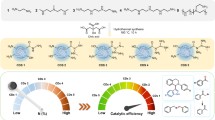
Abstract
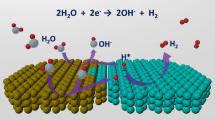
Regulating the electronic structure of metal sites to develop high-performance catalysts for selective hydrogenation reactions is very important for sustainable organic synthesis, yet still challenging. Herein, we adopt a facile method to rationally design an ultrafine PtRu alloy cocatalyst on CdS nanorods (PtRu/CdS) for the efficient photocatalytic hydrogenation reaction with water as a proton source. The ultrafast transient absorption spectroscopy studies uncover that alloying promotes the separation of photoexcited carriers, and density functional theory calculations demonstrate that alloying reduces the energy barrier of the rate-determining step, thus enhancing the hydrogenation activity. This work opens a new avenue to rationally design highly-active metal-modified photocatalysts via modulating electronic structures of co-catalysts.

摘要
调控金属活性位点的电子结构来发展高活性的选择性加氢反应 催化剂对于可持续有机合成非常重要. 但时至今日, 这仍然是非常具有 挑战性的课题. 在本文中, 我们将具有电子结构调节特性的超细PtRu合 金纳米颗粒负载在CdS纳米棒上, 成功构建了PtRu/CdS复合催化剂并 研究了其光催化芳香族硝基化合物氢化还原性能. 我们发现当以水为 质子源时, 所得复合催化剂表现出高效的光催化加氢活性. 超快光谱研 究和密度泛函理论计算揭示了合金的形成促进了光生载流子的分离并 降低了氢化还原反应中决速步的能垒, 从而显著提高了加氢活性. 本研 究通过合金化效应调节助催化剂的电子结构, 为高活性助催化剂/光催 化剂体系的理性设计开辟了一条新途径.
Similar content being viewed by others
References
Liu L, Corma A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem Rev, 2018, 118: 4981–5079
Wang H, Chen J, Lin Y, et al. Electronic modulation of non-van der Waals 2D electrocatalysts for efficient energy conversion. Adv Mater, 2021, 33: 2008422
Subbaraman R, Tripkovic D, Strmcnik D, et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science, 2011, 334: 1256–1260
Hu B, Sun K, Zhuang Z, et al. Distinct crystal-facet-dependent behaviors for single-atom palladium-on-ceria catalysts: Enhanced Stabilization and catalytic properties. Adv Mater, 2022, 34: 2107721
Wan J, Zhao Z, Shang H, et al. In situ phosphatizing of triphenyl-phosphine encapsulated within metal-organic frameworks to design atomic Co1-P1N3 interfacial structure for promoting catalytic performance. J Am Chem Soc, 2020, 142: 8431–8439
Yin XP, Wang HJ, Tang SF, et al. Engineering the coordination environment of single-atom platinum anchored on graphdiyne for optimizing electrocatalytic hydrogen evolution. Angew Chem Int Ed, 2018, 57: 9382–9386
Qin J, Liu H, Zou P, et al. Altering ligand fields in single-atom sites through second-shell anion modulation boosts the oxygen reduction reaction. J Am Chem Soc, 2022, 144: 2197–2207
Li X, Sun Y, Xu J, et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat Energy, 2019, 4: 690–699
Zhou S, Jang H, Qin Q, et al. Boosting hydrogen evolution reaction by phase engineering and phosphorus doping on Ru/P-TiO2. Angew Chem Int Ed, 2022, 61: e202212196
Xin H, Lin L, Li R, et al. Overturning CO2 hydrogenation selectivity with high activity via reaction-induced strong metal-support interactions. J Am Chem Soc, 2022, 144: 4874–4882
Wang X, Chu M, Wang M, et al. Unveiling the local structure and electronic properties of PdBi surface alloy for selective hydrogenation of propyne. ACS Nano, 2022, 16: 16869–16879
Greiner MT, Jones TE, Beeg S, et al. Free-atom-like d states in singleatom alloy catalysts. Nat Chem, 2018, 10: 1008–1015
Wang L, Wang H, Zhang S, et al. Manipulating the electronic structure of nickel via alloying with iron: Toward high-kinetics sulfur cathode for Na-S batteries. ACS Nano, 2021, 15: 15218–15228
Cheruvathoor Poulose A, Zoppellaro G, Konidakis I, et al. Fast and selective reduction of nitroarenes under visible light with an earth-abundant plasmonic photocatalyst. Nat Nanotechnol, 2022, 17: 485–492
Corma A, Serna P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science, 2006, 313: 332–334
Oger C, Balas L, Durand T, et al. Are alkyne reductions chemo-, regio-, and stereoselective enough to provide pure (Z)-olefins in poly-functionalized bioactive molecules? Chem Rev, 2013, 113: 1313–1350
Zhang Q, Kusada K, Wu D, et al. Crystal structure control of binary and ternary solid-solution alloy nanoparticles with a face-centered cubic or hexagonal close-packed phase. J Am Chem Soc, 2022, 144: 4224–4232
Zhang H, Zhang P, Qiu M, et al. Ultrasmall MoOx clusters as a novel cocatalyst for photocatalytic hydrogen evolution. Adv Mater, 2019, 31: 1804883
Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B, 1996, 54: 11169–11186
Blöchl PE. Projector augmented-wave method. Phys Rev B, 1994, 50: 17953–17979
Perdew JP, Chevary JA, Vosko SH, et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys Rev B, 1992, 46: 6671–6687
Perdew JP, Wang Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B, 1992, 45: 13244–13249
Monkhorst HJ, Pack JD. Special points for Brillouin-zone integrations. Phys Rev B, 1976, 13: 5188–5192
Wang V, Xu N, Liu JC, et al. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput Phys Commun, 2021, 267: 108033
Hao S, Sheng H, Liu M, et al. Torsion strained iridium oxide for efficient acidic water oxidation in proton exchange membrane electrolyzers. Nat Nanotechnol, 2021, 16: 1371–1377
Norskov JK, Rossmeisl J, Logadottir A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B, 2004, 108: 17886–17892
Qiao S, He Q, Zhou Q, et al. Interfacial electronic interaction enabling exposed Pt(110) facets with high specific activity in hydrogen evolution reaction. Nano Res, 2023, 16: 174–180
Yang Z, Pedireddy S, Lee HK, et al. Manipulating the d-band electronic structure of platinum-functionalized nanoporous gold bowls: Synergistic intermetallic interactions enhance catalysis. Chem Mater, 2016, 28: 5080–5086
Su L, Jin Y, Gong D, et al. The role of discrepant reactive intermediates on Ru-Ru2P heterostructure for pH-universal hydrogen oxidation reaction. Angew Chem Int Ed, 2023, 62: e202215585
Luo D, Luo Y, Lu X, et al. Cooperative selective benzyl alcohol oxidation and hydrogen production over Pd6(SC12H25)12 cluster-coupled CdS nanorods: The key role of water in photocatalytic benzyl alcohol splitting. J Mater Chem A, 2022, 10: 15941–15948
Pelicano CM, Saruyama M, Takahata R, et al. Bimetallic synergy in ultrafine cocatalyst alloy nanoparticles for efficient photocatalytic water splitting. Adv Funct Mater, 2022, 32: 2202987
Zhang H, Zuo S, Qiu M, et al. Isolated cobalt centers on W18O49 nanowires perform as a reaction switch for efficient CO2 photoreduction. J Am Chem Soc, 2021, 143: 2173–2177
Lu S, Hu Y, Wan S, et al. Synthesis of ultrafine and highly dispersed metal nanoparticles confined in a thioether-containing covalent organic framework and their catalytic applications. J Am Chem Soc, 2017, 139: 17082–17088
Wu K, Du Y, Tang H, et al. Efficient extraction of trapped holes from colloidal CdS nanorods. J Am Chem Soc, 2015, 137: 10224–10230
Hu YZ, Koch SW, Lindberg M, et al. Biexcitons in semiconductor quantum dots. Phys Rev Lett, 1990, 64: 1805–1807
Klimov V, Hunsche S, Kurz H. Biexciton effects in femtosecond nonlinear transmission of semiconductor quantum dots. Phys Rev B, 1994, 50: 8110–8113
Klimov VI. Optical nonlinearities and ultrafast carrier dynamics in semiconductor nanocrystals. J Phys Chem B, 2000, 104: 6112–6123
Sheng J, He Y, Huang M, et al. Frustrated Lewis pair sites boosting CO2 photoreduction on Cs2CuBr4 perovskite quantum dots. ACS Catal, 2022, 12: 2915–2926
Acknowledgements
This work was supported by the University Annual Scientific Research Plan of Anhui Province (2022AH010013).
Author information
Authors and Affiliations
Contributions
Author contributions Ni Y directed this project. Shi M and Luo D conceived and designed the experiments. Shi M, Wei J, and Guo S carried out the syntheses, characterizations and performance tests of the materials. Wu P performed the DFT calculations and Huang Y reviewed the calculation results. Shen Y performed the ultrafast TA measurements and Lu Z analyzed the TA data. Shi M wrote the original draft and Ni Y revised and reviewed the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper.
Manman Shi is currently pursuing a PhD degree at the College of Chemistry and Materials Science, Anhui Normal University. Her research interest focuses on the synthesis of functional nanomaterials and their applications in solar energy conversion.
Dian Luo is a PhD student at Anhui Normal University. His current interests focus on the synthesis of nanocrystals and their applications in energy conversion.
Peng Wu is a Master student at the College of Chemistry and Materials Science, Anhui Normal University. His current interest focuses on the theoretical computation of materials for catalysis.
Yucheng Huang received his PhD degree from Nanjing University. He joined Anhui Normal University in 2005, and now is a professor of the College of Chemistry and Materials Science. His current interests focus on the theoretical computation of materials for catalysis and theoretical studies of the optical, electrical and magnetic properties of functional nanomaterials.
Zhou Lu graduated from the University of California, San Diego in 2007 with a PhD degree. He worked as a postdoctoral researcher at the University of Southern California and the Pacific Northwest National Laboratory from 2007 to 2013, and as a researcher at the Institute of Chemistry, Chinese Academy of Sciences from 2013 to 2019. In 2019, he joined the School of Physics and Electronic Information, Anhui Normal University as a Professor. His research interests include ultrafast spectroscopy, interfacial nonlinear spectroscopy and microscopic reaction kinetics.
Yonghong Ni received his PhD degree from the University of Science and Technology of China in 2001. He worked as a postdoctoral researcher at the National Key Laboratory of Coordination Chemistry, Nanjing University from 2002 to 2004. In 2004, he joined the chemistry faculty at Anhui Normal University. In 2005, he was promoted to professor in chemistry. His research is primarily on the development of functional inorganic micro/nano-materials that are both fundamentally important and potentially useful for environmental detection and protection, clean and renewable energy applications.
Supporting Information
40843_2023_2739_MOESM1_ESM.pdf
Manipulating the electronic structure of platinum via alloying with ruthenium to boost photocatalytic selective hydrogenation with water as a proton source
Rights and permissions
About this article
Cite this article
Shi, M., Luo, D., Wu, P. et al. Manipulating the electronic structure of platinum via alloying with ruthenium to boost photocatalytic selective hydrogenation with water as a proton source. Sci. China Mater. 67, 824–832 (2024). https://doi.org/10.1007/s40843-023-2739-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2739-4