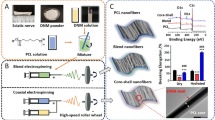
Abstract
Purpose
Peripheral nerve injury (PNI) and its regeneration continue to remain a significant medical burden worldwide. The current treatment strategies used to treat PNI are often associated with multiple complications and yet do not achieve complete motor and sensory functions. Recently, synthetic biodegradable nerve conduits have become one the most commonly used conduits to repair small gaps in nerve injury. But they have not shown better results than nerve grafts possibly because of the lack of biological microenvironment required for axonal growth. Schwann cells play a very crucial role in peripheral nerve regeneration where activated SCs produce multiple neurotrophic factors that help in remyelination and immune modulation during nerve repair. Studies have shown that nanofibrous scaffolds have better bioactivity and more closely mimic the native structure of the extracellular matrix. Therefore, the present study was focused on designing a nanofibrous scaffold that would cover the roles of both structural support for the cells that can provide a microenvironment with biological cues for nerve growth and regeneration.
Methods
Decellularized Schwann cell ECM were spin-coated on polycaprolactone random and aligned nanofibrous scaffolds and their compatibility was evaluated using Schwann cells.
Results
Schwann cells displayed growth in the direction of the aligned PCL nanofibers and ACM treated exhibited appropriate bipolar morphology indicating that these modified fibers could provide directional cues making them highly suitable for neuronal cell growth.
Conclusion
Our results indicate that the fabricated aligned SC-ACM treated PCL scaffolds would be a potential biomaterial to treat peripheral nerve injuries and promote regeneration.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Peripheral Nerve Injury (PNI) continues to be one of the most common medical burdens worldwide. Even with the advancement in medical technology, treatment and management of nerve injuries remain expensive and ineffective. [1,2,3,4] Most traumatic PNIs need surgical intervention which is often associated with multiple complications and side effects. Even then none of the current therapeutic options can fully achieve complete recovery of motor and sensory functions. Nerve grafting even though has proven to be one of the most effective treatments currently available, is not completely devoid of shortcomings. Nerve grafts are often associated with transplant rejections thus making the patient prone to more risks and complications. [5, 6] Therefore, it calls for an urgent need for new therapeutic options for the treatment of peripheral nerve injury.
Healing during peripheral nerve injury is a complicated biological process that concerns replacing damaged tissue with a living one. Thus, restoring tissue integrity is quite difficult by itself. In the self-healing process, the interaction of immune cells, as well as neural extracellular matrices (ECM) components such as fibronectin, glycosaminoglycans, proteoglycans, thrombospondins, tenascin, vitronectin, or collagens, takes place. The mentioned cell interactions with neural ECM components are subject to the regulation of biochemical mediators, numerous cytokines, and growth factors. [7,8,9]
The ECM in our body with which cells interact has topography at the nanoscale that guides axonal growth and regeneration. Thus, ECM-based biomaterials are proposed to quickly regain healing cues and suppress or halt immune reactions to implant sites. [10]
In the peripheral nervous system, Schwann cells play a very important role in nerve regeneration. Activated Schwann cells during an injury produce multiple neurotrophic factors such as Nerve Growth Factor (NGF), brain-derived growth factor, ciliary neurotrophic factor, and neuropeptide Y all of which play a crucial role in axonal regeneration and myelin formation. [11, 12] In addition to secreting neurotrophic factors, SCs are also responsible for secreting the extracellular matrix (ECM) components thus providing a platform for neuronal cell growth and regeneration. [13]
Recently, nerve conduits are being developed as a possible therapy for nerve damage that acts as the scaffolding platform for bridging the gap between the proximal and distal ends of the injured nerves. Synthetic, biodegradable nerve conduits have become one the most commonly used conduits to repair small gaps. [14,15,16] Some of the biomaterials used for this purpose are commonly made from polymers like Poly-caprolactone (PCL), Polyglycolic acid (PGA), and Poly Lactic acid (PLA). PCL is FDA approved (non-toxic), has a low melting point and its semi-crystalline form is a rubbery state that provides it excellent mechanical properties thus, making it highly suitable for application in regenerative therapy. [17]
The electro spinning technique produces fibrous scaffolds that can provide high surface area for attachment of cells, the porous network for cell migration, and a 3D environment for cell-to-cell interaction. This technique can produce aligned fibers that provide topographical cues for the alignment of cells for the regeneration of axons. This also ensures to help in the unidirectional conduction of nerve impulses. This method can consistently produce nanofibers while also being economical. Thus, the nanofibers formed by electrospinning mimic the ECM forming a similar microenvironment around the cells. [18, 19]
Even though synthetic conduits are cost-effective and are associated with fewer complications, in terms of nerve regeneration, they have not shown better results than nerve grafts possibly because of the lack of biological microenvironment required for axonal growth. [6] Therefore, in order to provide nerve cells with both structural support and a microenvironment with biological cues for growth and regeneration, in this study we fabricated electrospun poly-caprolactone nanofibers coated with decellularized extracellular matrix (ACM) of Schwann cells to use as a nerve conduit for the treatment of peripheral nerve injury that would potentially be financially feasible, with fewer side effects and highly effective for filling large nerve gaps. To study the effect of ACM on the synthesized nanofibers we then carried out various biochemical assays using Schwann cells.
2 Materials and Methods
2.1 Materials
Polycaprolactone was purchased from Sigma (MW 80,000); Methanol, Chloroform, and Dimethyl sulfoxide were purchased from HiMedia; Rat Schwann cells (CRL-2765 RSC96) were purchased from ATCC; Fetal Bovine Serum, antibiotics penicillin-streptomycin, anti-anti, and Dulbecco’s Eagle Medium were purchased from Gibco; cytochemical stains (Alcian Blue, Masson’s Trichrome, Picrosirius Red, Safranin O) were purchased from AMD Labs; Coomassie Blue; Methylene Blue were purchased from HiMedia, Phosphate Buffer Saline tablets were purchased from Sigma Life science; Live/Dead reagent (lot no. 2015555), Rhodamine-Phalloidin (lot no. 2157163), Secondary Antibody (Alexa Fluor 488 rabbit anti-mouse) (Catalogue no. A11059), Anti-α-tubulin (bovine), mouse IgG1, monoclonal (214775)7 were purchased from Invitrogen; MTT (TC191-1G), Triton-X, Tween- 20 and DAPI (4’,6 Diamidino-2-phenylindole dihydrochloride) (MB097-10MG) were purchased from HiMedia.
2.2 Fabrication of PCL Random and Aligned Fibers by Electrospinning
10% (w/v) PCL solution was prepared in a 8:2 ratio of chloroform and methanol. The solution was loaded into a 5-ml glass syringe of needle size 24G. Random fibers were synthesized at a flow rate of 0.002 ml/min at an applied voltage of 18 kV using an electrospinning machine (HO-NFES-040) with a stationary metal collector. Aligned nanofibers were prepared with the same parameters on a rotating mandrel at a constant speed of 2500 rpm. The nanofiber mats obtained were 0.25 mm in thickness. The synthesized mats were stored in a vacuum desiccator for further characterization.
2.3 Schwann Cell Culture and Extraction of Acellular Matrix (ACM)
Rat Schwann cells (RSCs) procured from American Type Culture Collection (ATCC) (CRL-2765 RSC96) were cultured in a growth media comprising Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin and maintained at 37 °C in 5% carbon dioxide. Confluent monolayer of Schwann cells was subjected to serum starvation by replacing the whole media with serum-free DMEM media for 5 days. Sterile distilled water was then added to the serum-starved cells and incubated for 1–2 min. After that 0.1% of ammonia solution was added and incubated until the cells detached. The ammonia solution with the cells was discarded and the remaining ECM was scrapped out in PBS using a cell scraper [23]. The concentration of protein in the extracted ACM was determined to be 227 µg/ml using Bradford protein assay. The obtained ACM was then stored in a -20 ºC freezer for about a week.
2.4 Spin Coating ACM on PCL Scaffolds
The extracted ACM was uniformly spread on the scaffolds using the spin coating machine (HO-TH-05) The sterilized scaffolds were first electrospun on the circular coverslips. The coverslip with the scaffold was placed on the spin coating machine and the rotator was spun at 300 rpm for 20 s. 20 µl of 227 µg/ml of ACM dissolved in PBS was added to the center of the spinning coverslip. A thin uniform layer of ACM was coated onto the scaffolds. The ACM-coated scaffolds were further UV sterilized for half an hour on each of the sides before further characterization.
2.5 Cytochemical Characterization of ACM on scaffolds
To qualitatively analyze the coating of SC-ACM on the scaffolds, cytochemical staining for different components of the extracellular matrix was carried out. To visualize glycoproteins, the ACM was stained using Alcian blue and picrosirius red and to visualize collagen, the scaffolds were treated with Mason’s trichome and safranin O. To visualize the total protein content, the ACM-coated scaffolds, and the control were treated with Coomassie blue. Since an ideal decellularized matrix should not contain any cells, the scaffolds were treated with methylene blue to check for any left-over cells by staining DNA. For all the staining, scaffolds treated with PBS were used as a control.
2.5.1 Alcian Blue staining
Cells were fixed with 4% formaldehyde for 30 min, washed with PBS and stained with acetic acid (pH 2.5) and alcian blue staining solution, washed with ethanol twice, and observed under the microscope with distilled water.
2.5.2 Picrosirius red staining
Cells were fixed in bovine fixative for 30 min, washed with PBS, and incubated in hematoxylin solution for 10 min. Cells were then stained with picrosirius red dye for 1 h. Cells were then finally washed with acidic water and observed under the microscope.
2.5.3 Mason’s Trichome staining
Cells were fixed in bovine fixative for 1 h washed with PBS and incubated with wright’s hematoxylin for 10 min. The cells are then stained with Fuchsin’s reagent, Phosphomolybdic acid, methyl blue for 5 min each. Cells were then washed with distilled water and 1% acetic acid and observed under the microscope.
2.5.4 Safranin O staining
Cells were fixed with 0.1% glutaraldehyde for 20 min and washed with PBS. The cells were then stained with Safranin O for 5 min and washed with PBS before observing under the microscope.
2.6 Cell seeding on Scaffolds
The electrospun PCL aligned and random nanofibers were sterilized for 3 h in UV for each side. Both ACM coated and non-ACM coated PCL random and aligned fibers were placed in a 24 well plate. 50,000 cells were seeded for cell adhesion and proliferation studies and 10,000 cells seeded for microscopic studies. Tissue culture polystyrene (TCPS) was used as a control, and the growth medium was changed every other day.
2.7 Cell Adhesion and Proliferation
Cell-scaffold interaction was qualitatively evaluated using a Scanning Electron microscope (SEM) after 2, 4, and 8 h of cell seeding on scaffolds. Briefly, after each time-point, the samples were washed with PBS and fixed with 2.5% glutaraldehyde overnight at 4 °C. The samples were then washed with PBS and dried in a vacuum desiccator. The dried samples were sputter‐coated with gold and observed under FE‐SEM (ESEM, AFMM, IISc).
In order to confirm the cytocompatibility of scaffolds, a DNA quantification assay was also performed on cells cultured on scaffolds and tissue culture polystyrene (TCPS) control using the DNA Quantification Kit (SIGMA, catalog number: DNAQF). Briefly, cells were cultured on the nanofibrous scaffolds for two different time points (day 1 and day 3). The cultured cells are extracted from the scaffolds using trypsin and centrifuged for 1400 rpm for 5 min. The supernatant is discarded and fluorescent dye, bisbenzimide H33258, is added to the pellet. DNA present in the cells was quantified by measuring fluorescence excitation at 360 nm and emission at 460 nm using a multimode plate reader - Perkin Elmer (Ensight) multi-mode plate reader (HH34000000).
2.8 Cell Viability Assay
Cell viability of cells seeded on scaffolds was assessed after 1 and 3 days of seeding using live /dead assay which is a two-colored fluorescent dye assay that differentially labels both live and dead cells. The stock solution of live /dead reagent was prepared by adding 4 µl of EthD-1 and 1 µl of Calcein-AM in 2ml PBS. 100-150ul of live/dead reagent was added to the scaffolds and incubated at room temperature for 1 h. The well plate was observed under the fluorescence microscope (Nikon Eclipse-TE2000-U).
2.9 Phalloidin Staining
Phalloidin staining was done to visualize the actin cytoskeletal spreading and morphology of Schwann cells seeded on the fabricated nanofibers. For actin cytoskeletal staining, the scaffolds were fixed with 4% paraformaldehyde for 30 min, permeabilized using 0.3% Triton -X 100 for 15 min, blocked with 3% BSA for 30–40 min. The scaffolds were then incubated with rhodamine-phalloidin (1:200; Invitrogen) for 1 h at room temperature. All samples were then stained with DAPI (1:1000; HiMedia) for nuclear staining for 5 min and imaged under a fluorescence microscope at 20X magnification. (Nikon Eclipse-TE2000-U)
2.10 10 Confocal Microscopic Studies
The cell-seeded on scaffolds were stained for microtubules after 3 days of culture. The adhered cells on scaffolds were fixed with 4% paraformaldehyde for 30 min, permeabilized using 0.3% Triton -X 100 for 15 min, blocked with 3% Bovine serum albumin (BSA) for 1 h at RT. The scaffolds were then incubated with alpha-tubulin antibody (1:1000) (Invitrogen 2,147,757) overnight at 4 C. The scaffolds were thoroughly washed with PBS and incubated with anti-mouse IgG- Alexa flour 488 (Invitrogen) for 2 h at RT. The samples were stained with DAPI for nuclear staining for 5 min and observed under laser scanning confocal microscope at 20X magnification (Leica TCS SP8, IISc). Three-dimensional images were obtained using Z‐ sectioning to assess cellular infiltration through the scaffolds.
3 Results
3.1 Surface Morphology of PCL Random and Aligned Nanofibers
From Scanning electron microscopic images (Fig. 1), it can be observed that uniform and beadless PCL random nanofibers were obtained with a fiber diameter range of about 400–600 nm. PCL-aligned nanofibers also appeared in similar morphology without beads and were found to be having highly oriented morphology.
3.2 Preparation and Cytochemical Characterization of ACM
For the preparation of the ACM, cells were incubated for 5 days with FBS deficient DMEM media. On the 5th day, the media was removed from the cells, and cells were washed with PBS. After the wash, autoclaved distilled water was added to the cells for 2 min until the cells appeared round. 0.1% of ammonia solution was then added to the cells for decellularization. Once the cells started detaching, the ammonia solution containing the cells was removed slowly and PBS wash was given. ACM was scraped out in PBS with a cell scraper. The extracted ACM was stored at -20ºC until for a week for further characterization. Figure 2 represents the steps for ECM extraction from Schwann cells. Obtained ACM was spin-coated onto various scaffolds and cytochemical characterization was carried out. Staining using Alcian blue, Picrosirius Red, Masson’s Trichrome, and Safranin O dyes were performed which showed a significant difference in the intensity of the color on the surface of the scaffolds treated with SC-ACM compared to PBS coated scaffolds indicating the presence of intact ECM contents on the scaffolds. Alcian Blue: the treated samples had clearly shown the blue intensive color of the stain stating the presence of proteoglycans and glycosaminoglycans than the control. Masson’s Trichrome: the treated samples were mauve-colored on being observed under a microscope, showing the impact of connective tissue and collagen. Picrosirius Red: the treated sample showed reddish orange-like when stained with PR indicating that the dECM contained collagen. Safranin O: this dye stains for the presence of glycosaminoglycans and thus the treated samples were effectively showing an orange color than those of control. In (Fig. 3) Cytochemical characterization can be visualized, left-hand side is the Control (PBS coated scaffolds); the right-hand side is the Treated (ACM coated scaffolds). AB- Alcian Blue, MT- Masson’s Trichrome, PR- Picrosirius Red, and SO- Safranin O.
3.3 Cell adhesion and cell proliferation
The scanning electron microphotographs (Fig. 4) of SC cultured on scaffolds at different time points (2 h, 4 h and 8 h) show that the cells adhered to all the scaffolds starting from just 2 h of cell seeding indicating that the nanofibrous scaffolds support cell adhesion.
DNA quantification graph (Fig. 5) represents the relative fluorescence units with respect to the DNA present in the cells cultured on the nanofibrous scaffolds. From this graph, we observe that there is an increase in the DNA concentration from day 1 to day 3 from which we can imply that there is a gradual increase in the cell viability showing the cytocompatibility of the scaffolds comparable to the control - TCPS. There was a slight increase in the fluorescence between TCPS, random and aligned fibers showing the affinity of the cells towards the oriented topography. Also, there was a prominent increase observed in the treated scaffolds especially in the aligned group showing the positive effect of the Schwann cell ECM on controlling the cell proliferation. However, there was no statistically significant difference observed between the groups.
3.4 Cell Viability Assay
The viability or cytotoxicity assay carried out using live/dead reagent (Fig. 6) clearly showed that there was a significantly higher number of live cells compared to dead cells on each of the scaffolds indicating that both the treated (ACM coated) and untreated (uncoated) scaffolds supported Schwann cell growth indicating their cytocompatibility.
3.5 Actin Staining
Actin cytoskeleton staining (Fig. 7) with Rhodamine phalloidin of SCs seeded on scaffolds showed that cells showed clear elongated morphology on day 3 on all the scaffolds. Moreover, cells also seemed to show better morphology and grow in the direction of the aligned fibers which was more significantly observed on treated scaffolds.
3.6 Confocal Microscopic Studies
Immunostaining of alpha-tubulin was performed to observe the cytoskeletal morphology of the cells cultured on the scaffolds. Confocal microscopic images in (Fig. 8) showed that the cells have gained their typical extended morphology on each of the scaffolds after 3 days of culture. They also showed clear alignment in the direction of the fibers observed from the DIC images with the nanofiber orientation in the background. We could also observe the microtubule cytoskeleton with better-extended morphology on the aligned scaffolds than on the random scaffolds. This could be more noticeably appreciated in the aligned ACM treated scaffolds compared to the randomly treated scaffolds indicating the effect of the ACM on retaining cell morphology in an improved manner.
4 Discussion
Peripheral nerve injury unfortunately still remains one of the biggest medical burdens of our time even with the current advancement in medical technology and continuous research on the topic for several decades. One of the most important reasons for this might be the complicated biological processes associated with the healing of nerve injuries. The healing process involves interaction between immune cells and the extracellular matrix components such as fibronectins, glycosaminoglycans, and collagens which are further subjected to regulation by biochemical mediators, cytokines, and growth factors. [7, 9] Due to the involvement of several players in the regeneration process, intervention associated with peripheral nerve injury must consider several factors. In our current study, we attempted a novel approach to fabricate synthetic biodegradable nanofibrous aligned scaffolds infused with ECM extracted from Schwann cells. We hypothesized that the fabricated scaffolds would provide the neurons with (i) Directional cue for their growth in the right direction due to the alignment of the fibers, (ii) Biological cues for their proper growth provided by the Schwann cell ECM and (iii) Fewer side effects and cost feasibility provided by the FDA approved polymer Polycaprolactone used for the fabrication of the nanofibers. PCL nanofibers, both aligned and random were produced using the electrospinning technique. The SEM images of the fabricated aligned and random fibers showed a clear difference between the morphology of the aligned and random nanofibers with a fiber diameter of 400–600 nm.
Schwann cells are the main glial cells of the peripheral nervous system and play a very important role in nerve regeneration wherein ECM synthesized and assembled by SC guides and promotes neurite outgrowth of the peripheral neurons [20]. Therefore, in this study, we utilized rat Schwann cells to produce ECM which is coated onto the PCL nanofibrous scaffolds. Further, SCs themselves were used to assess their adhesion, proliferation, and compatibility with the scaffolds as a preliminary step to understand their interaction with neuronal co-cultures in future studies. The extracellular matrix was extracted using the protocol described in Wasnik et al. [24]. The extracted ECM was characterized by cytochemical staining of ECM proteins such as Collagen and glycoproteins. The staining confirmed the presence of ECM components such as collagen, proteoglycans, and glycosaminoglycans.
The cell viability assay carried out with live/dead reagent showed that there were more live cells compared to dead cells on each of the scaffolds. This clearly indicated that both ACM-coated and uncoated scaffolds were non-toxic and found to be biocompatible with Schwann cells. The cell number increased from day 1 to day 3 indicating that the fibers supported cell proliferation similar to the TCPS control. However, there was no significant difference in cell proliferation observed between the ACM-coated and non-coated scaffolds. This observation was similar to the results obtained by Reid et al. where PCL was combined with aortic ECM [25] and Slavic et al. where PCL-based electrospun fibers were compared with porcine liver ECM [26]. Overall, it could be deciphered that ACM treatment has a positive influence on the Schwann cell differentiation and maturation to myelin-promoting cells. Scanning electron microscope images showed that the RSCs adhered to all the scaffolds within just 2 h of seeding showing a very strong cell-scaffold interaction, clearly indicating that the fibrous morphology of the scaffolds plays an important role in guiding cell adhesion. The cytoskeleton staining of actin filaments and microtubules of the RSCs showed that they gained typical bipolar extended morphology on the ECM coated scaffolds. Especially the cells gained extended morphology in the direction of the aligned fibers clearly indicating that the aligned nanofibrous scaffold morphology can provide the cells with the directional cue for growth. This property of the aligned fibers makes it highly suitable for nerve cell growth and contact guidance. This type of behavior was also seen in a study where keratinocytes were have exhibited elongated morphology on PCL scaffolds combined with corneal extracellular matrix (ECM) compared to the plain PCL scaffolds [27]. They attributed this phenomenon to the additional cell-binding sites in PCL/ECM scaffolds being absent in plain PCL scaffolds. Similarly, in another study by Zhu et al., ECM scaffolds with aligned microchannels were prepared and compared with PCL control scaffolds [28]. Here, Schwann cells (RSC96) grew longitudinally along the microchannels throughout ECM scaffolds whereas, across the control scaffold, cells were distributed randomly. This was due to the physical guidance cues provided to the cells which is a combinatorial effect of parallel microchannels and oriented ECM nanofibers which improved the cell migration and efficient nutrient transport. Thus, we believe that the Schwann cell ECM functionalized aligned PCL nanofibrous scaffolds fabricated in this study would pave the way to design novel biomaterials which could be used for peripheral nerve regeneration applications.
5 Conclusion
Random and aligned PCL nanofibrous scaffolds were successfully fabricated using electrospinning and were surface coated with an acellularized Schwann cell-extracellular matrix (SC-ACM). The cells showed to have strong cell-scaffold interaction as the cells adhered to scaffolds from just 2 h after seeding. The RSCs also showed their typical extended bipolar morphology with very few dead cells on each of the scaffolds indicating that the scaffolds were biocompatible. In addition to that RSCs showed growth in the direction of the aligned PCL nanofibers indicating that these fibers could provide directional cues for the growth of cells making them highly suitable for neuronal cell growth. Our results indicate that the fabricated aligned SC-ACM treated PCL scaffolds are highly suitable for the growth of nerve cells and have the potential to be used in the application of peripheral nerve repair.
Data Availability
Not Applicable.
Code Availability
Not Applicable.
References
Robinson, L. R. (2000). Traumatic injury to peripheral nerves. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine, 23(6), 863–873
Scholz, T., Krichevsky, A., Sumarto, A., Jaffurs, D., Wirth, G. A., Paydar, K., & Evans, G. R. (2009). Peripheral nerve injuries: an international survey of current treatments and future perspectives. Journal of reconstructive microsurgery, 25(06), 339–344
Bergmeister, K. D., Große-Hartlage, L., Daeschler, S. C., Rhodius, P., Böcker, A., Beyersdorff, M., & Harhaus, L. (2020). Acute and long-term costs of 268 peripheral nerve injuries in the upper extremity. PloS one, 15(4), e0229530.
Kouyoumdjian, J. A., Graça, C. R., & Ferreira, V. F. (2017). Peripheral nerve injuries: A retrospective survey of 1124 cases. Neurology India, 65(3), 551
Evans, G. R. (2000). Challenges to nerve regeneration. Seminars in surgical oncology (19 vol., pp. 312–318). New York: John Wiley & Sons, Inc. 3
Carvalho, C. R., Reis, R. L., & Oliveira, J. M. (2020). Fundamentals and Current Strategies for Peripheral Nerve Repair and Regeneration. Advances in experimental medicine and biology, 1249, 173–201. https://doi.org/10.1007/978-981-15-3258-0_12
Palumbo, C., Massa, R., Panico, M., Di Muzio, A., Sinibaldi, P., Bernardi, G., & Modesti, A. (2002). Peripheral nerve extracellular matrix remodeling in Charcot-Marie-Tooth type I disease. Acta neuropathologica, 104(3), 287–296
Dzyubenko, E., Manrique-Castano, D., Kleinschnitz, C., Faissner, A., & Hermann, D. M. (2018). Role of immune responses for extracellular matrix remodeling in the ischemic brain. Therapeutic advances in neurological disorders, 11, 1756286418818092
Caddeo, S., Boffito, M., & Sartori, S. (2017). Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Frontiers in bioengineering and biotechnology, 5, 40
Gonzalez-Perez, F., Udina, E., & Navarro, X. (2013). Extracellular matrix components in peripheral nerve regeneration. International review of neurobiology, 108, 257–275
Jessen, K. R., Mirsky, R., & Lloyd, A. C. (2015). Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harbor perspectives in biology, 7(7), a020487. https://doi.org/10.1101/cshperspect.a020487
Höke, A., Redett, R., Hameed, H., Jari, R., Zhou, C., Li, Z. B., & Brushart, T. M. (2006). Schwann cells express motor and sensory phenotypes that regulate axon regeneration. Journal of Neuroscience, 26(38), 9646–9655
Chernousov, M. A., & Carey, D. J. (2000). Schwann cell extracellular matrix molecules and their receptors. Histology and histopathology, 15(2), 593–601. https://doi.org/10.14670/HH-15.593
Chang, W., Shah, M. B., Lee, P., & Yu, X. (2018 Jun). Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration. Acta Biomater, 73, 302–311. doi: https://doi.org/10.1016/j.actbio.2018.04.046. Epub 2018 Apr 24. PMID: 29702292
Xu, H., Yan, Y., & Li, S. (2011). PDLLA/chondroitin sulfate/chitosan/NGF conduits for peripheral nerve regeneration. Biomaterials, 32(20), 4506–4516
Bian, Y. Z., Wang, Y., Aibaidoula, G., Chen, G. Q., & Wu, Q. (2009). Evaluation of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials, 30(2), 217–225
Wang, S., & Cai, L. (2010). Polymers for fabricating nerve conduits. International Journal of Polymer Science, 2010
Li, W. J., Laurencin, C. T., Caterson, E. J., Tuan, R. S., & Ko, F. K. (2002). Electrospun nanofibrous structure: a novel scaffold for tissue engineering. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 60(4), 613–621
Koh, H. S., Yong, T., Teo, W. E., Chan, C. K., Puhaindran, M. E., Tan, T. C., Ramakrishna,S. (2010). In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. Journal of Neural Engineering, 7(4), 046003
Ard, M. D., Bunge, R. D., & Bunge, M. R. (1987). Comparison of the Schwann cell surface and Schwann cell extracellular matrix as promoters of neurite outgrowth. Journal of Neurocytology, 16, 539–555
Salzer, J. L., & Bunge, R. D. (1980). Studies of Schwann Cell Proliferation in I. An Analysis in Tissue Culture of Proliferation during Development, Wallerian Degeneration, and Direct Injury. Journal of Cell Biology© The Rockefeller University Press, 84, 739–752
Divieto, C., & Sassi, M. P. (2015). A first approach to evaluate the cell dose in highly porous scaffolds by using a nondestructive metabolic method.Future science OA, 1(4)
Chang, H. I., & Wang, Y. (2011). Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative medicine and tissue engineering-cells and biomaterials. InTechOpen
Wasnik, S., Kantipudi, S., Kirkland, M. A., & Pande, G. (2016). Enhanced. Expansion of Human Hematopoietic Progenitors on Native and Spin Coated Acellular Matrices Prepared from Bone Marrow Stromal Cells. Stem Cells International, 7231567, 13
Reid, J. A., & Callanan, A. (2019). Influence of aorta extracellular matrix in electrospun polycaprolactone scaffolds. J. Appl. Polym. Sci, 4818, 2–8
Slivac, I., Zdraveva, E., Ivančić, F., Žunar, B., Holjevac Grgurić, T., Gaurina Srček, V. … Mijović, B. (2021). Bioactivity Comparison of Electrospun PCL Mats and Liver Extracellular Matrix as Scaffolds for HepG2 Cells. Polymers, 13, 279
Fernández-Péreza, J., Kadora, K. E., Lyncha, A. P., & Ahearne, M. (2020). Characterization of extracellular matrix modified poly(ε-caprolactone) electrospun scaffolds with differing fiber orientations for corneal stroma regeneration. Materials Science & Engineering C, 108, 110415
Zhu, M., Li, W., Dong, X., Yuan, X., Midgley, A. C., Chang, H. … Kong, D. (2019). In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nature Communications, 10, 4620
Acknowledgements
The authors would like to acknowledge the Department of Science & Technology for the SERB start-up research grant (SRG/2019/002130) and Manipal Academy of Higher Education (MAHE) seed money grant for faculty research for financial support. We would also like to thank Ms. Srividya H and Ms. Michelle Abraham for the help during material preparation and cell culture experiments. We also would extend thanks to the Manipal Institute of Regenerative Medicine for the infrastructural support.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. The authors would like to acknowledge the Department of Science & Technology for the SERB start-up research Grant (SRG/2019/002130) for financial support. We would also like to thank the MAHE seed money grant for faculty research.
Author information
Authors and Affiliations
Contributions
MN has designed the experiments and prepared the manuscript draft. MB and AN have performed the experiments and prepared the manuscript figures.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical Approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nune, M., Bhat, M. & Nagarajan, A. Design of ECM Functionalized Polycaprolactone Aligned Nanofibers for Peripheral Nerve Tissue Engineering. J. Med. Biol. Eng. 42, 147–156 (2022). https://doi.org/10.1007/s40846-022-00699-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-022-00699-3