Abstract
Purpose
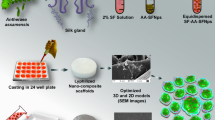
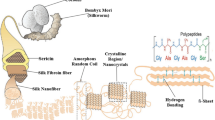
Biopolymeric materials, especially composites, are extensively used as wound healing scaffolds in tissue engineering due to their ability to mimic the essential properties of the native tissue. This research aims to investigate the usability of honeybee silk (HS), which could be an alternative silk source to silkworm silk, in tissue engineering (TE) applications. HS, which has not been used in scaffold fabrication, and chitosan (CH), frequently used in TE, were combined to produce a novel and cost-effective biocompatible CH–HS scaffold.
Methods
HS, CH and CH–HS were characterized using XRD, FTIR and SEM to determine structure and functional groups. SEM analysis was performed for different CH concentrations (0.5%, 1% and 2%) and different ratios of CH:HS (1:2, 1:1 and 2:1, respectively). The antioxidant properties, antibacterial activity and as well as biofilm formation and ability to destroy mature biofilm activity of HS and CH–HS were shown. The human breast cancer MDA-MB231 cells were used to investigate possible effects on cell viability proliferation.
Results
The smallest pore size was determined to be 70.7 µm on average at a ratio of 1:1 at 1% CH concentration. The antioxidant properties of HS and CH–HS were shown. The CH–HS showed antibacterial activity against Escherichia coli and Pseudomonas aeruginosa, as well as inhibition of biofilm formation and destruction of mature biofilm. Additionally, the MDA-MB-231 cells appeared significantly elongated and denser when seeded on the CH–HS over 24 h and 48 h.
Conclusion
This study demonstrated the usability of honeybee silk, a promising but underutilized material, tissue engineering and its potential for future studies. Considering the materials used and our promising results, the synthesized CH–HS scaffold was observed to have microbiological and cellular effects that may be useful in future biomedical applications for wound healing.







Similar content being viewed by others
Data Availability
The raw/processed data are available on reasonable request.
References
Di Silvio, L. (2007). 15—Bone tissue engineering and biomineralization. In A. R. Boccaccini & J. E. Gough (Eds.), Tissue engineering using ceramics and polymers (pp. 319–331). Woodhead Publishing.
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260, 920–926. https://doi.org/10.1126/science.8493529
Pearson, R. G., Bhandari, R., Quirk, R. A., & Shakesheff, K. M. (2017). Recent advances in tissue engineering. Journal of Long-Term Effects of Medical Implants, 27, 199–231. https://doi.org/10.1615/JLongTermEffMedImplants.v27.i2-4.70
Berthiaume, F., Maguire, T. J., & Yarmush, M. L. (2011). Tissue engineering and regenerative medicine: History, progress, and challenges. Annual Review of Chemical and Biomolecular Engineering, 2, 403–430. https://doi.org/10.1146/annurev-chembioeng-061010-114257
Olson, J. L., Atala, A., & Yoo, J. J. (2011). Tissue engineering: Current strategies and future directions. Chonnam Medical Journal, 47, 1–13. https://doi.org/10.4068/cmj.2011.47.1.1
O’Brien, F. J. (2011). Biomaterials & scaffolds for tissue engineering. Materials Today, 14, 88–95. https://doi.org/10.1016/S1369-7021(11)70058-X
Litowczenko, J., Woźniak-Budych, M. J., Staszak, K., Wieszczycka, K., Jurga, S., & Tylkowski, B. (2021). Milestones and current achievements in development of multifunctional bioscaffolds for medical application. Bioactive Materials, 6, 2412–2438. https://doi.org/10.1016/j.bioactmat.2021.01.007
Tottoli, E. M., Dorati, R., Genta, I., Chiesa, E., Pisani, S., & Conti, B. (2020). Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics, 12, 735.
Al-Himdani, S., Jessop, Z. M., Al-Sabah, A., Combellack, E., Ibrahim, A., Doak, S. H., Hart, A. M., Archer, C. W., Thornton, C. A., & Whitaker, I. S. (2017). Tissue-engineered solutions in plastic and reconstructive surgery: Principles and practice. Frontiers in Surgery, 4, 4. https://doi.org/10.3389/fsurg.2017.00004
Fasiku, V. O., Omolo, C. A., Devnarain, N., Ibrahim, U. H., Rambharose, S., Faya, M., Mocktar, C., Singh, S. D., & Govender, T. (2021). Chitosan-based hydrogel for the dual delivery of antimicrobial agents against bacterial methicillin-resistant Staphylococcus aureus biofilm-infected wounds. ACS Omega, 6, 21994–22010. https://doi.org/10.1021/acsomega.1c02547
Krishani, M., Shin, W. Y., Suhaimi, H., & Sambudi, N. S. (2023). Development of scaffolds from bio-based natural materials for tissue regeneration applications: A review. Gels, 9, 100.
Nardo, T., Carmagnola, I., Ruini, F., Caddeo, S., Calzone, S., Chiono, V., & Ciardelli, G. (2017). Chapter 65—Synthetic biomaterial for regenerative medicine applications. In G. Orlando, G. Remuzzi, & D. F. Williams (Eds.), Kidney transplantation, bioengineering and regeneration (pp. 901–921). Academic Press.
Jenkins, T. L., & Little, D. (2019). Synthetic scaffolds for musculoskeletal tissue engineering: Cellular responses to fiber parameters. NPJ Regenerative Medicine, 4, 15.
Jensen, G., Morrill, C., & Huang, Y. (2018). 3D tissue engineering, an emerging technique for pharmaceutical research. Acta Pharmaceutica Sinica B, 8, 756–766. https://doi.org/10.1016/j.apsb.2018.03.006
Lee, D. H., Kim, W., Song, J. E., & Khang, G. (2022). Chapter 14—Prospects of collagen scaffolds for muscle regeneration. In C. P. Sharma, T. Chandy, V. Thomas, & F. G. Thankam (Eds.), Tissue engineering (pp. 347–361). Academic Press.
Sanchez-Rubio, A., Jayawarna, V., Maxwell, E., Dalby, M. J., & Salmeron-Sanchez, M. (2023). Keeping it organized: Multicompartment constructs to mimic tissue heterogeneity. Advanced Healthcare Materials, 12, 2202110.
He, W., & Benson, R. (2017). Polymeric biomaterials. In W. He & R. Benson (Eds.), Applied plastics engineering handbook (pp. 145–164). Elsevier.
Negut, I., Dorcioman, G., & Grumezescu, V. (2020). Scaffolds for wound healing applications. Polymers, 12, 2010.
Qu, H., Fu, H., Han, Z., & Sun, Y. (2019). Biomaterials for bone tissue engineering scaffolds: A review. RSC Advances, 9, 26252–26262. https://doi.org/10.1039/c9ra05214c
Arif, M. M., Khan, S. M., Gull, N., Tabish, T. A., Zia, S., Khan, R. U., Awais, S. M., & Butt, M. A. (2021). Polymer-based biomaterials for chronic wound management: Promises and challenges. International Journal of Pharmaceutics, 598, 120270.
Das, P., Manna, S., Roy, S., Nandi, S. K., & Basak, P. (2023). Polymeric biomaterials-based tissue engineering for wound healing: A systemic review. Burns & Trauma. https://doi.org/10.1093/burnst/tkac058
Kohane, D. S., & Langer, R. (2008). Polymeric biomaterials in tissue engineering. Pediatric Research, 63, 487–491.
Liu, X., Ma, L., Mao, Z., & Gao, C. (2011). Chitosan-based biomaterials for tissue repair and regeneration. Chitosan for Biomaterials, II, 81–127.
Niculescu, A.-G., & Grumezescu, A. M. (2022). An up-to-date review of biomaterials application in wound management. Polymers, 14, 421.
Patel, D. K., Dutta, S. D., Hexiu, J., Ganguly, K., & Lim, K.-T. (2022). 3D-printable chitosan/silk fibroin/cellulose nanoparticle scaffolds for bone regeneration via M2 macrophage polarization. Carbohydrate Polymers, 281, 119077. https://doi.org/10.1016/j.carbpol.2021.119077
Farokhi, M., Mottaghitalab, F., Fatahi, Y., Saeb, M. R., Zarrintaj, P., Kundu, S. C., & Khademhosseini, A. (2019). Silk fibroin scaffolds for common cartilage injuries: Possibilities for future clinical applications. European Polymer Journal, 115, 251–267. https://doi.org/10.1016/j.eurpolymj.2019.03.035
Kundu, B., Rajkhowa, R., Kundu, S. C., & Wang, X. (2013). Silk fibroin biomaterials for tissue regenerations. Advanced Drug Delivery Reviews, 65, 457–470. https://doi.org/10.1016/j.addr.2012.09.043
Chouhan, D., & Mandal, B. B. (2020). Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomaterialia, 103, 24–51. https://doi.org/10.1016/j.actbio.2019.11.050
Hepburn, H. R., Duangphakdee, O., & Pirk, C. W. (2013). Physical properties of honeybee silk: A review. Apidologie, 44, 600–610.
Kumar, M., Jain, D., Bhardwaj, N., Gupta, P., Nandi, S. K., & Mandal, B. B. (2016). Native honeybee silk membrane: A potential matrix for tissue engineering and regenerative medicine. RSC Advances, 6, 54394–54403. https://doi.org/10.1039/C6RA10738A
Hepburn, H. R., & Kurstjens, S. (1988). The combs of honeybees as composite materials. Apidologie, 19, 25–36.
Zhang, K., Si, F. W., Duan, H. L., & Wang, J. (2010). Microstructures and mechanical properties of silks of silkworm and honeybee. Acta Biomaterialia, 6, 2165–2171. https://doi.org/10.1016/j.actbio.2009.12.030
Reddy, N., & Yang, Y. (2015). Honeybee silks. In N. Reddy & Y. Yang (Eds.), Innovative biofibers from renewable resources (pp. 201–203). Springer.
Tuwalska, A., Grabska-Zielińska, S., & Sionkowska, A. (2022). Chitosan/silk fibroin materials for biomedical applications—A review. Polymers, 14, 1343.
Kavaz, D., Idris, M., & Onyebuchi, C. (2019). Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. International Journal of Biological Macromolecules, 123, 837–845. https://doi.org/10.1016/j.ijbiomac.2018.11.177
Li, D.-W., Lei, X., He, F.-L., He, J., Liu, Y.-L., Ye, Y.-J., Deng, X., Duan, E., & Yin, D.-C. (2017). Silk fibroin/chitosan scaffold with tunable properties and low inflammatory response assists the differentiation of bone marrow mesenchymal stem cells. International Journal of Biological Macromolecules, 105, 584–597.
Al-Bakri, A. G., & Afifi, F. U. (2007). Evaluation of antimicrobial activity of selected plant extracts by rapid XTT colorimetry and bacterial enumeration. Journal of Microbiological Methods, 68, 19–25. https://doi.org/10.1016/j.mimet.2006.05.013
Bali, E. B., Türkmen, K. E., Erdönmez, D., & Sağlam, N. (2019). Comparative study of inhibitory potential of dietary phytochemicals against quorum sensing activity of and biofilm formation by Chromobacterium violaceum 12472, and swimming and swarming behaviour of Pseudomonas aeruginosa PAO1. Food technology and biotechnology, 57, 212–221. https://doi.org/10.17113/ftb.57.02.19.5823
Sivaraj, C., Aashinya, Y., Sripriya, R., & Arumugam, P. (2018). Antioxidant activities and thin layer chromatographic analysis of aqueous extract of tubers of Drynaria quercifolia (L). J. Sm, Free Radicals and Antioxidants, 8, 26–31.
Perumal, A., Krishna, S., & Madhusree, M. (2018). GC-MS analysis, antioxidant and antibacterial activities of ethanol extract of leaves of Aegle marmelos (L.) corraša. Journal of Drug Delivery and Therapeutics, 8, 247–255.
Li, M., Liang, Y., He, J., Zhang, H., & Guo, B. (2020). Two-pronged strategy of biomechanically active and biochemically multifunctional hydrogel wound dressing to accelerate wound closure and wound healing. Chemistry of Materials, 32, 9937–9953. https://doi.org/10.1021/acs.chemmater.0c02823
Hu, L., Zou, L., Liu, Q., Geng, Y., Xu, G., Chen, L., Pan, P., & Chen, J. (2022). Construction of chitosan-based asymmetric antioxidant and anti-inflammatory repair film for acceleration of wound healing. International Journal of Biological Macromolecules, 215, 377–386. https://doi.org/10.1016/j.ijbiomac.2022.06.103
Gianak, O., Pavlidou, E., Sarafidis, C., Karageorgiou, V., & Deliyanni, E. (2018). Silk fibroin nanoparticles for drug delivery: Effect of bovine serum albumin and magnetic nanoparticles addition on drug encapsulation and release. Separations, 5, 25.
Singh, V., Tripathi, D. K., Sharma, V. K., Srivastava, D., Kumar, U., Poluri, K. M., Singh, B. N., Kumar, D., & Venkatesh Kumar, R. (2023). Silk fibroin hydrogel: A novel biopolymer for sustained release of vancomycin drug for diabetic wound healing. Journal of Molecular Structure. https://doi.org/10.1016/j.molstruc.2023.135548
Weisman, S., Haritos, V. S., Church, J. S., Huson, M. G., Mudie, S. T., Rodgers, A. J., Dumsday, G. J., & Sutherland, T. D. (2010). Honeybee silk: Recombinant protein production, assembly and fiber spinning. Biomaterials, 31, 2695–2700.
Murphy, C. M., & O’Brien, F. J. (2010). Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhesion & Migration, 4, 377–381. https://doi.org/10.4161/cam.4.3.11747
Zeng, S., Liu, L., Shi, Y., Qiu, J., Fang, W., Rong, M., Guo, Z., & Gao, W. (2015). Characterization of silk fibroin/chitosan 3D porous scaffold and in vitro cytology. PLoS ONE, 10, e0128658. https://doi.org/10.1371/journal.pone.0128658
Vishwanath, V., Pramanik, K., & Biswas, A. (2016). Optimization and evaluation of silk fibroin-chitosan freeze-dried porous scaffolds for cartilage tissue engineering application. Journal of Biomaterials Science Polymer edition, 27, 657–674. https://doi.org/10.1080/09205063.2016.1148303
Kweon, H., Ha, H. C., Um, I. C., & Park, Y. H. (2001). Physical properties of silk fibroin/chitosan blend films. Journal of Applied Polymer Science, 80, 928–934. https://doi.org/10.1002/app.1172
Huang, Y., Zhang, B., Xu, G., & Hao, W. (2013). Swelling behaviours and mechanical properties of silk fibroin–polyurethane composite hydrogels. Composites Science and Technology, 84, 15–22. https://doi.org/10.1016/j.compscitech.2013.05.007
Azmy, E. A. M., Hashem, H. E., Mohamed, E. A., & Negm, N. A. (2019). Synthesis, characterization, swelling and antimicrobial efficacies of chemically modified chitosan biopolymer. Journal of Molecular Liquids, 284, 748–754. https://doi.org/10.1016/j.molliq.2019.04.054
Lin, Y.-J., Lee, G.-H., Chou, C.-W., Chen, Y.-P., Wu, T.-H., & Lin, H.-R. (2015). Stimulation of wound healing by PU/hydrogel composites containing fibroblast growth factor-2. Journal of Materials Chemistry B, 3, 1931–1941.
Moradi, S., Nazarian, S., Milan, P. B., Nilforoushzadeh, M. A., & Zargan, J. (2023). Silk fibroin based core-shell nanofibers loaded with ZnO nanoparticles: An ideal candidate for designing a medicated wound dressing. Journal of Medical and Biological Engineering, 43, 689–705.
Khosravimelal, S., Chizari, M., Farhadihosseinabadi, B., Moosazadeh Moghaddam, M., & Gholipourmalekabadi, M. (2021). Fabrication and characterization of an antibacterial chitosan/silk fibroin electrospun nanofiber loaded with a cationic peptide for wound-dressing application. Journal of Materials Science: Materials in Medicine, 32, 114. https://doi.org/10.1007/s10856-021-06542-6
Cai, Z.-X., Mo, X.-M., Zhang, K.-H., Fan, L.-P., Yin, A.-L., He, C.-L., & Wang, H.-S. (2010). Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. International Journal of Molecular Sciences, 11, 3529–3539.
Eivazzadeh-Keihan, R., Radinekiyan, F., Aliabadi, H. A. M., Sukhtezari, S., Tahmasebi, B., Maleki, A., & Madanchi, H. (2021). Chitosan hydrogel/silk fibroin/Mg(OH)2 nanobiocomposite as a novel scaffold with antimicrobial activity and improved mechanical properties. Scientific Reports, 11, 650. https://doi.org/10.1038/s41598-020-80133-3
Liang, Y., Liang, Y., Zhang, H., & Guo, B. (2022). Antibacterial biomaterials for skin wound dressing. Asian Journal of Pharmaceutical Sciences, 17, 353–384. https://doi.org/10.1016/j.ajps.2022.01.001
Subramani, R., & Jayaprakashvel, M. (2019). Bacterial quorum sensing: biofilm formation, survival behaviour and antibiotic resistance. In P. V. Bramhachari (Ed.), Implication of quorum sensing and biofilm formation in medicine, agriculture and food industry (pp. 21–37). Springer.
Sutherland, T. D., Young, J. H., Weisman, S., Hayashi, C. Y., & Merritt, D. J. (2010). Insect silk: One name, many materials. Annual Review of Entomology, 55, 171–188.
Aramwit, P., Napavichayanum, S., Pienpinijtham, P., Rasmi, Y., & Bang, N. (2020). Antibiofilm activity and cytotoxicity of silk sericin against Streptococcus mutans bacteria in biofilm: An in vitro study. Journal of Wound Care, 29, S25–S35.
Muthuchamy, M., Govindan, R., Shine, K., Thangasamy, V., Alharbi, N. S., Thillaichidambaram, M., Khaled, J. M., Wen, J.-L., & Alanzi, K. F. (2020). Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa and K. pneumoniae. Carbohydrate Polymers, 230, 115646. https://doi.org/10.1016/j.carbpol.2019.115646
Alrouji, M., Kuriri, F. A., Alqasmi, M. H., AlSudais, H., Alissa, M., Alsuwat, M. A., Asad, M., Joseph, B., & Almuhanna, Y. (2023). A simple in-vivo method for evaluation of antibiofilm and wound healing activity using excision wound model in diabetic Swiss albino mice. Microorganisms, 11, 692.
Baliyan, S., Mukherjee, R., Priyadarshini, A., Vibhuti, A., Gupta, A., Pandey, R. P., & Chang, C. M. (2022). Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules (Basel, Switzerland), 27, 1326.
Wang, G., Yang, F., Zhou, W., Xiao, N., Luo, M., & Tang, Z. (2023). The initiation of oxidative stress and therapeutic strategies in wound healing. Biomedicine & Pharmacotherapy, 157, 114004. https://doi.org/10.1016/j.biopha.2022.114004
Fitzmaurice, S. D., Sivamani, R. K., & Isseroff, R. R. (2011). Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Skin Pharmacology and Physiology, 24, 113–126. https://doi.org/10.1159/000322643
Abd El-Hack, M. E., El-Saadony, M. T., Shafi, M. E., Zabermawi, N. M., Arif, M., Batiha, G. E., Khafaga, A. F., Abd El-Hakim, Y. M., & Al-Sagheer, A. A. (2020). Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. International Journal of Biological Macromolecules, 164, 2726–2744. https://doi.org/10.1016/j.ijbiomac.2020.08.153
Schreiber, S. B., Bozell, J. J., Hayes, D. G., & Zivanovic, S. (2013). Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocolloids, 33, 207–214.
Woranuch, S., & Yoksan, R. (2013). Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydrate Polymers, 96, 495–502.
Saha, J., Mondal, M., Sheikh, M. K., & Habib, M. (2019). Extraction, structural and functional properties of silk sericin biopolymer from Bombyx mori silk cocoon waste. Journal of Textile Science & Engineering. https://doi.org/10.4172/2165-8064.1000390
Selvaraj, S., & Fathima, N. N. (2017). Fenugreek incorporated silk fibroin nanofibers—A potential antioxidant scaffold for enhanced wound healing. ACS Applied Materials & Interfaces, 9, 5916–5926. https://doi.org/10.1021/acsami.6b16306
Wang, F., Pang, Y., Chen, G., Wang, W., & Chen, Z. (2020). Enhanced physical and biological properties of chitosan scaffold by silk proteins cross-linking. Carbohydrate Polymers, 229, 115529. https://doi.org/10.1016/j.carbpol.2019.115529
Acknowledgements
This research was supported by the Cancer Research Foundation of TRNC.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Sual Tatlisulu: Conceptualization, investigation, methodology, writing—original draft. Erkay Ozgor: Conceptualization, supervision, writing—review and editing. Doga Kavaz: Methodology, supervision, writing—review and editing. Mustafa B. A. Djamgoz: Conceptualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
No ethics approval is required for this study.
Consent for Publication
All authors read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tatlisulu, S., Ozgor, E., Kavaz, D. et al. Honeybee Silk and Chitosan: A Promising Biocomposite for Wound Healing Applications. J. Med. Biol. Eng. (2024). https://doi.org/10.1007/s40846-024-00853-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40846-024-00853-z