairobi, Kenya.
Temperature and rainfall data collection
Monthly rainfall, maximum and minimum temperature data for the sampling sites during April 2015 was provided by the Kenya Meteorological Department (KMD). To investigate the long term trend in temperature and rainfall in the sampling sites, data was obtained from KMD as indicated in Table 2. Analysis of nematode data was performed on the average of the three 200 g soil sub-samples. Differences in nematode genera abundance between the counties (pooled nematode data per county) were determined by analysis of variance (ANOVA) using Genstat v 17.1 statistical software (VSN International Ltd) with the Fisher’s LSD post hoc analysis. The absolute frequency (Number of samples containing a nematode genus/Number of samples collected) X 100), prominence values (Absolute density X square root (absolute frequency); Absolute density = Mean number of nematodes/100 cm3 soil) and relative prominence values (Prominence value of a nematode genus/sum of prominence values for all nematode genera X 100) of nematode genera were calculated (Norton 1978). The nematode data was transformed by log (x + 1) before analysis to reduce heterogeneity of variances. Hierarchical cluster analysis based on Bray-Curtis dissimilarity matrix and Ward’s clustering algorithm which produces well defined clusters was used to infer the structure of plant parasitic nematodes (PPN) populations in sampled sweet potato fields from 28 villages. The ‘heatmap.2’ function was used to pair the dendrograms with a heat map of nematode abundance using the gplots package of R (R Development Core Team 2013). Differences in soil physical and chemical properties were analyzed using ANOVA. Canonical correspondence analysis (CCA) was used to investigate relationships between PPN and soil properties, rainfall, maximum and minimum temperature (for weather data collected in April 2015 during collection of soil samples) using vegan in R package. Seasonal and annual trends for long term rainfall and temperature data were analyzed using the Mann-Kendall non-parametric test with the ‘Kendall’ package in R environment. Seasons were considered as March, April, May (MAM), June, July, August (JJA), September, October, November (SON) and December, January, February (DJF). A positive and negative Kendall tau coefficient (P < 0.05) indicates an increasing and decreasing trend respectively.
Results
Thirteen PPN genera were identified across the five counties with a significant difference in the abundance of Meloidogyne (F = 4.480[4, 48]; P = 0.004). Makueni county had the highest number (11) of nematode genera. Kisii county had the highest number of Meloidogyne population and the least was in Teso county (Table 3). Some nematode genera were exclusively observed in a single county. These are Criconemella (Makueni county), Hoplolaimus (Teso county) and Trichodorus (Makueni county). Helicotylenchus, Meloidogyne, Pratylenchus, Rotylenchulus, Rotylenchus and Tylenchorhynchus were observed in all the Counties. The nematode genera, Filenchus, Scutellonema and Tylenchus were recorded in 80% of the sampled fields.
Relative prominence (RP) values (Table 4) which indicate the frequency of occurrence of nematode genera compared to that of all genera were different in the five counties. The most prevalent genera (RP >10%) across all Counties were Meloidogyne, Pratylenchus and Rotylenchus. Paratylenchus was not recorded in Embu and Makueni counties. The nematode genera Tylenchus and Filenchus had high RP values in Makueni county. The highest RP values for Pratylenchus (16.52%) and Rotylenchulus (13.4%) were recorded in Embu and Busia counties respectively (Table 4).
The PPN population structure in sweet potato fields in 28 villages of Busia, Embu, Kisii, Makueni and Teso counties was inferred using a heat map (Fig. 2). There was a clear grouping of the villages into two major clusters. Each cluster comprised of villages from all the five counties. The distribution and abundance of nematode genera depicted in the heat map showed that Meloidogyne, Rotylenchus and Pratylenchus were abundant in similar sites to those identified through the relative prominence analysis. Sweet potato fields in Nyabungututu, Nyakobaria (Kisii county), Buroboi A, Buroboi B, Nabisiongo, Namikoe (Busia county), Iveche (Embu county), Kinui, Kalima, Kiinze (Makueni county) and Angorom (Teso county) villages were clustered together. The soil samples from these villages did not have Rotylenchulus, Hoplolaimus or Scutellonema populations. In the same cluster, Kinui and Kiinze villages (Makueni county) were the only areas out of the five counties where the nematode genera Trichodorus and Criconemella were recorded. The highest populations of Meloidogyne and Rotylenchus were observed in Emaseno village which was part of the second major cluster. Njukiri village was also part of this cluster and it had the highest number of Pratylenchus. The nematode genera were also grouped into two main clusters with the smallest cluster consisting of Trichodorus, Criconemella and Tylenchus which were absent from most counties.
Heatmap of parasitic nematode genera in sweet potato fields in Nyabungututu, Nyakobaria, Bonyagatenyi, Kiaboega, Mwabagaka, Mwamasarore, Mwamoja (Kisii county), Buroboi A, Buroboi B, Nabisiongo, Namikoe, Emaseno, Buruba (Busia county), Iveche, Mariamairi, Kangaru, Githungururu, Njukiri (Embu county), Kinui, Kikuu, Kalima, Kiinze, Kyanguu, Inyeke (Makueni county), Angorom, Andungos, Andukumut and Alomodoi villages (Teso county). Ward’s clustering algorithm was applied to the Bray-Curtis dissimilarity matrix of nematode abundance in sweet potato fields in 28 villages. Dendrogram of villages where sweet potato fields were sampled is along the left axis. Upper dendrogram represents the nematode genera. The color key scale represents normalized nematode abundances with intensity of color representing nematode abundance 200 cm−3
Across the five counties, significant differences in soil physical and chemical properties were noted except for copper and potassium (Table 5). In the CCA analysis (Fig. 3) on the relationship between soil properties and PPN abundance, the first two axes accounted for 75.6% of the variance. There was a significant (P < 0.05) relationship between PPN abundance and sodium, calcium and iron. The nematode genera Criconemella and Trichodorus which were observed in Makueni county showed a non-significant correlation with sand (Sd).
Canonical correspondence analysis of the relationship between nematode communities and soil properties in Busia, Embu, Kisii, Makueni and Teso counties. The first two axes explain 75.1% of the variance. Arrows represent potassium (K), phosphorous (P), sodium (Na), total nitrogen (TN), pH, total organic carbon (TOC), zinc (Zn), calcium (Ca), copper (Cu), iron (Fe), magnesium (Mg), manganese (Mn), silt (St), sand (Sd) and clay (Cy). The nematode genera are Aphelenchoides (Aphd), Aphelenchus (Aphe), Criconemella (Cric), Ditylenchus (Dity), Filenchus (File), Helicotylenchus (Heli), Hoplolaimus (Hopl), Longidorus (Long), Meloidogyne (Melo), Paratylenchus (Para), Pratylenchus (Praty), Radopholus (Rado), Rotylenchulus (Rotys), Rotylenchus (Rotyl), Scutellonema (Scut), Trichodorus (Tric), Tylenchorhynchus (Tyler), Tylenchus (Tyle) and Xiphinema (Xiph)
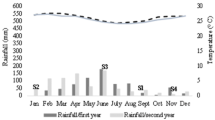
During the sampling period (April, 2015) the mean monthly rainfall was 368.5 mm, 158.4 mm, 139.2 mm, 71.4 mm and 368.5 mm in Busia, Embu, Kisii, Makueni and Teso counties respectively. Maximum temperature in Busia, Embu, Kisii, Makueni and Teso counties was 28 °C, 25 °C, 24.5 °C, 30.7 °C and 28 °C respectively while the minimum temperature was 13 °C, 11.7 °C, 15.7 °C, 17.7 °C and 13 °C respectively. There was a significant difference (P <0.05) in rainfall, maximum and minimum temperature across the five counties. Makueni county had the lowest amount of rainfall and the highest maximum and minimum temperature.
Canonical correspondence analysis was used to evaluate the relationship between nematode genera abundance and rainfall, maximum and minimum temperature. The first axis accounted for 53.2% of the variance and the second axis accounted for 15.8% of the variance. Permutation tests for climatic variables revealed that the relationship between rainfall and nematode genera was significant (P <0.05) while maximum and minimum temperatures were not significant (P = 0.61 and P = 0.63 respectively). Rainfall was highly correlated with CCA1 (Fig. 4). The nematode genera Aphelenchoides (Aphd), Criconemella (Cric), Filenchus (Filen), Scutellonema (Scut), Trichodorus (Tric) and Tylenchus (Tyle) were associated with maximum and minimum temperature but the relationship was not statistically significant.
Canonical correspondence analysis of the relationship between nematode communities and climate variables in Busia, Embu, Kisii, Makueni and Teso counties. The first axis explains 53.2% of the variance while the second axis explains 15.8% of the variance. Arrows represent rainfall, maximum and minimum temperature. The nematode genera are Aphelenchoides (Aphd), Aphelenchus (Aphe), Criconemella (Cric), Ditylenchus (Dity), Filenchus (File), Helicotylenchus (Heli), Hoplolaimus (Hopl), Longidorus (Long), Meloidogyne (Melo), Paratylenchus (Para), Pratylenchus (Praty), Radopholus (Rado), Rotylenchulus (Rotys), Rotylenchus (Rotyl), Scutellonema (Scut), Trichodorus (Tric), Tylenchorhynchus (Tyler), Tylenchus (Tyle) and Xiphinema (Xiph)
There was an annual increase in rainfall across all the Counties but the increase was not significant (Table 6). For each respective record period of long term temperature data across the five counties, a significant increase in maximum temperature was observed annually. During MAM season, a significant rise in maximum temperature was recorded in all the Counties. Kakamega, Kisii and Makueni counties had a significant increase in maximum temperature during the JJA and DJF seasons. A significant increase in annual minimum temperature was recorded in all counties. Embu and Kakamega counties had a significant increase in minimum temperature during JJA and SON seasons. There was a significant rise in minimum temperature during all seasons in Kisii county (Table 6).
Discussion
Plant parasitic nematodes belonging to the orders Tylenchida and Dorylaimida were identified in Kenyan sweet potato growing regions. These nematodes cause global yield losses of > $120 billion p.a. (Chitwood 2011) and there are challenges in controlling the nematodes through application of sustainable nematode management strategies (Thoden et al. 2011).
Economic losses caused by PPN associated with sweet potato may increase due to ineffective management and increased damage resulting from their interaction with fungal, bacterial and viral pathogens (Barker et al. 1994). Nematode genera reported in this study have been previously observed in association with sweet potato (Njuguna and Bridge 1998; Coyne et al. 2003; Marais and Swart 2007; Haougui et al. 2011). Meloidogyne, Pratylenchus and Rotylenchus were the most predominant nematode genera in all counties with an RP value greater than 10%. Rotylenchus is associated with 80–100% crop losses (Robinson et al. 1997) and has been reported to infect sweet potato in Uganda (Coyne et al. 2003). Pratylenchus and Meloidogyne are common endoparasites in most crops in Kenya. The lesion nematode, Pratylenchus had a frequency of occurrence of 64% in soils collected from cabbage farms in Kenya (Waceke 2007) while Meloidogyne has been reported in association with beans (Kimenju et al. 1999), coffee (Nzesya et al. 2014), and Sesbania (Desaeger and Rao 1999). The PPN Meloidogyne is distributed globally and is more damaging in tropical regions (Jones et al. 2013) with the potential to rapidly spread to different geographic locations (Bebber et al. 2014). The juveniles penetrate the roots and over time galls are formed on the plant roots and this reduces the uptake of water and nutrients leading to stunted growth and low yields. Kisii county had the highest number of Meloidogyne populations. Planting susceptible sweet potato genotypes in this county may result in low yields. Reproduction of Meloidogyne on sweet potato is high during the initial growth stages due to availability of penetration sites. For those roots that reach maturity, cracks occur on the surface reducing their quality and market value (Lawrence et al. 1986). Unlike Meloidogyne which is a sedentary endoparasite, Pratylenchus is a migratory endoparasite and is the third most important PPN worldwide. This endoparasite causes stunting in plants as a result of reduced root growth (Jones et al. 2013).
The reniform nematode, Rotylenchulus was recorded in all counties with Busia having the highest PV value (13.4%). Development of Rotylenchulus on sweet potato is different from that of Meloidogyne but causes similar root cracking. This species causes yield reduction through a “pruning effect” on roots (Clark and Wright 1983). Understanding the interaction between nematode species is key to effective management, for example, reproduction of Rotylenchulus is inhibited by the presence of Meloidogyne during concomitant infections on sweet potato (Thomas and Clark 1983). Soil properties may also affect the competitive balance of these nematodes (Godefroid et al. 2013). In the heatmap analysis, Criconemella was recorded in Kinui and Kalima villages of Makueni county where the monthly rainfall was lower (71.4 mm) than in the other four counties. The low rainfall may be one of the factors contributing to the presence of this genus in Makueni county. The observed correlation of PPN abundance with Sodium, Iron and Calcium has been recorded in other studies (Ardakani et al. 2014; Fiscus and Neher 2002; Yavuzaslanoglu et al. 2012). Understanding the effect of soil properties on PPN communities in sweet potato agroecosystems is an important step in their management since the relationship between soil properties and PPN assemblages is complex and it is influenced by climate (Nielsen et al. 2014).
Nematode abundance and composition is also significantly influenced by rainfall and temperature. In the current study, rainfall was significantly associated with the abundance of nematodes during the sampling period but temperature did not affect the abundance of nematodes. The effect of temperature on nematode abundance may have been masked by other environmental factors. The effect of rainfall on nematode abundance may become more pronounced with the predicted extreme precipitation and differences between wet and dry seasons (IPCC 2013). On a global scale, the abundance of PPN increases with a rise in mean annual temperature (Nielsen et al. 2014). Analysis of long term temperature data in the regions where soil samples were collected showed that maximum and minimum temperature increased annually in all the five counties. Annual increase in maximum and minimum temperature in Makueni, Kakamega and Kisii has been previously reported (Mugalavai and Kipkorir 2013; Omoyo et al. 2015) with Kenya experiencing an increase in temperature by 1 °C in the last 2 decades. Maximum and minimum temperature are also expected to increase by 1.8–4.3 °C in the 21st century (Hoang et al. 2014).
If the trend in increasing temperature continues it may affect the PPN assemblages in sweet potato fields. Temperatures greater than 25 °C cause an increase in the rate of development and reproduction of Meloidogyne (Bird 1972) and Pratylenchus (Duyck et al. 2012). Rotylenchus is also well adapted to warm climate and it is capable of feeding at temperatures between 0.5 and 42.4 °C (Boag 1980). Furthermore, warming may lead to an increase in fungal pathogens (Pritchard 2011) which would further decrease sweet potato yields due to formation of disease complexes between fungi and nematodes. Low rainfall results in an increase in abundance of Pratylenchus and Rotylenchulus (Boag 1980). In New Zealand, climate warming is considered a potential risk to crop production due to the possible increase in abundance of Meloidogyne spp. (Watson and Pottinger 1990). A shift in virus vector nematodes due to global warming was predicted in Great Britain (Boag et al. 1991) and an increase in infestation of Meloidogyne in Brazil coffee agroecosystems was also attributed to warming (Ghini et al. 2008). Shifts in geographic distribution of PPN associated with sweet potato due to an increase in temperature may take a long time (Boag et al. 1991; Neilson and Boag 1996) but movement of PPN between fields may be accelerated by the exchange of planting materials between farmers.
Based on the current study, Kenyan sweet potato fields have a high diversity of economically damaging PPN. The trend in rainfall and temperature increase/decrease in these regions is also changing and this may significantly influence PPN abundance and composition (Nielsen et al. 2014). The effect of changes in temperature and moisture on PPN in sweet potato agro-ecosystems may result from several direct and indirect factors including modification of plant community and soil properties (Colagiero and Ciancio 2011; Kardol et al. 2011; Pritchard 2011). In addition, an interplay of various processes linked to host presence, abundance and susceptibility may also affect the distribution of sweet potato PPN. The potential impact of changes in temperature and moisture on distribution and abundance of PPN in Kenyan sweet potato agro-ecosystems is not easy to predict. The nematode abundance reported in this study may also vary depending on the extraction method used (Den Nijs and Van Den Berg (2013). However, the baseline data in this study provides a starting point from which appropriate nematode management strategies can be put in place in order to mitigate yield losses that may result from PPN.
References
Ardakani A, Mafi Z, Heser A, Goltappeh E (2014) Relationship between soil properties and abundance of Tylenchulus semipenetrans in citrus orchards, Kohgilouyeh va Boyerahmad Province. J Agric Sci Technol 16:1699–1710
Bakonyi G, Nagy P, Kovacs-Lang E, Kovacs E, Barabas S, Repasi V, Seres A (2007) Soil nematode community structure as affected by temperature and moisture in a temperate semiarid shrubland. Appl Soil Ecol 37:31–40
Barker KR, Hussey RS, Krusberg LR, Bird GW, Dunn RA, Ferris H, Ferris V, Freckman DW, Gabriel CJ, Grewal PS, Macguidwin AE, Riddle DL, Roberts PA, Schmitt DP (1994) Plant and soil nematodes: Societal impact and focus for the future. J Nematol 26:127–137
Bebber DP, Holmes T, Gurr SJ (2014) The global spread of crop pests and pathogens. Glob Ecol Biogeogr 23:1398–1407
Bird F (1972) Influence of temperature on embryogenesis in Meloidogyne javanica. J Nematol 4:206–213
Boag B (1980) Effects of temperature on rate of feeding of the plant parasitic nematodes Rotylenchus robustus, Xiphinema diversicaudatum, and Hemicycliophora conida. J Nematol 12:193–195
Boag B, Crawford JW, Neilson R (1991) The effect of potential climatic changes on the geographical distribution of the plant parasitic nematodes Xiphinema and Longidorus in Europe. Nematologica 37:312–323
Chitwood DJ (2011) Research on plant-parasitic nematode biology conducted by he United States Department of Agriculture-Agricultural Research Service. Pest Manag Sci 59:748–753
Clark CA, Wright VL (1983) Effect and reproduction of Rotylenchulus reniformis on sweet potato selections. J Nematol 15:197–203
Colagiero M, Ciancio A (2011) Climate changes and nematodes: Expected effects and perspectives for plant protection. REDIA 94:113–118
Coyne DL, Talwana HAL, Maslen NR (2003) Plant-parasitic nematodes associated with root and tuber crops in Uganda. Afr Plant Protect 9:87–98
Den Nijs LJMF, Van Den Berg W (2013) The added value of proficiency tests: choosing the proper method for extracting Meloidogyne second-stage juveniles from soil. Nematology 15:143–151
Desaeger J, Rao MR (1999) The root-knot nematode problem in Sesbania fallows and scope for managing it in western Kenya. Agrofor Syst 47:273–288
Development Core Team R (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Duyck PF, Dortel E, Tixier P, Vinatier F, Loubana PM, Chabrier C, Quénéhervé P (2012) Niche partitioning based on soil type and climate at the landscape scale in a community of plant-feeding nematodes. Soil Biol Biochem 44:49–55
Fiscus D, Neher D (2002) Distinguishing sensitivity of free living soil nematode genera to physical and chemical disturbances. Ecol Appl 12:565–575
Ghini R, Hamada E, Júnior MJP, Marengo JA, Ribeiro R, Goncalves D (2008) Risk analysis of climate change on coffee nematodes and leaf miner in Brazil. Pesq Agrop Bras 43:187–194
Godefroid M, Delaville L, Marie-Luce S, Quénéhervé P (2013) Spatial stability of a plant-feeding nematode community in relation to macro-scale soil properties. Soil Biol Biochem 57:173–181
Griffin GD, Asay KH, Horton WH (1996) Factors affecting population trends of plant-parasitic nematodes on rangeland grasses. J Nematol 28:107–114
Haougui A, Doumma A, Toufique B, Kollo IA (2011) Survey of plant parasitic nematodes associated with sweet potato in Niger. Asian J Agric Sci 3:32–36
Hoang MHH, Namirembe S, van Noordwijk M, Catacutan D, Öborn I, Perez-Teran ASS, Nguyen HQQ, Dumas-Johansen MKK (2014) Farmer portfolios, strategic diversity management and climate-change adaptation – implications for policy in Vietnam and Kenya. Clim Dev 6:216–225
Hooper DJ (1990) Extraction and processing of plant and soil nematodes. In: Luc M, Sikora RA, Bridge J (eds) Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. CAB International, Wallingford, pp 45–68
IPCC (2013) Summary for Policymakers. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J (eds) Climate change 2013: The physical science basis. contribution of working group I to the fifth assessment report of the intergovernmental panel on climate Change. Cambridge University Press, Cambridge
Jaetzold R, Schmidt H (1983) Farm management handbook of Kenya, vol II/C. Ministry of Agriculture, Nairobi. 78 pp
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WIMML, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961
Kardol P, Reynolds WN, Norby RJ, Classen AT (2011) Climate change effects on soil microarthropod abundance and community structure. Appl Soil Ecol 47:37–44
Kimenju JW, Karanja NK, Macharia I (1999) Plant parasitic nematodes associated with common bean in Kenya and the effect of Meloidogyne infection on bean nodulation. Afr Crop Sci J 7:503–510
Lawrence G, Clark C, Wright V (1986) Influence of Meloidogyne incognita on resistant and susceptible sweet potato cultivars. J Nematol 18:59–65
Maina JM, Waceke JW, Kariuki GM (2011) Plant parasitic nematodes: A threat to vegetable production in Kenya. Afr Crop Sci Conf Proc 10:205–208
Marais M, Swart A (2007) Plant nematodes in South Africa. 8. Bizana, Lusikisiki and Port St Johns area, Eastern Cape Province. Afr Plant Protect 13:16–27
Mugalavai EM, Kipkorir EC (2013) Assessing the potential of maize growing seasons for Western Kenya using agro-climatic indices. Int J Disaster Manag Risk Reduction 5:53–73
Mwangi JM, Waceke JW, Kariuki GM (2014) Occurence and abundance of plant parasitic nematodes in cabbage-based cropping systems in Kenya. J Agric Biol Sci 9:326–332
Mwololo JK, Mburu MK, Njeru RW, Ateka EM, Kiarie N, Munyua JK, Muinga RW, Kapinga R, Lemaga B (2007) Resistance of sweet potato genotypes to sweet potato virus disease in coastal Kenya. Afr Crop Sci Conf Proc 8:2083–2086
Neilson R, Boag B (1996) The predicted impact of possible climatic change on virus-vector nematodes in Great Britain. Eur J Plant Pathol 102:193–199
Nielsen UN, Ayres E, Wall DH, Li G, Bardgett RD, Wu T, Garey JR (2014) Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob Ecol Biogeogr 23:968–978
Njuguna LK, Bridge J (1998) Plant parasitic nematodes of Irish potatoes (Solanum tuberosum) in Central Province and sweet potatoes (Ipomoea batatas) in Central, Nyanza and Coast Provinces of Kenya. Int J Nematol 8:21–26
Norton DC (1978) Ecology of plant-parasitic nematodes. Wiley-Interscience, New York
Nzesya MJ, Wangai KJ, Maina MW, Peter WM, Elijah GK (2014) Plant parasitic nematodes associated with coffee in Kenya and factors influencing their occurrence, abundance and diversity. J Biol Agric Healthc 4:120–129
Olabiyi TI (2007) Susceptibility of sweet potato (Ipomea batatas) varieties to root knot nematode, Meloidogyne incognita. Am Eurasian J Agric Environ Sci 2:318–320
Omoyo NN, Wakhungu J, Otengi S (2015) Effects of climate variability on maize yield in the arid and semi arid lands of lower eastern Kenya. Agric Food Sec 4:8–21
Onkendi EM, Kariuki GM, Marais M, Moleleki LN (2014) The threat of root-knot nematodes (Meloidogyne spp.) in Africa : a review. Plant Pathol 63:727–737
Pritchard SG (2011) Soil organisms and global climate change. Plant Pathol 60:82–99
Roberts PA, Gundy SD, Van Mckinney HE (1979) Effects of soil temperature and planting date of wheat on Meloidogyne incognita reproduction, soil populations, and grain yield. J Nematol 13:338–345
Robinson AF, Inserra RN, Caswell-Chen EP, Vovlas N, Troccoli A (1997) Rotylenchulus species: Identification, distribution, host ranges, and crop plant resistance. Nematropica 27:127–180
Stamps WT, Linit MJ (1998) Neutral storage lipid and exit behavior of Bursaphelenchus xylophilus fourth stage dispersal juveniles from their beetle vectors. J Nematol 30:255–261
Strange RN, Scott PR (2005) Plant disease : A threat to global food security. Annu Rev Phytopathol 43:83–116
Thoden TC, Korthals GW, Termorshuizen AJ (2011) Organic amendments and their influences on plant parasitic and free living nematodes: A promising method for nematode management? Nematology 13:133–153
Thomas RJ, Clark CA (1983) Effects of concomitant development on reproduction of Meloidogyne incognita and Rotylenchulus reniformis on sweet potato. J Nematol 15:215–221
Waceke JW (2007) Plant parasitic nematodes associated with cabbages in Kenya. Afr Crop Sci Conf Proc 8:1071–1074
Watson RN, Pottinger RP (1990) Impact of climate change on invertebrate pests in New Zealand. Weather Clim 10:61–66
Wiesel L, Daniell TJ, King D, Neilson R (2015) Determination of the optimal soil sample size to accurately characterise nematode communities in soil. Soil Biol Biochem 80:89–91
Woolfe J (2002) Sweet potato: An untapped food resource. Cambridge University Press, Cambridge
Yavuzaslanoglu E, Elekcioglu H, Nicol J, Yorgancilar O, Hodson D, Yildirim A, Yorgancilar A, Bolat N (2012) Distribution, frequency and occurrence of cereal nematodes on the central Anatolian plateau in Turkey and their relationship with soil physicochemical properties. Nematology 14:8839–854
Acknowledgements
The authors would like to express gratitude to the Department for International Development (DfID) under the Climate Impact Research Capacity and Leadership Enhancement (CIRCLE) programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Isabel Abrantes
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Karuri, H.W., Olago, D., Neilson, R. et al. Plant parasitic nematode assemblages associated with sweet potato in Kenya and their relationship with environmental variables. Trop. plant pathol. 42, 1–12 (2017). https://doi.org/10.1007/s40858-016-0114-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-016-0114-4