Abstract
Sugarcane orange rust was recently introduced into Brazil and its control is based on the use of resistant varieties. This study aimed to determine the reaction of Brazilian sugarcane varieties to the disease in the field and to compare artificial inoculation methods. Rust severity was assessed in 17 varieties at a 15-day interval. The maximum disease severity (MS%) and the area under the disease progress curve (AUDPC) were determined for each genotype. The artificial inoculation methods tested were: spraying of a spore suspension on 60-day-old plants in the greenhouse, or placing the spore suspension into the leaf whorl of 5-month-old field-grown plants. Nine out of the 17 varieties studied were resistant to the disease, including the most widely grown in new plantings, RB867515 and RB966928. Varieties RB72454, SP89-1115 and SP79-2233 were susceptible, while RB925211 and SP81-3250 were moderately susceptible. Varieties RB855156, RB92579 and SP83-2847 showed an intermediate reaction. Both inoculation methods correlated well with field results. Spray inoculation discriminates better the responses of the varieties and enables the evaluation of more disease variables. Leaf whorl inoculation allows the use of field-grown plants and generates results in a shorter time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brazil is the world’s largest sugarcane producer, with a production estimated in 654 million tons from more than 9 million ha under cultivation (CONAB 2015). Sugarcane production is an important source of income for Brazil, arising from domestic consumption, sugar and ethanol exports, and the generation of employment and energy for the country (Matsuoka et al. 2009).
Several diseases limit sugarcane yield so that host resistance is a focus on breeding programs (Walker 1987). One of the most important diseases is orange rust, caused by the fungus Puccinia kuehnii (W. Krüger) E. J. Butler. Orange rust heavily impacted the sugarcane industry in Australia in the early 2000s, causing yield losses up to 36 % (Magarey et al. 2004). According to Zhao et al. (2011), the fungus affects plant growth and yield by reducing chlorophyll content of leaves, carbon sequestration efficiency, stomatal conductance, leaf transpiration rate and net photosynthetic rate.
The first report of P. kuehnii in the western hemisphere occurred in Florida, USA, in 2007 (Comstock et al. 2008). In 2009, the disease was detected in São Paulo state, Brazil (Barbasso et al. 2010). Since then, it has spread to other regions of the country, such as the northeast (Chaves et al. 2013).
The main control measure for orange rust is planting of resistant varieties (Magarey 2000); thus, knowing the resistance of major varieties grown in a particular region is essential. In Guatemala, after disease detection, a field trial was conducted to evaluate the reaction of 68 sugarcane genotypes to the disease in natural infection conditions. The results showed that 50 % of genotypes were highly resistant, with disease severity reaching 40 % on the seventh fully expanded leaf in the susceptible variety SP79-2233 (Ovalle et al. 2009).
Varietal resistance to orange rust can also be determined by artificial inoculation. Sood et al. (2009) placed 0.5 mL of a P. kuehnii spore suspension (104 viable spores /mL) into the leaf whorl of field-grown plants. Four weeks after inoculation, symptoms were observed in susceptible varieties (as grouped pustules). Of 1,470 genotypes evaluated, 965 (66 %) were resistant. The method enabled the evaluation of a large number of genotypes in a short time period with low inoculum use and minimal labor.
Few data on orange rust resistance are available for the main varieties cultivated in Brazil (Araújo et al. 2013; Klosowski et al. 2015). Therefore, this study was conducted with the following objectives: 1) determine the orange rust resistance of important sugarcane varieties cultivated in Brazil under field conditions, and 2) evaluate and compare two different artificial inoculation methods for determining resistant reaction.
Material and methods
Natural infection in the field
Two experiments were conducted at an experimental area in Araras, São Paulo state, Brazil (22°18′S; 47°23′W; average elevation of 611 m). The climate is classified as Cwa mesothermic (Köppen classification) with hot and humid summers and dry winters. The annual average temperature in the experimental area is 21.5 °C ranging from 17.9 °C in the coldest month (July) to 24.2 °C in the hottest month (February) with an average annual precipitation of 1,435 mm.
The municipality of Araras is located in a region classified as of moderate-high to high risk of orange rust epidemics (Santos 2013). In addition, according to Moreira (2013), in a study involving P. kuehnii populations in three Brazilian states, the population collected in Araras was the most aggressive.
Seventeen varieties were evaluated: RB72454, RB835054, RB855156, RB855453, RB855536, RB867515, RB92579, RB925211, RB935744, RB966928, SP79-2233, SP80-1842, SP80-3280, SP81-3250, SP83-2847, SP89-1115 and SP91-1049. Sixteen of them were selected based on the planted area in the states of São Paulo and Mato Grosso do Sul in 2010 (Chapola et al. 2011); these states together accounted for 60 % of the national sugarcane production in 2015 (CONAB 2015). Variety SP79-2233 was included in the tests due to its susceptibility to orange rust as reported in other countries (Ovalle et al. 2008, 2009; Chavarría et al. 2009; Garcés et al. 2014). Similarly, varieties RB72454 and SP89-1115 were considered susceptible to the disease in Brazil (Barbasso et al. 2010).
The first experiment was planted in April 2011 and the second, which consisted of a replication of the first, in July of the same year. The plots corresponded to double-row 2-m long with 1.4 m spacing between the rows. The plantings were made using 3-4-bud seed pieces, distributed in the rows at a density of 12–15 buds /m. The susceptible variety SP89-1115 was planted as border / spreader rows every six plots to increase the inoculum pressure within the experimental area. The experiment was conducted in a randomized complete block design with four replications.
Disease severity was determined on the +3 leaf (Araújo et al. 2013) by estimating the percentage of leaf area affected by symptoms. Assessments were carried out biweekly by four experienced evaluators, beginning 3–4 months after planting (or harvesting, in the case of the ratoon) and continuing until symptom expression peaked in susceptible varieties. In each assessment, 40 plants of each variety were evaluated, i. e, 10 plants per replicate.
The experiments were evaluated during the plant crop; harvest was in May 2012. Then, the second experiment was evaluated during the first ratoon. After harvest in October 2013, another evaluation during the second ratoon crop was made in the second experiment. Therefore, four different growing cycles were considered: 1 – planting in April 2011 and evaluation during the plant crop (16 assessments - from 4 to 11 months after planting); 2 – planting in July 2011 and evaluation during the plant crop (12 assessments - from 4 to 9 months after planting); 3 – harvest in May 2012 and evaluation during the first ratoon (17 assessments - from 3 to 11 months after harvesting); 4 – harvest in October 2013 and evaluation during the second ratoon (seven assessments - from 3 to 7 months after harvesting). For each variety, the area under the disease progress curve (AUDPC) was calculated by trapezoidal integration (Campbell and Madden 1990) with the maximum disease severity (MS%) determined; both variables were defined across all four growing cycles.
Artificial inoculation 1: spraying
This experiment was conducted in the facilities of the Piracicaba campus, ESALQ -Universidade de São Paulo, São Paulo state, Brazil. Six varieties with different disease resistance reactions were selected based on the results of the field experiments. Single-bud seed pieces were planted in plastic trays containing sterile substrate (composed of soil, sand and cattle manure). A completely randomized experimental design with four replicates was used; plots consisted of three plants.
The inoculum was prepared with spores of P. kuehnii collected from symptomatic leaves of varieties SP81-3250 and SP89-1115 growing in Araras; to collect spores, a brush was used (Klosowski et al. 2015). The spores were kept in the dark and at room temperature for 12 h. Spore viability was tested in four Petri dishes on 2 % water agar medium. The Petri dishes were divided into four fields; 50 spores per field were counted on a light microscope, i. e, 200 spores per Petri dish (Minchio et al. 2011). Spores with a germination tube longer than their diameter were considered germinated.
Spores were added to distilled water and the suspension was homogenized in a magnetic stirrer for 15 min. The suspension was adjusted to 104 viable spores /mL using a hemacytometer (Sood et al. 2009). Inoculation was carried out on 60-day-old plants using a manual sprayer; inoculation of primarily the abaxial surface of leaves continued until run-off. After inoculation, the plants remained for 24 h in a dew chamber at an average temperature 22 °C, conditions considered optimal for spore germination (Minchio et al. 2011) and infection (Infante et al. 2009; Martins 2010). Subsequently, the plants were transferred to a greenhouse under ambient conditions and irrigated as needed.
The following variables were assessed: incubation period (time between inoculation and appearance of first symptoms) and latent period (time between inoculation and sporulation in more than 50 % of pustules); number of pustules /cm2 on the +1 leaf using a grid with two “windows” of 1 cm2 each, considering the average of two counts for the analysis; and disease severity on the +1 leaf. Severity rating was assigned as percent diseased leaf area with the help of a leaf area meter (LI-3000, LI-COR) according to Díaz et al. (2001).
Artificial inoculation 2: leaf whorl
This experiment was conducted in the facilities of the Araras campus, CCA - Universidade Federal de São Carlos, São Paulo state, Brazil, using plants from one of the field trials. The varieties evaluated, the methods for collecting spores, the germination test and inoculum preparation were the same as used in the spray inoculation experiment. Five hundred microliters of a suspension containing 104 viable spores /mL were placed into the leaf whorl of 5-month-old plants, as per the method described by Sood et al. (2009). Inoculated plants were identified with red ribbons and the leaf tips were cut with scissors. The plots were composed of three plants with three replicates per variety.
Disease severity was assessed a month after inoculation and scores were attributed based on the scale proposed by Sood et al. (2009) according to the symptoms observed in the inoculated area of the leaf: score 1 – chlorotic spots; score 2 – discoloration without sporulation; score 3 – presence of pustules with sporulation; score 4 – massive sporulation or necrosis. The presence of sporulation was confirmed using a 10× loupe.
Statistical analysis
For the field experiment, AUDPC data were transformed using [(log x + 1) +1] and MS% was transformed into [√(x + 1)] for the statistical analysis. Data from all experiments were subjected to analysis of variance and means were compared by the Scott-Knott test at the 5 % level of significance, using SISVAR software.
Results
Natural infection in the field
The results in Table 1 showed significant differences (p < 0.01) among varieties for both AUDPC and MS%. Seven out of the 17 varieties showed no symptoms during the assessments: RB855453, RB855536, RB867515, RB966928, SP80-1842, SP80-3280 and SP91-1049. Two others, RB835054 and RB935744, exhibited symptoms in some assessments with MS% always below 1 %. Similar results were obtained by Araújo et al. (2013) in the municipality of Valparaiso, São Paulo state, and Klosowski et al. (2015) in Paranavaí, Paraná state, Brazil.
Eight other varieties exhibited a range of severities. RB72454 and SP89-1115 were the most susceptible ones, confirming the observations of Barbasso et al. (2010) and Araújo et al. (2013). SP79-2233 was also susceptible, which is corroborated by Ovalle et al. (2008, 2009), Chavarría et al. (2009) and Garcés et al. (2014), although its AUDPC and MS% were lower than that of RB72454 and SP89-1115 in our study.
Varieties RB925211, SP81-3250, RB855156, RB92579 and SP83-2847 presented AUDPC and MS% lower than that of susceptible varieties, but higher than that of resistant varieties (Table 1). RB925211 and SP81-3250 were the most susceptible and did not differ for AUDPC, but RB925211 exhibited the highest MS%. SP83-2847 was the most resistant of this group, while RB92579 showed higher resistance than RB855156 considering AUDPC, although their MS% did not differ statistically.
Figure 1 shows the genotype x environment interaction for the two variables evaluated. In the case of AUDPC (Fig. 1a), this interaction was more noticeable in SP81-3250, which exhibited greater susceptibility in the ratoon (growing cycles 3 and 4), and between RB835054 and RB935744, which showed a reversal ranking position in growing cycle 4. For MS% (Fig. 1b), the interaction among genotypes and environment (cycles) was greater.
Area under the disease progress curve (AUDPC) and maximum severity (MS%) of orange rust in sugarcane varieties in four different growing cycles (1 – planting in April 2011 and evaluation during the plant crop; 2 – planting in July 2011 and evaluation during the plant crop; 3 – harvest in May 2012 and evaluation during the first ratoon; 4 – harvest in October 2013 and evaluation during the second ratoon): A. AUDPC - Data transformed as [(log x + 1) +1]; B. MS%. The error bars denote standard deviations based on four replications
Figure 2 shows the disease progress curve in the four growing cycles considering the average severity of the most susceptible varieties, RB72454 and SP89-1115. An increase in the disease intensity was noted from the end of November with peaks of severity between February and April. Magarey et al. (2004) stated that conditions of high humidity and high temperatures favor orange rust development; the severity peaks of which usually occur between January and April in Australia. Orange rust development coincided with an increase in the number of favorable hours for the disease recorded from February to May (Araújo et al. 2013), reinforcing the results of our study.
Progress of orange rust severity considering the mean of the susceptible varieties RB72454 and SP89-1115 in four different growing cycles (1 – planting in April 2011 and evaluation during the plant crop; 2 – planting in July 2011 and evaluation during the plant crop; 3 – harvest in May 2012 and evaluation during the first ratoon; 4 – harvest in October 2013 and evaluation during the second ratoon)
Based on the information obtained in four different growing cycles, a relationship among AUDPC intervals and categories of orange rust varietal resistance was established (Table 2). In the classification proposed, RB72454, SP79-2233 and SP89-1115 were susceptible to orange rust (AUDPC > 4.0) followed by RB925211 and SP81-3250, both considered moderately susceptible (4.0 ≥ AUDPC > 3.5). Genotypes RB855156, RB92579 and SP83-2847 were classified as intermediate (3.5 ≥ AUDPC > 2.5). On the other hand, RB835054 and RB935744 were resistant (2.5 ≥ AUDPC > 1.5), while the others (RB855453, RB855536, RB867515, RB966928, SP80-1842, SP80-3280 and SP91-1049) were highly resistant to the disease (AUDPC ≤ 1.5).
Artificial inoculation 1: spraying
Spore viability based on germination was 42 %, similar to that obtained by Sood et al. (2009). The varieties used in this experiment were: RB72454 and SP89-1115 (susceptible); SP81-3250 (moderately susceptible); RB855156 (intermediate); RB935744 (resistant); and RB966928 (highly resistant). The first symptoms observed in the susceptible varieties were chlorotic spots 15 days after inoculation; at 18 days the first pustules sporulated. Minchio et al. (2011), working with temperatures of 10–30 °C, observed a lower spore germination at 10 °C. In our study, during the first 15 days after inoculation, temperatures below 10 °C were recorded in 10 separate days (8 days in a row - from 8 to 15 days after inoculation) and the highest temperature did not surpass 20 °C from 7 to 10 days after inoculation (Fig. 3), which likely influenced pathogen development, incubation and latent periods.
Maximum, minimum and average temperatures in Piracicaba during the spray inoculation study for the period of 7/16/2013 (0 d after inoculation) to 7/31/2013 (15 d after inoculation) according to the agro-meteorological station situated at the Biosystems Engineering Department, ESALQ/USP. Available at http://www.leb.esalq.usp.br/anos.html
There were significant treatment effects (p < 0.01) for the four variables analyzed. The susceptible varieties RB72454 and SP89-1115 had the shortest incubation and latent periods (Table 3). SP81-3250 and RB855156 did not differ from each other for incubation period; however, the latent period was shorter with SP81-3250. No symptoms were observed in the resistant varieties RB935744 and RB966928.
Latent period differences among genotypes were more pronounced than for the incubation period. Latent period measurements confirmed greater susceptibility of RB72454 and SP89-1115 as compared with the others, and also suggested a greater resistance of RB855156 compared to SP81-3250 - as observed in field trials.
There were no significant differences among RB72454, SP89-1115 and SP81-3250 for number of pustules /cm2 and severity. On the other hand, RB855156 showed a smaller number of pustules and lower severity scores than the three other varieties.
Genotype reactions obtained by spray inoculation were similar to the results of field trials. Susceptible varieties RB72454 and SP89-1115 had the shortest incubation and latent periods, the highest disease severity and the largest number of pustules /cm2. SP81-3250 did not differ from susceptible varieties regarding the number of pustules and severity, but it had longer incubation and latent periods; this was consistent with its lower susceptibility in the field compared to RB72454 and SP89-1115. However, SP81-3250 was more susceptible than RB855156 when taking into account three of the four variables evaluated, corroborating field observations. Varieties RB935744 and RB966928 showed no symptoms, confirming their resistance observed in natural infection conditions. Table 4 shows a significant correlation among the disease variables for all possible combinations. This indicated that it is possible to determine the genotype resistance reaction to orange rust by choosing any of the variables evaluated.
Artificial inoculation 2: leaf whorl
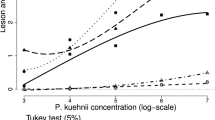
The viability of the spores collected was 46 %, similar to that in the spray inoculation test. The coefficient of variation (CV%) was 8.41 %, indicating good experimental precision. There were significant differences (p < 0.01) among varieties, similar to field and spray inoculation experiments. Figure 4 showed that RB72454 and SP89-1115 were the most susceptible with the highest disease severity. Within a month from inoculation, the inoculated leaves of both varieties exhibited abundant pathogen sporulation. Unlike the spray inoculation, there was no significant difference between SP81-3250 and RB855156.
Orange rust severity scores based on the scale proposed by Sood et al. (2009), determined in six sugarcane varieties artificially inoculated by a spore suspension of Puccinia kuehnii into the leaf whorl of field-grown plants (values followed by the same letters are not significantly different by Scott-Knott test at 0.05 level; the error bars denote standard deviation based on three replications)
The score for RB935744 was higher than that for RB966928. However, inoculated leaves of RB935744 did not show sporulation, indicating disease resistance. This result was similar to that observed in the field, where RB935744 presented symptoms in some assessments, but at severity levels always below 1 %. There was a high and significant correlation among the field and the two artificial inoculation techniques (Table 5). This showed that the inoculation methods tested have good potential to rapidly identify resistant genotypes, because both were effective in distinguishing varieties that were susceptible and resistant under field conditions.
Discussion
Nine (53 %) out of the 17 varieties evaluated in the field were resistant to the disease, consistent with the results obtained by Araújo et al. (2013) and Klosowski et al. (2015). Considering that the susceptibility of three varieties selected for the study (RB72454, SP79-2233 and SP89-1115) was already known, 64 % of the remaining 14 varieties were resistant.
These nine varieties accounted for nearly 50 % of the sugarcane area of São Paulo and Mato Grosso do Sul states in 2013 (Chapola et al. 2014). According to the authors, two varieties that proved resistant in our study were the most widely grown in new plantings (RB867515 and RB966928). This information suggests that the growing of resistant varieties predominates in Brazil, since São Paulo and Mato Grosso do Sul accounted for 60 % of the national sugarcane production in 2015 (CONAB 2015).
The classification of the varieties RB925211, SP81-3250, RB855156, RB92579 and SP83-2847 differed from that reported by Araújo et al. (2013). However, these authors conducted resistance tests in Valparaiso municipality, which is located in a region considered less favorable to orange rust (Santos 2013). Our study was conducted in a region more favorable to the disease, where the MS% reached values close to, or greater than, 50 % (Table 1), considerably above the levels obtained by Araújo et al. (2013). In the study of Klosowski et al. (2015), RB92579 was resistant, which differed from our results. This fact may be attributed to the higher inoculum pressure in our experiments, in which spreader rows of a susceptible variety (SP89-1115) were used.
In the field trials, the coefficient of variation (CV%) obtained for AUDPC was lower than that obtained for MS% (Table 1); in addition, the interaction among varieties and growing cycles was higher for MS% (Fig. 1). These observations indicated that AUDPC was the most suitable criterion to determine the orange rust resistance reaction of varieties. Its calculation takes into consideration several disease severity assessments during crop growth, making it a more reliable variable (Campbell and Madden 1990).
Severity assessments of orange rust in the south-center region in Brazil should be undertaken between November and April, regardless of the planting or harvesting time. During this period, temperatures and precipitation increase, favoring disease development. The results obtained in growing cycle 4 confirmed this observation, because with only seven assessments carried out between January and April, the classification of varieties was similar to the other three growing cycles, when the number of assessments ranged from 12 (growing cycle 2) to 17 (growing cycle 3).
The correlation among the disease variables evaluated in the spray inoculation technique indicated that it was possible to determine the genotype resistance reaction to orange rust by choosing any of the variables evaluated. For example, varieties with shorter incubation and latent periods exhibited larger number of pustules and higher severity than varieties with longer incubation and latent periods. This information is very useful for breeding programs, in which the number of genotypes to be evaluated can be high depending on the breeding stage; a single variable could be measured with minimal time inputs.
The high and significant correlation among natural infection and artificial inoculation results showed that the latter inoculation techniques have the potential to rapidly identify resistant genotypes. Both inoculation methods clearly distinguished susceptible and resistant varieties, saving time, space and labor.
Larger severity differences were noted among varieties with the spray inoculation technique, as measurement of more variables was possible. In the spray inoculation assay, SP81-3250 was more susceptible than RB855156, confirming the results obtained under field conditions. However, in the leaf whorl inoculation these two varieties did not differ. On the other hand, the leaf whorl inoculation allowed the use of field-grown plants, eliminating the need for greenhouse space, and the method generated quicker results.
Both inoculation techniques tested in our study may be included in sugarcane breeding programs to screening for orange rust resistance. The leaf whorl inoculation can be a useful tool at the early stages of breeding; according to Sood et al. (2009), this method enabled the evaluation of a large number of clones in a short time with low inoculum use and minimal labor. Besides, the technique did not require a greenhouse and a dew chamber before inoculation, since the leaf whorls acted protecting the spores from the effect of temperature fluctuation and increasing the availability of moisture, creating a favorable microenvironment for spore germination and infection (Sood et al. 2009).
On the other hand, the spray inoculation technique may be useful at the final stages of breeding, in which the number of clones to be evaluated is reduced and their characterization needs to be more refined. The advantage of this method compared to the leaf whorl inoculation was the possibility to measure more variables, enabling a more detailed characterization of the varietal resistance reaction. However, the measurement of many variables can turn the spray inoculation into a very laborious method. Furthermore, the number of varieties to be evaluated is limited by the size of the greenhouse available and the need of a dew chamber before the inoculation.
References
Araújo KL, Canteri MG, Gilio TAS, Neubauer RA, Sanches PB, Sumida CH, Giglioti EA (2013) Resistência genotípica e monitoramento da favorabilidade para ocorrência da ferrugem alaranjada da cana-de-açúcar. Summa Phytopathol 39:271–275
Barbasso D, Jordão H, Maccheroni W, Boldini J, Bressiani J, Sanguino A (2010) First report of Puccinia kuehnii, causal agent of orange rust of sugarcane, in Brazil. Plant Dis 94:1170
Campbell CL, Madden LV (1990) Introduction to plant disease epidemiology. Wiley-Interscience, New York
Chapola RG, Hoffmann HP, Bassinello AI, Fernandes AR Jr, Vieira MAS (2011) Censo varietal 2010 de cana-de-açúcar nos estados de São Paulo e Mato Grosso do Sul. STAB 29:42–45
Chapola RG, Hoffmann HP, Nunes IK, Fernandes AR Jr, Cursi DE, Bassinello AI (2014) Variedades de cana-de-açúcar mais cultivadas nos estados de São Paulo e Mato Grosso do Sul em 2013. STAB 32:26–30
Chavarría E, Subiros F, Vega J, Ralda G, Glynn NC, Comstock JC, Castlebury LA (2009) First report of orange rust caused by Puccinia kuehnii in Costa Rica and Nicaragua. Plant Dis 93:325
Chaves A, Simões Neto DE, Dutra Filho JA, Oliveira AC, Rodrigues WDL, Pedrosa EMR, Borges VJL, França PRP (2013) Presence of orange rust on sugarcane in the state of Pernambuco, Brazil. Trop Plant Pathol 38:443–446
Comstock JC, Sood SG, Glynn NC, Shine JM Jr, McKemy JM, Castlebury LA (2008) First report of Puccinia kuehnii, causal agent of orange rust of sugarcane, in the United States and Western Hemisphere. Plant Dis 92:175
CONAB (2015) Acompanhamento da safra brasileira cana-de-açúcar, v.2 – safra 2015/16, n.1 – primeiro levantamento. Available at: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/15_04_13_09_39_02_boletim_cana_portugues_-_1o_lev_-_15-16.pdf. Accessed on August 3, 2015
Díaz CG, Bassanezi RB, Godoy CV, Lopes DB, Bergamin Filho A (2001) Quantificação do efeito do crestamento bacteriano comum na eficiência fotossintética e na produção do feijoeiro. Fitopatol Bras 26:71–76
Garcés FF, Fiallos FF, Silva E, Martinez F, Aime MC, Comstock JC, Glynn NC, Castlebury LA (2014) First report of orange rust of sugarcane caused by Puccinia kuehnii in Ecuador. Plant Dis 98:842
Infante D, Martínez B, González E, González N (2009) Puccinia kuehnii (Krüger) Butler y Puccinia melanocephala H. Sydow y P. Sydow en el cultivo de la caña de azúcar. Revista de Protección Vegetal 24:22–28
Klosowski AC, Bespalhok Filho JC, Ruaro L, Fragoso RB, May de Mio LL (2015) Reação de cultivares e época de avaliação da ferrugem alaranjada da cana-de-açúcar. Biosci J 31:489–498
Magarey RC (2000) Orange rust disease of sugarcane. In: Rott P, Bailey RA, Comstock JC, Croft BJ, Saumtally AS (eds) A guide to sugarcane diseases. CIRAD, Montpellier, pp 121–125
Magarey RC, Neilsen WA, Bull JI (2004) The effect of orange rust on sugarcane yield in breeding selection trials in Central Queensland: 1999–2001. In: Conference of the Australian Society of Sugar Cane Technologists, Brisbane, Australia. ASSCT. Available at: assct.com.au/media/pdfs/2004_Ag_33.pdf. Accessed on January 5, 2015
Martins TD (2010) Aspectos epidemiológicos da ferrugem alaranjada da cana-de-açúcar. PhD Thesis, University of São Paulo. Piracicaba, SP, Brazil
Matsuoka S, Ferro J, Arruda P (2009) The Brazilian experience of sugarcane ethanol industry. In Vitro Cell Dev Biol Plant 45:372–381
Minchio CA, Canteri MG, Rocha JA (2011) Germinação de uredósporos de Puccinia kuehnii submetidos a diferentes temperaturas e tempos de incubação. Summa Phytopathol 37:211–214
Moreira AS (2013) Ferrugem alaranjada da cana-de-açúcar no Brasil: estudo de populações do patógeno e comportamento varietal. PhD Thesis, University of São Paulo. Piracicaba, SP, Brazil
Ovalle W, Comstock JC, Glynn NC, Castlebury LA (2008) First report of Puccinia kuehnii, causal agent of orange rust of sugarcane, in Guatemala. Plant Dis 92:973
Ovalle W, Orozco H, Quemé J, Melgar M, García S (2009) La roya naranja en Guatemala y estrategias para su manejo. Sugar J 72:18–23
Santos DL (2013) Zoneamento da favorabilidade climática para a ocorrência da ferrugem alaranjada da cana-de-açúcar nas principais regiões produtoras do Brasil e da Austrália. Master’s Thesis, University of São Paulo. Piracicaba, SP, Brazil
Sood SG, Comstock JC, Glynn NC (2009) Leaf whorl inoculation method for screening sugarcane rust resistance. Plant Dis 93:1335–1340
Walker DIT (1987) Breeding for disease resistance. In: Heinz DJ (ed) Sugarcane improvement through breeding. Elsevier Academic Press, Amsterdam, pp 455–502
Zhao D, Glynn NC, Glaz B, Comstock JC, Sood SG (2011) Orange rust effects on leaf photosynthesis and related characters of sugarcane. Plant Dis 95:640–647
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Harald Scherm
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chapola, R.G., Hoffmann, H.P. & Massola, N.S. Reaction of sugarcane varieties to orange rust (Puccinia kuehnii) and methods for rapid identification of resistant genotypes. Trop. plant pathol. 41, 139–146 (2016). https://doi.org/10.1007/s40858-016-0076-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-016-0076-6