Abstract
Purpose
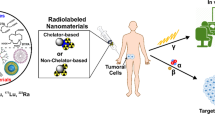
Although plenty of research and reviews focus on the medical applications of nanomaterials, the use of nanomaterials as pharmaceuticals is still rarely approved. Intravenous injections of theranostic nanomaterials are a common route for in vivo studies. However, the consideration of using nanomaterials for prevalent drug carriers is still limited because they are inevitably accumulated in vital organs. This review focused on contemporary radiolabeled nanomaterials and potent immunological responses that have been reported in preclinical and clinical studies.
Methods
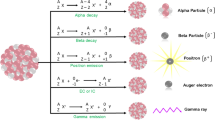
In general, nanomaterials can conjugate with different targeting or therapeutic entities to treat diseases in vivo, and certain materials may generate fluorescence or heat by external photoexcitation. Both inorganic and organic nanomaterials used for conjugation of radionuclides for diagnosis or therapy in vivo are discussed.
Results
Most nanomaterials are claimed to contain low cytotoxicity, and the immunological responses resulted from nanomaterials are versatile. The induced immunity is a benefit for adjuvant therapy in cancer treatment. However, the systemic effects are yet to be investigated. The organic polymer dots exhibit ultrabright near-infrared II fluorescence that can be used for imaging of tumor angiogenesis, and enhanced permeability and retention effects. This nanomaterial should be ideal for clinical application after conjugation with approved radionuclides.
Conclusion
Nanomaterials are candidates for theranostic purposes, although their biosafety is still a challenge. The immunomodulation by specific nanomaterials is particularly interesting because radionuclide-conjugated nanoparticles may contribute to the concept of combined radiotherapy and immunotherapy.

Permissions were not required according to the responses from journal publishers.)



Similar content being viewed by others
References
Price, E. W., & Orvig, C. (2014). Matching chelators to radiometals for radiopharmaceuticals. Chemical Society Reviews, 43(1), 260–290
Ni, D., Jiang, D., Ehlerding, E. B., Huang, P., & Cai, W. (2018). Radiolabeling Silica-Based Nanoparticles via Coordination Chemistry: Basic Principles, Strategies, and Applications. Accounts Of Chemical Research, 51(3), 778–788
Hwang, D. W., Ko, H. Y., Lee, J. H., Kang, H., Ryu, S. H., Song, I. C. … Kim, S. (2010). A nucleolin-targeted multimodal nanoparticle imaging probe for tracking cancer cells using an aptamer. Journal Of Nuclear Medicine, 51(1), 98–105
Cai, Z., Chattopadhyay, N., Yang, K., Kwon, Y. L., Yook, S., Pignol, J. P., & Reilly, R. M. (2016). (111)In-labeled trastuzumab-modified gold nanoparticles are cytotoxic in vitro to HER2-positive breast cancer cells and arrest tumor growth in vivo in athymic mice after intratumoral injection. Nuclear Medicine And Biology, 43(12), 818–826
Chen, C. C., Chang, D. Y., Li, J. J., Chan, H. W., Chen, J. T., Chang, C. H. … Wang, H. E. (2020). Investigation of biodistribution and tissue penetration of PEGylated gold nanostars and their application for photothermal cancer treatment in tumor-bearing mice. J Mater Chem B, 8(1), 65–77
Kao, H. W., Lin, Y. Y., Chen, C. C., Chi, K. H., Tien, D. C., Hsia, C. C. … Wang, H. E. (2014). Biological characterization of cetuximab-conjugated gold nanoparticles in a tumor animal model. Nanotechnology, 25(29), 295102
Zhang, Y., Hong, H., & Cai, W. (2011). PET tracers based on Zirconium-89. Curr Radiopharm, 4(2), 131–139
Dijkers, E. C., Kosterink, J. G., Rademaker, A. P., Perk, L. R., van Dongen, G. A., Bart, J. … de Vries, E. G. (2009). Lub-de Hooge MN: Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. Journal Of Nuclear Medicine, 50(6), 974–981
Rylova, S. N., Del Pozzo, L., Klingeberg, C., Tonnesmann, R., Illert, A. L., Meyer, P. T. … Holland, J. P. (2016). Immuno-PET Imaging of CD30-Positive Lymphoma Using 89Zr-Desferrioxamine-Labeled CD30-Specific AC-10 Antibody. Journal Of Nuclear Medicine, 57(1), 96–102
Karmani, L., Labar, D., Valembois, V., Bouchat, V., Nagaswaran, P. G., Bol, A., Gillart, J., Leveque, P., Bouzin, C., Bonifazi, D., et al. (2013). Antibody-functionalized nanoparticles for imaging cancer: influence of conjugation to gold nanoparticles on the biodistribution of 89Zr-labeled cetuximab in mice. Contrast Media & Molecular Imaging, 8(5), 402–408
Sobol, N. B., Korsen, J. A., Younes, A., Edwards, K. J., & Lewis, J. S. (2020). : ImmunoPET Imaging of Pancreatic Tumors with (89)Zr-Labeled Gold Nanoparticle-Antibody Conjugates. Mol Imaging Biol
Bansal, A., Pandey, M. K., Demirhan, Y. E., Nesbitt, J. J., Crespo-Diaz, R. J., Terzic, A. … DeGrado, T. R. (2015). Novel (89)Zr cell labeling approach for PET-based cell trafficking studies. EJNMMI Res, 5, 19
Man, F., Lim, L., Volpe, A., Gabizon, A., Shmeeda, H., Draper, B., Parente-Pereira, A. C., Maher, J., Blower, P. J., Fruhwirth, G. O., et al. (2019). In Vivo PET Tracking of (89)Zr-Labeled Vgamma9Vdelta2 T Cells to Mouse Xenograft Breast Tumors Activated with Liposomal Alendronate. Molecular Therapy, 27(1), 219–229
Menke-van der Houven, van Oordt, C. W., Gootjes, E. C., Huisman, M. C., Vugts, D. J., Roth, C., Luik, A. M., Mulder, E. R., Schuit, R. C., Boellaard, R., Hoekstra, O. S., et al. (2015). 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget, 6(30), 30384–30393
Lee, H. Y., Li, Z., Chen, K., Hsu, A. R., Xu, C., Xie, J. … Chen, X. (2008). PET/MRI Dual-Modality Tumor Imaging Using Arginine-Glycine-Aspartic (RGD)–Conjugated Radiolabeled Iron Oxide Nanoparticles. Journal of Nuclear Medicine, 49(8), 1371–1379
Lledos, M., Mirabello, V., Sarpaki, S., Ge, H., Smugowski, H. J., Carroll, L., Aboagye, E. O., Aigbirhio, F. I., Botchway, S. W., Dilworth, J. R., et al. (2018). : Synthesis, Radiolabelling and In Vitro Imaging of Multifunctional Nanoceramics. ChemNanoMat 4(4):361–372
Bhirde, A., Xie, J., Swierczewska, M., & Chen, X. (2011). Nanoparticles for cell labeling. Nanoscale, 3(1), 142–153
Li, H., Diaz, L., Lee, D., Cui, L., Liang, X., & Cheng, Y. (2014). In vivo imaging of T cells loaded with gold nanoparticles: a pilot study. La Radiologia Medica, 119(4), 269–276
Bhatnagar, P., Li, Z., Choi, Y., Guo, J., Li, F., Lee, D. Y., Figliola, M., Huls, H., Lee, D. A., Zal, T., et al. (2013). Imaging of genetically engineered T cells by PET using gold nanoparticles complexed to Copper-64. Integr Biol (Camb), 5(1), 231–238
Cheng, S. H., Yu, D., Tsai, H. M., Morshed, R. A., Kanojia, D., Lo, L. W., Leoni, L., Govind, Y., Zhang, L., Aboody, K. S., et al. (2016). Dynamic In Vivo SPECT Imaging of Neural Stem Cells Functionalized with Radiolabeled Nanoparticles for Tracking of Glioblastoma. Journal Of Nuclear Medicine, 57(2), 279–284
Song, L., Falzone, N., & Vallis, K. A. (2016). EGF-coated gold nanoparticles provide an efficient nano-scale delivery system for the molecular radiotherapy of EGFR-positive cancer. International Journal Of Radiation Biology, 92(11), 716–723
Zolata, H., Afarideh, H., & Davani, F. A. (2016). Triple Therapy of HER2(+) Cancer Using Radiolabeled Multifunctional Iron Oxide Nanoparticles and Alternating Magnetic Field. Cancer Biotherapy & Radiopharmaceuticals, 31(9), 324–329
Dash, A., Pillai, M. R., & Knapp, F. F. Jr. (2015). Production of (177)Lu for Targeted Radionuclide Therapy: Available Options. Nucl Med Mol Imaging, 49(2), 85–107
Strosberg, J., El-Haddad, G., Wolin, E., Hendifar, A., Yao, J., Chasen, B., Mittra, E., Kunz, P. L., Kulke, M. H., Jacene, H., et al. (2017). Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. New England Journal Of Medicine, 376(2), 125–135
Assadi, M., Pirayesh, E., Rekabpour, S. J., Zohrabi, F., Jafari, E., Nabipour, I. … Ahmadzadehfar, H. (2019). 177Lu-PSMA and 177Lu-DOTATATE Therapy in a Patient With Metastatic Castration-Resistant Prostate Cancer and Neuroendocrine Differentiation. Clinical Nuclear Medicine, 44(12), 978–980
Luna-Gutiérrez, M., Ferro-Flores, G., Ocampo-García, B. E., Santos-Cuevas, C. L., Jiménez-Mancilla, N., León-Rodríguez, D. … Isaac-Olivé, K. (2013). A therapeutic system of 177Lu-labeled gold nanoparticles-RGD internalized in breast cancer cells. Journal of the Mexican Chemical Society, 57(3), 212–219
Vilchis-Juarez, A., Ferro-Flores, G., Santos-Cuevas, C., Morales-Avila, E., Ocampo-Garcia, B., Diaz-Nieto, L. … Gomez-Olivan, L. (2014). Molecular targeting radiotherapy with cyclo-RGDFK(C) peptides conjugated to 177Lu-labeled gold nanoparticles in tumor-bearing mice. Journal Of Biomedical Nanotechnology, 10(3), 393–404
Yook, S., Cai, Z., Lu, Y., Winnik, M. A., Pignol, J. P., & Reilly, R. M. (2015). : Radiation Nanomedicine for EGFR-Positive Breast Cancer: Panitumumab-Modified Gold Nanoparticles Complexed to the beta-Particle-Emitter, (177) Lu Mol Pharm 12(11):3963–3972
Yook, S., Cai, Z., Lu, Y., Winnik, M. A., Pignol, J. P., & Reilly, R. M. (2016). Intratumorally Injected 177Lu-Labeled Gold Nanoparticles: Gold Nanoseed Brachytherapy with Application for Neoadjuvant Treatment of Locally Advanced Breast Cancer. Journal Of Nuclear Medicine, 57(6), 936–942
Lusic, H., & Grinstaff, M. W. (2013). X-ray-computed tomography contrast agents. Chemical Reviews, 113(3), 1641–1666
Hong, C. M., & Ahn, B. C. (2017). Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer for Reapplication of I-131 Therapy. Front Endocrinol (Lausanne), 8, 260
Kim, S. H., Kim, E. M., Lee, C. M., Kim, D. W., Lim, S. T., Sohn, M. H., & Jeong, H. J. (2012). Synthesis of PEG-Iodine-Capped Gold Nanoparticles and Their Contrast Enhancement in < i > In Vitro and < i > In Vivo for X-Ray/CT. Journal of Nanomaterials, 2012, 504026
Liu, Y., Ashton, J. R., Moding, E. J., Yuan, H., Register, J. K., Fales, A. M., Choi, J., Whitley, M. J., Zhao, X., Qi, Y., et al. (2015). A Plasmonic Gold Nanostar Theranostic Probe for In Vivo Tumor Imaging and Photothermal Therapy. Theranostics, 5(9), 946–960
Kim, Y. H., Jeon, J., Hong, S. H., Rhim, W. K., Lee, Y. S., Youn, H., Chung, J. K., Lee, M. C., Lee, D. S., Kang, K. W., et al. (2011). Tumor targeting and imaging using cyclic RGD-PEGylated gold nanoparticle probes with directly conjugated iodine-125. Small (Weinheim An Der Bergstrasse, Germany), 7(14), 2052–2060
Chen, M., Guo, Z., Chen, Q., Wei, J., Li, J., Shi, C. … Zheng, N. (2018). Pd nanosheets with their surface coordinated by radioactive iodide as a high-performance theranostic nanoagent for orthotopic hepatocellular carcinoma imaging and cancer therapy. Chemical Science, 9(18), 4268–4274
Walsh, A. A. (2017). Chemisorption of iodine-125 to gold nanoparticles allows for real-time quantitation and potential use in nanomedicine. Journal Of Nanoparticle Research, 19(4), 152
Shaffer, T. M., Wall, M. A., Harmsen, S., Longo, V. A., Drain, C. M., Kircher, M. F., & Grimm, J. (2015). Silica nanoparticles as substrates for chelator-free labeling of oxophilic radioisotopes. Nano Letters, 15(2), 864–868
Chen, F., Ma, K., Zhang, L., Madajewski, B., Zanzonico, P., Sequeira, S. … Bradbury, M. S. (2017). Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for Enhanced Cancer-Directed Uptake in Melanoma: A Comparison of Radiolabeling Strategies. Chemistry Of Materials, 29(19), 8269–8281
Chen, F., Goel, S., Valdovinos, H. F., Luo, H., Hernandez, R., Barnhart, T. E., & Cai, W. (2015). In Vivo Integrity and Biological Fate of Chelator-Free Zirconium-89-Labeled Mesoporous Silica Nanoparticles. Acs Nano, 9(8), 7950–7959
Ognjanovic, M., Radovic, M., Mirkovic, M., Prijovic, Z., Puerto Morales, M. D., Ceh, M. … Antic, B. (2019). (99m)Tc-, (90)Y-, and (177)Lu-Labeled Iron Oxide Nanoflowers Designed for Potential Use in Dual Magnetic Hyperthermia/Radionuclide Cancer Therapy and Diagnosis. Acs Applied Materials & Interfaces, 11(44), 41109–41117
Piotrowska, A., Męczyńska-Wielgosz, S., Majkowska-Pilip, A., Koźmiński, P., Wójciuk, G., Cędrowska, E. … Bilewicz, A. (2017). Nanozeolite bioconjugates labeled with (223)Ra for targeted alpha therapy. Nuclear Medicine And Biology, 47, 10–18
Czerwińska, M., Fracasso, G., Pruszyński, M., Bilewicz, A., Kruszewski, M., Majkowska-Pilip, A., & Lankoff, A. (2020). : Design and Evaluation of (223)Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy-Part I. Materials (Basel) 13(17)
Zhou, M., Zhang, R., Huang, M., Lu, W., Song, S., Melancon, M. P. … Li, C. (2010). A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. Journal Of The American Chemical Society, 132(43), 15351–15358
Zhou, M., Chen, Y., Adachi, M., Wen, X., Erwin, B., Mawlawi, O. … Li, C. (2015). Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer. Biomaterials, 57, 41–49
Sun, X., Huang, X., Guo, J., Zhu, W., Ding, Y., Niu, G., Wang, A., Kiesewetter, D. O., Wang, Z. L., Sun, S., et al. (2014). Self-illuminating 64Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging. Journal Of The American Chemical Society, 136(5), 1706–1709
Guo, W., Sun, X., Jacobson, O., Yan, X., Min, K., Srivatsan, A. … Chen, X. (2015). Intrinsically radioactive [64Cu]CuInS/ZnS quantum dots for PET and optical imaging: improved radiochemical stability and controllable Cerenkov luminescence. Acs Nano, 9(1), 488–495
Guryev, E. L., Volodina, N. O., Shilyagina, N. Y., Gudkov, S. V., Balalaeva, I. V., Volovetskiy, A. B., Lyubeshkin, A. V., Sen, A. V., Ermilov, S. A., Vodeneev, V. A., et al. (2018). Radioactive ((90)Y) upconversion nanoparticles conjugated with recombinant targeted toxin for synergistic nanotheranostics of cancer. Proc Natl Acad Sci U S A, 115(39), 9690–9695
Satterlee, A. B., Yuan, H., & Huang, L. (2015). A radio-theranostic nanoparticle with high specific drug loading for cancer therapy and imaging. Journal Of Controlled Release : Official Journal Of The Controlled Release Society, 217, 170–182
Satterlee, A. B., Rojas, J. D., Dayton, P. A., & Huang, L. (2017). Enhancing Nanoparticle Accumulation and Retention in Desmoplastic Tumors via Vascular Disruption for Internal Radiation Therapy. Theranostics, 7(2), 253–269
Chanda, N., Kan, P., Watkinson, L. D., Shukla, R., Zambre, A., Carmack, T. L., Engelbrecht, H., Lever, J. R., Katti, K., Fent, G. M., et al. (2010). Radioactive gold nanoparticles in cancer therapy: therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. Nanomedicine: The Official Journal Of The American Academy Of Nanomedicine, 6(2), 201–209
Shukla, R., Chanda, N., Zambre, A., Upendran, A., Katti, K., Kulkarni, R. R., Nune, S. K., Casteel, S. W., Smith, C. J., Vimal, J., et al. (2012). Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc Natl Acad Sci U S A, 109(31), 12426–12431
Gawęda, W., Pruszyński, M., Cędrowska, E., Rodak, M., Majkowska-Pilip, A., Gaweł, D. … Bilewicz, A. (2020). : Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy. Nanomaterials (Basel) 10(10)
McLaughlin, M. F., Robertson, D., Pevsner, P. H., Wall, J. S., Mirzadeh, S., & Kennel, S. J. (2014). LnPO4 nanoparticles doped with Ac-225 and sequestered daughters for targeted alpha therapy. Cancer Biotherapy & Radiopharmaceuticals, 29(1), 34–41
Cędrowska, E., Pruszyński, M., Gawęda, W., Żuk, M., Krysiński, P., Bruchertseifer, F. … Bilewicz, A. (2020). : Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with (225)Ac as a Perspective Tool for Combined α-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules 25(5)
Semmler-Behnke, M., Lipka, J., Wenk, A., Hirn, S., Schaffler, M., Tian, F. … Kreyling, W. G. (2014). Size dependent translocation and fetal accumulation of gold nanoparticles from maternal blood in the rat. Particle And Fibre Toxicology, 11, 33
Di Pasqua, A. J., Yuan, H., Chung, Y., Kim, J. K., Huckle, J. E., Li, C. … Lu, X. (2013). Neutron-activatable holmium-containing mesoporous silica nanoparticles as a potential radionuclide therapeutic agent for ovarian cancer. Journal Of Nuclear Medicine, 54(1), 111–116
Petriev, V. M., Tischenko, V. K., Mikhailovskaya, A. A., Popov, A. A., Tselikov, G., Zelepukin, I., Deyev, S. M., Kaprin, A. D., Ivanov, S., Timoshenko, V. Y., et al. (2019). Nuclear nanomedicine using Si nanoparticles as safe and effective carriers of (188)Re radionuclide for cancer therapy. Scientific Reports, 9(1), 2017
Pai-Scherf, L. H., Carrasquillo, J. A., Paik, C., Gansow, O., Whatley, M., Pearson, D., Webber, K., Hamilton, M., Allegra, C., Brechbiel, M., et al. (2000). : Imaging and Phase I Study of < sup > 111</sup > In-and < sup > 90</sup > Y-labeled Anti-Lewis < sup > Y</sup > Monoclonal Antibody B3. Clinical Cancer Research 6(5):1720–1730
Johari Daha, F., Shafiei, S., Sheibani, S., Tavakoli, Y. H., Mazidi, M., Mirfalah, M. H., & Babaei, M. H. (2010). Production of 177Lu and formulation of Ethylene diamine tetramethylene phosphonate (EDTMP) kits as a bone-seeking radiopharmaceutical. International Journal of Radiation Research, 7(4), 229–234
Yook, S., Lu, Y., Jeong, J. J., Cai, Z., Tong, L., Alwarda, R. … Reilly, R. M. (2016). Stability and Biodistribution of Thiol-Functionalized and (177)Lu-Labeled Metal Chelating Polymers Bound to Gold Nanoparticles. Biomacromolecules, 17(4), 1292–1302
Subramanian, S., Pandey, U., Gugulothu, D., Patravale, V., & Samuel, G. (2013). Modification of PLGA nanoparticles for improved properties as a 99mTc-labeled agent in sentinel lymph node detection. Cancer Biotherapy & Radiopharmaceuticals, 28(8), 598–606
89Zr-Nanocolloidal Albumin–Based PET/CT Lymphoscintigraphy for Sentinel Node Detection in Head and Neck Cancer: Preclinical Results. Journal of Nuclear Medicine 2011, 52(10):1580
Harrington, K. J., Rowlinson-Busza, G., Syrigos, K. N., Uster, P. S., Abra, R. M., & Stewart, J. S. W. (2000). Biodistribution and pharmacokinetics of 111In-DTPA-labelled pegylated liposomes in a human tumour xenograft model: implications for novel targeting strategies. British Journal of Cancer, 83(2), 232–238
Harrington, K. J., Mohammadtaghi, S., Uster, P. S., Glass, D., Peters, A. M., Vile, R. G., & Stewart, J. S. (2001). Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clinical Cancer Research, 7(2), 243–254
Hajiramezanali, M., Atyabi, F., Mosayebnia, M., Akhlaghi, M., Geramifar, P., Jalilian, A. R. … Beiki, D. (2019). (68)Ga-radiolabeled bombesin-conjugated to trimethyl chitosan-coated superparamagnetic nanoparticles for molecular imaging: preparation, characterization and biological evaluation. Int J Nanomedicine, 14, 2591–2605
Lee, D. E., Na, J. H., Lee, S., Kang, C. M., Kim, H. N., Han, S. J., Kim, H., Choe, Y. S., Jung, K. H., Lee, K. C., et al. (2013). Facile method to radiolabel glycol chitosan nanoparticles with (64)Cu via copper-free click chemistry for MicroPET imaging. Molecular Pharmaceutics, 10(6), 2190–2198
Lee, S., Kang, S. W., Ryu, J. H., Na, J. H., Lee, D. E., Han, S. J., Kang, C. M., Choe, Y. S., Lee, K. C., Leary, J. F., et al. (2014). Tumor-Homing Glycol Chitosan-Based Optical/PET Dual Imaging Nanoprobe for Cancer Diagnosis. Bioconjugate Chemistry, 25(3), 601–610
Akhlaghi, M., & Pourjavadi, A. (2011). Preparation and primary evaluation of 66Ga-DTPA-chitosan in fibrosarcoma bearing mice. Nukleonika, 56(1), 41–47
Mollarazi, E., Jalilian, A. R., Johari-Daha, F., & Atyabi, F. (2015). Development of (153) Sm-folate-polyethyleneimine-conjugated chitosan nanoparticles for targeted therapy. J Labelled Comp Radiopharm, 58(8), 327–335
Bao, A., Goins, B., Klipper, R., Negrete, G., & Phillips, W. T. (2003). 186Re-liposome labeling using 186Re-SNS/S complexes: in vitro stability, imaging, and biodistribution in rats. Journal Of Nuclear Medicine, 44(12), 1992–1999
Lin, L. T., Chang, C. H., Yu, H. L., Liu, R. S., Wang, H. E., Chiu, S. J. … Lee, Y. J. (2014). Evaluation of the therapeutic and diagnostic effects of PEGylated liposome-embedded 188Re on human non-small cell lung cancer using an orthotopic small-animal model. Journal Of Nuclear Medicine, 55(11), 1864–1870
Sofou, S., Kappel, B. J., Jaggi, J. S., McDevitt, M. R., Scheinberg, D. A., & Sgouros, G. (2007). Enhanced retention of the alpha-particle-emitting daughters of Actinium-225 by liposome carriers. Bioconjugate Chemistry, 18(6), 2061–2067
Anti–Prostate-Specific Membrane Antigen Liposomes Loaded with 225Ac for Potential Targeted Antivascular α-Particle Therapy of Cancer. Journal of Nuclear Medicine 2014, 55(1):107
Arora, G., Shukla, J., Ghosh, S., Maulik, S. K., Malhotra, A., & Bandopadhyaya, G. (2012). PLGA Nanoparticles for Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors: A Novel Approach towards Reduction of Renal Radiation Dose. PLOS ONE, 7(3), e34019
Gibbens-Bandala, B., Morales-Avila, E., Ferro-Flores, G., Santos-Cuevas, C., Meléndez-Alafort, L., Trujillo-Nolasco, M., & Ocampo-García, B. (2019). 177Lu-Bombesin-PLGA (paclitaxel): A targeted controlled-release nanomedicine for bimodal therapy of breast cancer. Materials Science and Engineering: C, 105, 110043
Soares, D. C., de Oliveira, M. C., dos Santos, R. G., Andrade, M. S., Vilela, J. M., Cardoso, V. N., & Ramaldes, G. A. (2011). Liposomes radiolabeled with 159Gd-DTPA-BMA: preparation, physicochemical characterization, release profile and in vitro cytotoxic evaluation. European Journal Of Pharmaceutical Sciences, 42(5), 462–469
Marik, J., Tartis, M. S., Zhang, H., Fung, J. Y., Kheirolomoom, A., Sutcliffe, J. L., & Ferrara, K. W. (2007). : Long-circulating liposomes radiolabeled with [18F]fluorodipalmitin ([18F]FDP). Nucl Med Biol 34(2):165–171
Sirianni, R. W., Zheng, M. Q., Patel, T. R., Shafbauer, T., Zhou, J., Saltzman, W. M. … Huang, Y. (2014). Radiolabeling of Poly(lactic-co-glycolic acid) (PLGA) Nanoparticles with Biotinylated F-18 Prosthetic Groups and Imaging of Their Delivery to the Brain with Positron Emission Tomography. Bioconjugate Chemistry, 25(12), 2157–2165
Liao, A. H., Wu, S. Y., Wang, H. E., Weng, C. H., Wu, M. F., & Li, P. C. (2013). Evaluation of 18F-labeled targeted perfluorocarbon-filled albumin microbubbles as a probe for microUS and microPET in tumor-bearing mice. Ultrasonics, 53(2), 320–327
Tian, L., Chen, Q., Yi, X., Wang, G., Chen, J., Ning, P. … Liu, Z. (2017). Radionuclide I-131 Labeled Albumin-Paclitaxel Nanoparticles for Synergistic Combined Chemo-radioisotope Therapy of Cancer. Theranostics, 7(3), 614–623
Liu, K., Zheng, D., Zhao, J., Tao, Y., Wang, Y., He, J. … Xi, X. (2018). pH-Sensitive nanogels based on the electrostatic self-assembly of radionuclide 131I labeled albumin and carboxymethyl cellulose for synergistic combined chemo-radioisotope therapy of cancer. Journal of Materials Chemistry B, 6(29), 4738–4746
Wang, H., & Sheng, W. (2017). (131)I-Traced PLGA-Lipid Nanoparticles as Drug Delivery Carriers for the Targeted Chemotherapeutic Treatment of Melanoma. Nanoscale Research Letters, 12(1), 365
Liang, C., Chao, Y., Yi, X., Xu, J., Feng, L., Zhao, Q. … Liu, Z. (2019). Nanoparticle-mediated internal radioisotope therapy to locally increase the tumor vasculature permeability for synergistically improved cancer therapies. Biomaterials, 197, 368–379
Hansen, A. E., Fliedner, F. P., Henriksen, J. R., Jørgensen, J. T., Clemmensen, A. E., Børresen, B. … Andresen, T. L. (2018). Liposome accumulation in irradiated tumors display important tumor and dose dependent differences. Nanomedicine: Nanotechnology Biology and Medicine, 14(1), 27–34
Akman, L., Biber Muftuler, F. Z., Bilgi, A., Yurt Kilcar, A., Gokulu, S. G., Medine, E. I., & Terek, M. C. (2016). Synthesis of a theranostic agent: radioiodinated PEGylated PLGA-indocyanine capsules and in vitro determination of their bioaffinity on ovarian, cervical and breast cancer cells. Journal of Radioanalytical and Nuclear Chemistry, 308(2), 659–670
Yildiz, G., Yurt Kilcar, A., Medine, E. I., Tekin, V., Kozgus Guldu, O., & Biber Muftuler, F. Z. (2017). PLGA encapsulation and radioiodination of indole-3-carbinol: investigation of anticancerogenic effects against MCF7, Caco2 and PC3 cells by in vitro assays. Journal of Radioanalytical and Nuclear Chemistry, 311(2), 1043–1052
Engudar, G., Schaarup-Jensen, H., Fliedner, F. P., Hansen, A. E., Kempen, P., Jølck, R. I., Kjæer, A., Andresen, T. L., Clausen, M. H., Jensen, A. I., et al. (2018). Remote loading of liposomes with a (124)I-radioiodinated compound and their in vivo evaluation by PET/CT in a murine tumor model. Theranostics, 8(21), 5828–5841
Mondal, N., Halder, K. K., Kamila, M. M., Debnath, M. C., Pal, T. K., Ghosal, S. K. … Ganguly, S. (2010). Preparation, characterization, and biodistribution of letrozole loaded PLGA nanoparticles in Ehrlich Ascites tumor bearing mice. International Journal Of Pharmaceutics, 397(1–2), 194–200
Ekinci, M., Ilem-Ozdemir, D., Gundogdu, E., & Asikoglu, M. (2015). Methotrexate loaded chitosan nanoparticles: Preparation, radiolabeling and in vitro evaluation for breast cancer diagnosis. Journal of Drug Delivery Science and Technology, 30, 107–113
Hawary, D. L., Motaleb, M. A., Farag, H., Guirguis, O. W., & Elsabee, M. Z. (2011). Water-soluble derivatives of chitosan as a target delivery system of 99mTc to some organs in vivo for nuclear imaging and biodistribution. Journal of Radioanalytical and Nuclear Chemistry, 290(3), 557–567
Polyák, A., Hajdu, I., Bodnár, M., Trencsényi, G., Pöstényi, Z., Haász, V. … Borbély, J. (2013). (99m)Tc-labelled nanosystem as tumour imaging agent for SPECT and SPECT/CT modalities. International Journal Of Pharmaceutics, 449(1–2), 10–17
Karpuz, M., Silindir-Gunay, M., Kursunel, M. A., Esendagli, G., Dogan, A., & Ozer, A. Y. (2020). Design and in vitro evaluation of folate-targeted, co-drug encapsulated theranostic liposomes for non-small cell lung cancer. Journal of Drug Delivery Science and Technology, 57, 101707
Therapeutic Efficacy Evaluation of 111In-Labeled PEGylated Liposomal Vinorelbine in Murine Colon Carcinoma with Multimodalities of Molecular Imaging. Journal of Nuclear Medicine 2009, 50(12):2073
Liu, S. Y., Lo, S. N., Lee, W. C., Hsu, W. C., Lee, T. W., & Chang, C. H. (2021). : Evaluation of Nanotargeted (111)In-Cyclic RGDfK-Liposome in a Human Melanoma Xenotransplantation Model. Int J Mol Sci 22(3)
Marques, F., Gano, L., Batista, M. K. S., Gomes, C. A. R., Gomes, P., & Santos, I. (2009). Radiochemical and biological evaluation of novel 153Sm/166Ho-amino acid–chitosan complexes. Journal of Labelled Compounds and Radiopharmaceuticals, 52(3), 79–83
Lohar, S., Jadhav, S., Chakravarty, R., Chakraborty, S., Sarma, H. D., & Dash, A. (2020). A kit based methodology for convenient formulation of (166)Ho-Chitosan complex for treatment of liver cancer. Applied Radiation And Isotopes, 161, 109161
Hamoudeh, M., Kamleh, M. A., Diab, R., & Fessi, H. (2008). Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer. Advanced Drug Delivery Reviews, 60(12), 1329–1346
Zhang, L., Chen, H., Wang, L., Liu, T., Yeh, J., Lu, G. … Mao, H. (2010). Delivery of therapeutic radioisotopes using nanoparticle platforms: potential benefit in systemic radiation therapy. Nanotechnol Sci Appl, 3, 159–170
Naahidi, S., Jafari, M., Edalat, F., Raymond, K., Khademhosseini, A., & Chen, P. (2013). Biocompatibility of engineered nanoparticles for drug delivery. Journal Of Controlled Release : Official Journal Of The Controlled Release Society, 166(2), 182–194
Dobrovolskaia, M. A., Aggarwal, P., Hall, J. B., & McNeil, S. E. (2008). Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Molecular Pharmaceutics, 5(4), 487–495
Jeon, J. (2019). : Review of Therapeutic Applications of Radiolabeled Functional Nanomaterials. Int J Mol Sci 20(9)
Leroi, N., Lallemand, F., Coucke, P., Noel, A., & Martinive, P. (2016). : Impacts of Ionizing Radiation on the Different Compartments of the Tumor Microenvironment. Frontiers in Pharmacology 7(78)
Balasubramanian, S. K., Jittiwat, J., Manikandan, J., Ong, C. N., Yu, L. E., & Ong, W. Y. (2010). Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials, 31(8), 2034–2042
Khan, H. A., Abdelhalim, M. A. K., Alhomida, A. S., & Al-Ayed, M. S. (2013). Effects of Naked Gold Nanoparticles on Proinflammatory Cytokines mRNA Expression in Rat Liver and Kidney. BioMed Research International, 2013, 590730
Chen, H., Dorrigan, A., Saad, S., Hare, D. J., Cortie, M. B., & Valenzuela, S. M. (2013). In vivo study of spherical gold nanoparticles: inflammatory effects and distribution in mice. PLoS One, 8(2), e58208
Grant, R. W., & Stephens, J. M. (2015). Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. American Journal Of Physiology. Endocrinology And Metabolism, 309(3), E205–213
Kosteli, A., Sugaru, E., Haemmerle, G., Martin, J. F., Lei, J., Zechner, R., & Ferrante, A. W. Jr. (2010). Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of Clinical Investigation, 120(10), 3466–3479
Schäffler, A., & Schölmerich, J. (2010). Innate immunity and adipose tissue biology. Trends In Immunology, 31(6), 228–235
Almeida, J. P., Lin, A. Y., Langsner, R. J., Eckels, P., Foster, A. E., & Drezek, R. A. (2014). In vivo immune cell distribution of gold nanoparticles in naïve and tumor bearing mice. Small (Weinheim An Der Bergstrasse, Germany), 10(4), 812–819
Tomić, S., Ðokić, J., Vasilijić, S., Ogrinc, N., Rudolf, R., Pelicon, P., Vučević, D., Milosavljević, P., Janković, S., Anžel, I., et al. (2014). Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS One, 9(5), e96584
Laskar, A., Ghosh, M., Khattak, S. I., Li, W., & Yuan, X. M. (2012). Degradation of superparamagnetic iron oxide nanoparticle-induced ferritin by lysosomal cathepsins and related immune response. Nanomedicine (Lond), 7(5), 705–717
Rojas, J. M., Sanz-Ortega, L., Mulens-Arias, V., Gutiérrez, L., Pérez-Yagüe, S., & Barber, D. F. (2016). Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomedicine: The Official Journal Of The American Academy Of Nanomedicine, 12(4), 1127–1138
Park, E. J., Kim, H., Kim, Y., Yi, J., Choi, K., & Park, K. (2010). Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology, 275(1–3), 65–71
Ban, M., Langonné, I., Huguet, N., Guichard, Y., & Goutet, M. (2013). Iron oxide particles modulate the ovalbumin-induced Th2 immune response in mice. Toxicology Letters, 216(1), 31–39
Park, E. J., Oh, S. Y., Lee, S. J., Lee, K., Kim, Y., Lee, B. S., & Kim, J. S. (2015). Chronic pulmonary accumulation of iron oxide nanoparticles induced Th1-type immune response stimulating the function of antigen-presenting cells. Environmental Research, 143, 138–147
Shah, A., & Dobrovolskaia, M. A. (2018). Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomedicine: nanotechnology biology and medicine, 14(3), 977–990
Gaharwar, U. S., Kumar, S., & Rajamani, P. (2020). Iron oxide nanoparticle-induced hematopoietic and immunological response in rats. RSC Advances, 10(59), 35753–35764
Parks, C. G., Conrad, K., & Cooper, G. S. (1999). Occupational exposure to crystalline silica and autoimmune disease. Environmental Health Perspectives, 107(Suppl 5(Suppl 5), 793–802
Barbarin, V., Xing, Z., Delos, M., Lison, D., & Huaux, F. (2005). Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. American Journal Of Physiology. Lung Cellular And Molecular Physiology, 288(5), L841–848
Oh, W. K., Kim, S., Choi, M., Kim, C., Jeong, Y. S., Cho, B. R. … Jang, J. (2010). Cellular Uptake, Cytotoxicity, and Innate Immune Response of Silica – Titania Hollow Nanoparticles Based on Size and Surface Functionality. Acs Nano, 4(9), 5301–5313
Lee, S., Kim, M. S., Lee, D., Kwon, T. K., Khang, D., Yun, H. S., & Kim, S. H. (2013). The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int J Nanomedicine, 8, 147–158
Heidegger, S., Gössl, D., Schmidt, A., Niedermayer, S., Argyo, C., Endres, S. … Bourquin, C. (2016). Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale, 8(2), 938–948
Hoppstädter, J., Seif, M., Dembek, A., Cavelius, C., Huwer, H., Kraegeloh, A., & Kiemer, A. K. (2015). : M2 polarization enhances silica nanoparticle uptake by macrophages. Frontiers in Pharmacology 6(55)
Gómez, D. M., Urcuqui-Inchima, S., & Hernandez, J. C. (2017). Silica nanoparticles induce NLRP3 inflammasome activation in human primary immune cells. Innate Immun, 23(8), 697–708
Abbaraju, P. L., Jambhrunkar, M., Yang, Y., Liu, Y., Lu, Y., & Yu, C. (2018). Asymmetric mesoporous silica nanoparticles as potent and safe immunoadjuvants provoke high immune responses. Chemical Communications, 54(16), 2020–2023
Kim, J., Li, W. A., Choi, Y., Lewin, S. A., Verbeke, C. S., Dranoff, G., & Mooney, D. J. (2015). Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nature Biotechnology, 33(1), 64–72
Nguyen, T. L., Choi, Y., & Kim, J. (2019). Mesoporous Silica as a Versatile Platform for Cancer Immunotherapy. Advanced Materials, 31(34), 1803953
Anselmo, A. C., & Mitragotri, S. (2019). Nanoparticles in the clinic: An update. Bioeng Transl Med, 4(3), e10143–e10143
van Rooijen, N., & van Nieuwmegen, R. (1977). Liposomes in immunology: the immune response against antigen-containing liposomes. Immunol Commun, 6(5), 489–498
Nakanishi, T., Kunisawa, J., Hayashi, A., Tsutsumi, Y., Kubo, K., Nakagawa, S. … Mayumi, T. (1997). Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochemical And Biophysical Research Communications, 240(3), 793–797
Yan, W., Chen, W., & Huang, L. (2007). Mechanism of adjuvant activity of cationic liposome: Phosphorylation of a MAP kinase, ERK and induction of chemokines. Molecular Immunology, 44(15), 3672–3681
Zhuang, Y., Ma, Y., Wang, C., Hai, L., Yan, C., Zhang, Y. … Cai, L. (2012). PEGylated cationic liposomes robustly augment vaccine-induced immune responses: Role of lymphatic trafficking and biodistribution. Journal of Controlled Release, 159(1), 135–142
Kaur, R., Bramwell, V. W., Kirby, D. J., & Perrie, Y. (2012). Manipulation of the surface pegylation in combination with reduced vesicle size of cationic liposomal adjuvants modifies their clearance kinetics from the injection site, and the rate and type of T cell response. Journal of Controlled Release, 164(3), 331–337
Shen, K. Y., Liu, H. Y., Li, H. J., Wu, C. C., Liou, G. G., Chang, Y. C. … Liu, S. J. (2016). A novel liposomal recombinant lipoimmunogen enhances anti-tumor immunity. Journal of Controlled Release, 233, 57–63
Jiang, P. L., Lin, H. J., Wang, H. W., Tsai, W. Y., Lin, S. F., Chien, M. Y. … Liu, D. Z. (2015). Galactosylated liposome as a dendritic cell-targeted mucosal vaccine for inducing protective anti-tumor immunity. Acta Biomaterialia, 11, 356–367
Yoshizaki, Y., Yuba, E., Sakaguchi, N., Koiwai, K., Harada, A., & Kono, K. (2017). pH-sensitive polymer-modified liposome-based immunity-inducing system: Effects of inclusion of cationic lipid and CpG-DNA. Biomaterials, 141, 272–283
Landesman-Milo, D., & Peer, D. (2012). Altering the immune response with lipid-based nanoparticles. Journal of Controlled Release, 161(2), 600–608
Zahednezhad, F., Saadat, M., Valizadeh, H., Zakeri-Milani, P., & Baradaran, B. (2019). Liposome and immune system interplay: Challenges and potentials. Journal of Controlled Release, 305, 194–209
Sabnani, M. K., Rajan, R., Rowland, B., Mavinkurve, V., Wood, L. M., Gabizon, A. A., & La-Beck, N. M. (2015). Liposome promotion of tumor growth is associated with angiogenesis and inhibition of antitumor immune responses. Nanomedicine: Nanotechnology Biology and Medicine, 11(2), 259–262
Rajan, R., Sabnani, M. K., Mavinkurve, V., Shmeeda, H., Mansouri, H., Bonkoungou, S. … La-Beck, N. M. (2018). Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate. Journal of Controlled Release, 271, 139–148
La-Beck, N. M., Liu, X., & Wood, L. M. (2019). : Harnessing Liposome Interactions With the Immune System for the Next Breakthrough in Cancer Drug Delivery. Frontiers in Pharmacology 10(220)
Zaharoff, D. A., Rogers, C. J., Hance, K. W., Schlom, J., & Greiner, J. W. (2007). Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine, 25(11), 2085–2094
Shi, G. N., Zhang, C. N., Xu, R., Niu, J. F., Song, H. J., Zhang, X. Y., Wang, W. W., Wang, Y. M., Li, C., Wei, X. Q., et al. (2017). Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials, 113, 191–202
Pei, M., Liang, J., Zhang, C., Wang, X., Zhang, C., Ma, G., & Sun, H. (2019). Chitosan/calcium phosphates nanosheet as a vaccine carrier for effective cross-presentation of exogenous antigens. Carbohydrate Polymers, 224, 115172
Castro, F., Pinto, M. L., Silva, A. M., Pereira, C. L., Teixeira, G. Q., Gomez-Lazaro, M. … Oliveira, M. J. (2017). Pro-inflammatory chitosan/poly(γ-glutamic acid) nanoparticles modulate human antigen-presenting cells phenotype and revert their pro-invasive capacity. Acta Biomaterialia, 63, 96–109
Castro, F., Pinto, M. L., Pereira, C. L., Serre, K., Barbosa, M. A., Vermaelen, K. … Oliveira, M. J. (2020). Chitosan/γ-PGA nanoparticles-based immunotherapy as adjuvant to radiotherapy in breast cancer. Biomaterials, 257, 120218
Li, X., Dong, W., Nalin, A. P., Wang, Y., Pan, P., Xu, B., Zhang, Y., Tun, S., Zhang, J., Wang, L. S., et al. (2018). The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. OncoImmunology, 7(6), e1431085
Gleeson, M. W., & Dickson, R. C. (2015). Albumin gains immune boosting credibility. Clin Transl Gastroenterol, 6(4), e86–e86
Garcia-Martinez, R., Andreola, F., Mehta, G., Poulton, K., Oria, M., Jover, M., Soeda, J., Macnaughtan, J., De Chiara, F., Habtesion, A., et al. (2015). Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure. Journal Of Hepatology, 62(4), 799–806
China, L., Maini, A., Skene, S. S., Shabir, Z., Sylvestre, Y., Colas, R. A., Ly, L., Becares Salles, N., Belloti, V., Dalli, J., et al. (2018). Albumin Counteracts Immune-Suppressive Effects of Lipid Mediators in Patients With Advanced Liver Disease. Clinical Gastroenterology and Hepatology, 16(5), 738–747e737
Sleep, D. (2015). Albumin and its application in drug delivery. Expert Opinion On Drug Delivery, 12(5), 793–812
Hoogenboezem, E. N., & Duvall, C. L. (2018). Harnessing albumin as a carrier for cancer therapies. Advanced Drug Delivery Reviews, 130, 73–89
Lamichhane, S., & Lee, S. (2020). Albumin nanoscience: homing nanotechnology enabling targeted drug delivery and therapy. Archives of Pharmacal Research, 43(1), 118–133
Zhu, G., Lynn, G. M., Jacobson, O., Chen, K., Liu, Y., Zhang, H., Ma, Y., Zhang, F., Tian, R., Ni, Q., et al. (2017). Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nature Communications, 8(1), 1954
Ai, S. L., He, X. Y., Liu, B. Y., Zhuo, R. X., & Cheng, S. X. (2019). Targeting Delivery of Oligodeoxynucleotides to Macrophages by Mannosylated Cationic Albumin for Immune Stimulation in Cancer Treatment. Molecular Pharmaceutics, 16(6), 2616–2625
Hamdy, S., Haddadi, A., Shayeganpour, A., Samuel, J., & Lavasanifar, A. (2011). Activation of antigen-specific T cell-responses by mannan-decorated PLGA nanoparticles. Pharmaceutical Research, 28(9), 2288–2301
Oyewumi, M. O., Kumar, A., & Cui, Z. (2010). Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Review Of Vaccines, 9(9), 1095–1107
Silva, A. L., Soema, P. C., Slütter, B., Ossendorp, F., & Jiskoot, W. (2016). PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Human Vaccines & Immunotherapeutics, 12(4), 1056–1069
Zhou, H., Ivanov, V. N., Gillespie, J., Geard, C. R., Amundson, S. A., Brenner, D. J. … Hei, T. K. (2005). : Mechanism of radiation-induced bystander effect: Role of the cyclooxygenase-2 signaling pathway. Proceedings of the National Academy of Sciences of the United States of America 102(41):14641
Najafi, M., Fardid, R., Hadadi, G., & Fardid, M. (2014). The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng, 4(4), 163–172
Yahyapour, R., Salajegheh, A., Safari, A., Amini, P., Rezaeyan, A., Amraee, A., & Najafi, M. (2018). Radiation-induced Non-targeted Effect and Carcinogenesis; Implications in Clinical Radiotherapy. J Biomed Phys Eng, 8(4), 435–446
Gandhi, S., & Chandna, S. (2017). Radiation-induced inflammatory cascade and its reverberating crosstalks as potential cause of post-radiotherapy second malignancies. Cancer and Metastasis Reviews, 36(2), 375–393
Barker, H. E., Paget, J. T. E., Khan, A. A., & Harrington, K. J. (2015). The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nature Reviews Cancer, 15(7), 409–425
Chabanon, R. M., Pedrero, M., Lefebvre, C., Marabelle, A., Soria, J. C., & Postel-Vinay, S. (2016). Mutational Landscape and Sensitivity to Immune Checkpoint Blockers. Clinical Cancer Research, 22(17), 4309
Duan, Q., Zhang, H., Zheng, J., & Zhang, L. (2020). Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends in Cancer, 6(7), 605–618
Demaria, S., Coleman, C. N., & Formenti, S. C. (2016). Radiotherapy: Changing the Game in Immunotherapy. Trends in cancer, 2(6), 286–294
Mole, R. H. (1953). Whole Body Irradiation—Radiobiology or Medicine? The British Journal of Radiology, 26(305), 234–241
Hagemann, T., Balkwill, F., & Lawrence, T. (2007). Inflammation and Cancer: A Double-Edged Sword. Cancer Cell, 12(4), 300–301
Kroemer, G., Galluzzi, L., Kepp, O., & Zitvogel, L. (2013). Immunogenic cell death in cancer therapy. Annual Review Of Immunology, 31, 51–72
Reits, E. A., Hodge, J. W., Herberts, C. A., Groothuis, T. A., Chakraborty, M., Wansley, E. K., Camphausen, K., Luiten, R. M., de Ru, A. H., Neijssen, J., et al. (2006). Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. Journal Of Experimental Medicine, 203(5), 1259–1271
Chakraborty, M., Abrams, S. I., Camphausen, K., Liu, K., Scott, T., Coleman, C. N., & Hodge, J. W. (2003). Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. The Journal Of Immunology, 170(12), 6338–6347
Jarosz-Biej, M., Smolarczyk, R., Cichoń, T., & Kułach, N. (2019). : Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. International Journal of Molecular Sciences 20(13)
Weichselbaum, R. R., Liang, H., Deng, L., & Fu, Y. X. (2017). Radiotherapy and immunotherapy: a beneficial liaison? Nature Reviews. Clinical Oncology, 14(6), 365–379
Zhao, X., & Shao, C. (2020). : Radiotherapy-Mediated Immunomodulation and Anti-Tumor Abscopal Effect Combining Immune Checkpoint Blockade. Cancers 12(10)
Ngwa, W., Irabor, O. C., Schoenfeld, J. D., Hesser, J., Demaria, S., & Formenti, S. C. (2018). Using immunotherapy to boost the abscopal effect. Nature Reviews Cancer, 18(5), 313–322
Azad, A., Yin Lim, S., D’Costa, Z., Jones, K., Diana, A., Sansom, O. J., Kruger, P., Liu, S., McKenna, W. G., Dushek, O., et al. (2017). PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. Embo Molecular Medicine, 9(2), 167–180
Pfannenstiel, L. W., McNeilly, C., Xiang, C., Kang, K., Diaz-Montero, C. M., Yu, J. S., & Gastman, B. R. (2019). Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. OncoImmunology, 8(1), e1507669
Helm, A., Tinganelli, W., Simoniello, P., Kurosawa, F., Fournier, C., Shimokawa, T., & Durante, M. : Reduction of Lung Metastases in a Mouse Osteosarcoma Model Treated With Carbon Ions and Immune Checkpoint Inhibitors. International Journal of Radiation Oncology, Biology, Physics
Chen, D., Yang, D., Dougherty, C. A., Lu, W., Wu, H., He, X. … Hong, H. (2017). In Vivo Targeting and Positron Emission Tomography Imaging of Tumor with Intrinsically Radioactive Metal-Organic Frameworks Nanomaterials. Acs Nano, 11, 4315–4327
Zhang, J., Niu, G., Lang, L., Li, F., Fan, X., Yan, X., Yao, S., Yan, W., Huo, L., Chen, L., et al. (2017). Clinical Translation of a Dual Integrin αvβ3-and Gastrin-Releasing Peptide Receptor-Targeting PET Radiotracer, 68Ga-BBN-RGD. Journal Of Nuclear Medicine, 58, 228–234
Liu, Z., Huang, J., Dong, C., Cui, L., Jin, X., Jia, B. … Wang, F. (2012). 99mTc-Labeled RGD-BBN Peptide for Small-Animal SPECT/CT of Lung Carcinoma. Mol Pharmaceutics, 9, 1409–1417
He, S., Song, J., Qu, J., & Cheng, Z. (2018). Crucial Breakthrough of Second Near-Infrared Biological Window Fluorophores: Design and Synthesis Toward Multimodal Imaging and Theranostics. Chemical Society Reviews, 47, 4258–4278
Liu, S., Ou, H., Li, Y., Zhang, H., Liu, J., Lu, X. … Tang, B. Z. (2020). Planar and Twisted Molecular Structure Leads to the High Brightness of Semiconducting Polymer Nanoparticles for NIR-IIa Fluorescence Imaging. Journal Of The American Chemical Society, 142, 15146–15156
Zhong, Y., Ma, Z., Wang, F., Wang, X., Yang, Y., Liu, Y., Zhao, X., Li, J., Du, H., Zhang, M., et al. (2019). Vivo Molecular Imaging for Immunotherapy using Ultra-Bright Near-Infrared-IIb Rare-Earth Nanoparticles. Nature Biotechnology, 37, 1322–1331
Wan, H., Du, H., & Dai, F. W. (2019). Molecular Imaging in the Second Near-Infrared Window. Advanced Functional Materials, 29, 1900566
Yin, C., Lu, X., Fan, Q., & Huang, W. (2021). Organic Semiconducting Nanomaterials-Assisted Phototheranostics in Near-Infrared-II Biological Window. VIEW, 2, 20200070
Zhou, H., Zeng, X., Li, A., Zhou, W., Tang, L., Hu, W., Fan, Q., Meng, X., Deng, H., Duan, L., et al. (2020). Upconversion NIR-II Fluorophores for Mitochondria-Targeted Cancer Imaging and Photothermal Therapy. Nature Communications, 11, 6183
Cai, Y., Wei, Z., Song, C., Tang, C., Han, W., & Dong, X. (2019). Optical Nano-Agents in the Second Near-Infrared Window for Biomedical Applications. Chemical Society Reviews, 48, 22–37
Li, Y., Cai, Z., Liu, S., Zhang, H., Wong, S. T. H., Lam, J. W. Y. … Tang, B. Z. (2020). Design of AIEgens for Near-infrared IIb Imaging through Structural Modulation at Molecular and Morphological Levels. Nature Communications, 11, 1255
Li, D., Liu, Q., Qi, Q., Shi, H., Hsu, E. C., Chen, W., Yuan, W., Wu, Y., Lin, S., Zeng, Y., et al. (2020). Gold Nanoclusters for NIR-II Fluorescence Imaging of Bones. Small (Weinheim An Der Bergstrasse, Germany), 16, 2003851
Hu, Z., Fang, C., Li, B., Zhang, Z., Cao, C., Cai, M., Su, S., Sun, X., Shi, X., Li, C., et al. (2020). First-in-Human Liver-Tumour Surgery Guided by Multispectral Fluorescence Imaging in the Visible and Near-Infrared-I/II Windows. Nat Biomed Eng, 4, 259–271
Li, C., Chen, G., Zhang, Y., Wu, F., & Wang, Q. (2020). Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. Journal Of The American Chemical Society, 142, 14789–14804
Zhang, Q., Yu, P., Fan, Y., Sun, C., He, H., Liu, X. … Zhang, F. (2021). Bright and Stable NIR-II J‐Aggregated AIE Dibodipy‐Based Fluorescent Probe for Dynamic In Vivo Bioimaging. Angewandte Chemie Int Ed, 60, 3967–3973
Lei, Z., & Zhang, F. (2020). : Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection. Angew Chem Int Ed ASAP
Li, B., Zhao, M., & Zhang, F. (2020). Rational Design of Near-Infrared-II Organic Molecular Dyes for Bioimaging and Biosensing. ACS Materials Lett, 2, 905–917
Chen, D., Liu, Y., Zhang, Z., Liu, Z., Fang, X., He, S., & Wu, C. (2021). NIR-II Fluorescence Imaging Reveals Bone Marrow Retention of Small Polymer Nanoparticles. Nano Letters, 21, 798–805
Zhang, Z., Fang, X., Liu, Z., Liu, H., Chen, D., He, S., Zheng, J., Yang, B., Qin, W., Zhang, X., et al. (2020). Semiconducting Polymer Dots with Dual-Enhanced NIR‐IIa Fluorescence for Through-Skull Mouse‐Brain Imaging. Angewandte Chemie Int Ed, 59, 3691–3698
Liu, S., Li, Y., Kwok, R. T. K., Lam, J. W. Y., & Tang, B. Z. (2021). : Structural and Process Controls of AIEgens for NIR-II Theranostics. Chem Sci ASAP
Yang, R. Q., Lou, K. L., Wang, P. Y., Gao, Y. Y., Zhang, Y. Q., Chen, M. … Zhang, G. J. (2021). : Surgical Navigation for Malignancies Guided by Near-Infrared-II Fluorescence Imaging. Small Methods ASAP
Englman, R., & Jortner, J. (1970). The Energy Gap Law for Radiationless Transitions in Large Molecules. Molecular Physics, 18, 145–164
Chen, W. C., Chou, P. T., & Cheng, Y. C. (2019). Low Internal Reorganization Energy of the Metal – Metal-to-Ligand Charge Transfer Emission in Dimeric Pt(II) Complexes. Journal Of Physical Chemistry C, 123, 10225–10236
Wei, Y. C., Wang, S. F., Hu, Y., Liao, L. S., Chen, D. G., Chang, K. H., Wang, C. W., Liu, S. H., Chan, W. H., Liao, J. L., et al. (2020). Overcoming the Energy Gap Law in Near-Infrared OLEDs by Exciton-Vibration Eecoupling. Nature Photonics, 14, 570–577
Ly, K. T., Chen-Cheng, R. W., Lin, H. W., Shiau, Y. J., Liu, S. H., Chou, P. T. … Chi, Y. (2017). Near-Infrared Organic Light-Emitting Diodes with Very High External Quantum Efficiency and Radiance. Nature Photonics, 11, 63–68
Kenry, Duan, Y., & Liu, B. (2018). Recent Advances of Optical Imaging in the Second Near-Infrared Window. Advanced Materials, 30, 1802394
Ma, H., Liu, C., Hu, Z., Yu, P., Zhu, X., Ma, R., Sun, Z., Zhang, C. H., Sun, H., Zhu, S., et al. (2020). Propylenedioxy Thiophene Donor to Achieve NIR-II Molecular Fluorophores with Enhanced Brightness. Chemistry Of Materials, 32, 2061–2069
Nani, R. R., Kelley, J. A., Ivanic, J., & Schnermann, M. J. (2015). Reactive Species Involved in the Regioselective Photooxidation of Heptamethine Cyanines. Chemical Science, 6, 6556–6563
Liu, M. H., Zhang, Z., Yang, Y. C., & Chan, Y. H. (2021). Polymethine-Based Semiconducting Polymer Dots with Narrow-Band Emission and Absorption/Emission Maxima at NIR-II for Bioimaging. Angewandte Chemie Int Ed, 60, 983–989
Yu, J., Wu, C., Zhang, X., Ye, F., Gallina, M. E., Rong, Y. … Chiu, D. T. (2012). Stable Functionalization of Small Semiconducting Polymer Dots via Covalent Cross-Linking and Their Application for Specific Cellular Imaging. Advanced Materials, 24, 3498–3504
Liu, H. Y., Wu, P. J., Kuo, S. Y., Chen, C. P., Chang, E. H., Wu, C. Y., & Chan, Y. H. (2015). Quinoxaline-Based Polymer Dots with Ultrabright Red to Near-Infrared Fluorescence for In Vivo Biological Imaging. Journal Of The American Chemical Society, 137, 10420–10429
Liou, S. Y., Ke, C. S., Chen, J. H., Luo, Y. W., Kuo, S. Y., Chen, Y. H. … Chan, Y. H. (2016). Tuning the Emission of Semiconducting Polymer Dots from Green to Near-Infrared by Alternating Donor Monomers and Their Applications for in Vivo Biological Imaging. Acs Macro Letters, 5, 154–157
Ke, C. S., Fang, C. C., Yan, J. Y., Tseng, P. J., Pyle, J. R., Chen, C. P. … Chan, Y. H. (2017). Molecular Engineering and Design of Semiconducting Polymer Dots with Narrow-Band, Near-Infrared Emission for in Vivo Biological Imaging. Acs Nano, 11, 3166–3177
Tsai, W. K., & Chan, Y. H. (2019). Semiconducting Polymer Dots as Near-Infrared Fluorescent Probes for Bioimaging and Sensing. Journal Of The Chinese Chemical Society, 10, 9–20
Tsai, W. K., Wang, C. I., Liao, C. H., Yao, C. N., Kuo, T. J., Liu, M. H., Hsu, C. P., Lin, S. Y., Wu, C. Y., Pyle, J. R., et al. (2019). Molecular Design of Near-Infrared Fluorescent Pdots for Tumor Targeting: Aggregation-Induced Emission versus Anti-Aggregation-Caused Quenching. Chemical Science, 10, 198–207
Gupta, N., Chan, Y. H., Saha, S., & Liu, M. H. (2021). Near-Infrared-II Semiconducting Polymer Dots for Deep-tissue Fluorescence Imaging. Chemistry - An Asian Journal, 16, 175–184
Wu, C., & Chiu, D. T. (2013). Highly Fluorescent Semiconducting Polymer Dots for Biology and Medicine. AngewChem Int Ed, 52, 3086–3109
Chan, Y. H., & Wu, P. J. (2015). Semiconducting Polymer Nanoparticles as Fluorescent Probes for Biological Imaging and Sensing. Particle & Particle Systems Characterization, 32, 11–28
Li, K., & Liu, B. (2014). Polymer-Encapsulated Organic Nanoparticles for Fluorescence and Photoacoustic Imaging. Chemical Society Reviews, 43, 6570–6597
Pu, K., Shuhendler, A. J., Jokerst, J. V., Mei, J., Gambhir, S. S., Bao, Z., & Rao, J. (2014). Semiconducting Polymer Nanoparticles as Photoacoustic Molecular Imaging Probes in Living Mice. Nature Nanotechnology, 9, 233–239
Pu, K., Chattopadhyay, N., & Rao, J. (2016). Recent Advances of Semiconducting Polymer Nanoparticles in In Vivo Molecular Imaging. Journal Of Controlled Release : Official Journal Of The Controlled Release Society, 240, 312–322
Lim, X. (2016). The Nanolight Revolution is Coming. Nature, 531, 26–28
Feng, L., Zhu, C., Yuan, H., Liu, L., Lv, F., & Wang, S. (2013). Conjugated Polymer Nanoparticles: Preparation, Properties, Functionalization and Biological Applications. Chemical Society Reviews, 43, 6620–6633
Miao, Q., Xie, C., Zhen, X., Lyu, Y., Duan, H., Liu, X. … Pu, K. (2017). Molecular Afterglow Imaging with Bright, Biodegradable Polymer Nanoparticles. Nature Biotechnology, 35, 1102–1110
Yu, J., Rong, Y., Kuo, C. T., Zhou, X. H., & Chiu, D. T. (2017). Recent Advances in the Development of Highly Luminescent Semiconducting Polymer Dots and Nanoparticles for Biological Imaging and Medicine. Analytical Chemistry, 89, 42–56
Peng, H. S., & Chiu, D. T. (2015). Soft Fluorescent Nanomaterials for Biological and Biomedical Imaging. Chemical Society Reviews, 44, 4699–4722
Xuan Yi, M. X., Zhou, H., Xiong, S., Qian, R., & Chai, Z. (2018). Li Zhao,* and Kai Yang: Ultrasmall Hyperbranched Semiconducting Polymer Nanoparticles with Different Radioisotopes Labeling for Cancer Theranostics. ACS Nano 12:9142 – 9151
Dijkstra, K. K., Voabil, P., Schumacher, T. N., & Voest, E. E. (2016). Genomics- and Transcriptomics-Based Patient Selection for Cancer Treatment With Immune Checkpoint Inhibitors: A Review. JAMA Oncology, 2(11), 1490–1495
Keek, S. A., Leijenaar, R. T. H., Jochems, A., & Woodruff, H. C. (2018). A review on radiomics and the future of theranostics for patient selection in precision medicine. The British Journal of Radiology, 91(1091), 20170926
Grassberger, C., Ellsworth, S. G., Wilks, M. Q., Keane, F. K., & Loeffler, J. S. (2019). Assessing the interactions between radiotherapy and antitumour immunity. Nature Reviews Clinical Oncology, 16(12), 729–745
Hwang, W. L., Pike, L. R. G., Royce, T. J., Mahal, B. A., & Loeffler, J. S. (2018). Safety of combining radiotherapy with immune-checkpoint inhibition. Nature Reviews Clinical Oncology, 15(8), 477–494
Lin, L. T., Chang, C. Y., Chang, C. H., Wang, H. E., Chiou, S. H., Liu, R. S. … Lee, Y. J. (2016). Involvement of let-7 microRNA for the therapeutic effects of Rhenium-188-embedded liposomal nanoparticles on orthotopic human head and neck cancer model. Oncotarget, 7(40), 65782–65796
Lin, M. Y., Hsieh, H. H., Chen, J. C., Chen, C. L., Sheu, N. C., Huang, W. S. … Wu, C. Y. (2021). : Brachytherapy Approach Using (177)Lu Conjugated Gold Nanostars and Evaluation of Biodistribution, Tumor Retention, Dosimetry and Therapeutic Efficacy in Head and Neck Tumor Model. Pharmaceutics 13(11)
Acknowledgements
Lee Y-J was supported by a grant of Ministry of Science and Technology of Taiwan (109-2314-B-010-021-MY3); Chiang H.K. was supported by a grant of Ministry of Science and Technology of Taiwan (110-2124-M-A49A-501); Chuang H-Y has received research grants from the Ministry of Science and Technology of Taiwan (MOST 109-2314-B-010 -006 -MY2) and Yen Tjing Ling Medical Foundation (grant number: CI-109-9).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, CC., Chan, YH., Lin, SL. et al. Theranostic Radiolabeled Nanomaterials for Molecular Imaging and potential Immunomodulation Effects. J. Med. Biol. Eng. 42, 555–578 (2022). https://doi.org/10.1007/s40846-022-00715-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-022-00715-6