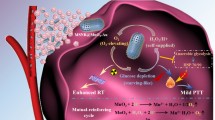
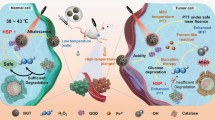
Abstract
Multimodal therapy presents one of the most promising strategies for combining multiple therapies to treat the usually complex and insidious tumor tissue. Although multifunctional nanomaterials have been designed for the construction of multimodal therapies, the generally existing inadequate coordination among components might result in low synergistic therapeutic effects and prevent the realization of their full clinical potential. Herein, inspired by the controllable “cluster bomb” model, we designed an intelligent, biocompatible, and multifunctional nanofactory system (PDA@GOx@MnO2-PEG) that encapsulates a variety of nanoagents to achieve high destruction efficiency against tumor. The stimulus-responsive outer MnO2 acts as the shell of “bomb” triggering the cascade catalytic reaction and forms a self-sustainable ring catalytic chain with glucose oxidase (GOx). Polydopamine (PDA) as a substrate with excellent protein carrying capacity achieves high GOx loading. Meanwhile, its efficient photothermal conversion efficiency exhibits the potential of low-temperature (∼45°C) to further enhance GOx enzymatic activity. Notably, the internal GOx is like a “sub-bomb” that is released in a controlled manner to increase the accumulation at tumor hypoxic sites, and gives full play to its glucose consumption capacity for starvation therapy under the help of sufficient oxygen and low hyperthermia. In this system, various nanoagents cooperate and advance layer by layer to fully exploit their power, forming a self-sufficient nanofactory model, and achieving excellent low-temperature photothermal-starvation synergistic therapy through a synergistic strategy. Moreover, the nanocomposite exhibits trimodal imaging capability for sensitive diagnosis and real-time monitoring of therapy. This study provides new insights for designing biocompatible and intelligent theranostic nanoplatforms to maximize the multi-modal therapeutic effect in precision medicine.

摘要
多模态疗法是结合多种疗法治疗通常复杂而隐蔽的肿瘤组织的最有希望的策略之一. 尽管多功能纳米材料已被设计用于构建多模态疗法, 但普遍存在的各组成部分之间的不充分协调可能导致协同治疗效果不佳, 并妨碍其充分实现临床潜力. 在此, 受可控“集束炸弹”模型的启发, 我们设计了一种智能、 生物相容、 多功能的纳米工厂系统(PDA@GOx@MnO2-PEG), 它封装了多种纳米试剂, 以达到对肿瘤组织的高破坏效率. 刺激反应性的外层二氧化锰作为“炸弹”的外壳可触发级联催化反应, 并与GOx形成一个自给自足的环形催化链. PDA作为一种具有良好蛋白质携带能力的基质, 实现了高的GOx负载. 同时, 其高效的光热转换效率显示了低温(~45°C)进一步提高GOx酶活性的潜力. 值得注意的是, 内部的GOx就像一个“子炸弹”, 通过控制释放来增加肿瘤缺氧部位的积累, 并在充足的氧气和低热度的帮助下充分发挥其葡萄糖消耗能力进行饥饿治疗. 在这个体系中, 各种纳米试剂相互配合, 层层推进, 充分发挥其威力, 形成了一个自给自足的纳米工厂模型, 通过协同策略实现了良好的低温光热-饥饿协同治疗. 此外, 该纳米复合材料表现出三态成像能力, 可用于敏感诊断和实时监控治疗. 这项研究为设计生物相容性和智能治疗纳米平台提供了新的见解, 使精准医疗中的多模式治疗效果最大化.
Similar content being viewed by others
References
Liang S, Deng X, Ma P, et al. Recent advances in nanomaterial-assisted combinational sonodynamic cancer therapy. Adv Mater, 2020, 32: 2003214
Guo X, Yang N, Ji W, et al. Mito-bomb: Targeting mitochondria for cancer therapy. Adv Mater, 2021, 33: 2007778
Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotech, 2007, 2: 751–760
Guo S, Vieweger M, Zhang K, et al. Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nat Commun, 2020, 11: 972
Gao G, Jiang YW, Guo Y, et al. Enzyme-mediated tumor starvation and phototherapy enhance mild-temperature photothermal therapy. Adv Funct Mater, 2020, 30: 1909391
Gholami Derami H, Gupta P, Weng KC, et al. Reversible photothermal modulation of electrical activity of excitable cells using polydopamine nanoparticles. Adv Mater, 2021, 33: 2008809
Chen W, Sun Z, Jiang C, et al. An all-in-one organic semiconductor for targeted photoxidation catalysis in hypoxic tumor. Angew Chem Int Ed, 2021, 60: 16641–16648
He X, Hao Y, Chu B, et al. Redox-activatable photothermal therapy and enzyme-mediated tumor starvation for synergistic cancer therapy. Nano Today, 2021, 39: 101174
Song N, Zhang Z, Liu P, et al. Pillar[5]arene-modified gold nanorods as nanocarriers for multi-modal imaging-guided synergistic photodynamic-photothermal therapy. Adv Funct Mater, 2021, 31: 2009924
Chang M, Wang M, Wang M, et al. A multifunctional cascade bioreactor based on hollow-structured Cu2MoS4 for synergetic cancer chemo-dynamic therapy/starvation therapy/phototherapy/immunotherapy with remarkably enhanced efficacy. Adv Mater, 2019, 31: 1905271
Chen Y, Li ZH, Pan P, et al. Tumor-microenvironment-triggered ion exchange of a metal-organic framework hybrid for multimodal imaging and synergistic therapy of tumors. Adv Mater, 2020, 32: 2001452
Li Z, Hu Y, Miao Z, et al. Dual-stimuli responsive bismuth nanoraspberries for multimodal imaging and combined cancer therapy. Nano Lett, 2018, 18: 6778–6788
Chen L, Gao H, Bai Y, et al. Colorimetric biosensing of glucose in human serum based on the intrinsic oxidase activity of hollow MnO2 nanoparticles. New J Chem, 2020, 44: 15066–15070
Yang X, Yang Y, Gao F, et al. Biomimetic hybrid nanozymes with self-supplied H+ and accelerated O2 generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett, 2019, 19: 4334–4342
He T, Xu H, Zhang Y, et al. Glucose oxidase-instructed traceable self-oxygenation/hyperthermia dually enhanced cancer starvation therapy. Theranostics, 2020, 10: 1544–1554
Lu Z, Gao JY, Fang C, et al. Porous Pt nanospheres incorporated with GOx to enable synergistic oxygen-inductive starvation/electrodynamic tumor therapy. Adv Sci, 2020, 7: 2001223
Jo SM, Wurm FR, Landfester K. Oncolytic nanoreactors producing hydrogen peroxide for oxidative cancer therapy. Nano Lett, 2020, 20: 526–533
Li SY, Cheng H, Xie BR, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano, 2017, 11: 7006–7018
Chang K, Liu Z, Fang X, et al. Enhanced phototherapy by nanoparticleenzyme via generation and photolysis of hydrogen peroxide. Nano Lett, 2017, 17: 4323–4329
Fu LH, Qi C, Lin J, et al. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem Soc Rev, 2018, 47: 6454–6472
Zhang P, Hou Y, Zeng J, et al. Coordinatively unsaturated Fe3+ based activatable probes for enhanced MRI and therapy of tumors. Angew Chem, 2019, 131: 11205–11213
Chang M, Hou Z, Wang M, et al. Single-atom Pd nanozyme for ferroptosis-boosted mild-temperature photothermal therapy. Angew Chem Int Ed, 2021, 60: 12971–12979
Lee DY, Kim JY, Lee Y, et al. Black pigment gallstone inspired platinum-chelated bilirubin nanoparticles for combined photoacoustic imaging and photothermal therapy of cancers. Angew Chem, 2017, 129: 13872–13876
Fan R, Chen C, Hou H, et al. Tumor acidity and near-infrared light responsive dual drug delivery polydopamine-based nanoparticles for chemo-photothermal therapy. Adv Funct Mater, 2021, 31: 2009733
Wang S, Lin J, Wang Z, et al. Core-satellite polydopamine-gadolinium-metallofullerene nanotheranostics for multimodal imaging guided combination cancer therapy. Adv Mater, 2017, 29: 1701013
Gong F, Yang N, Wang X, et al. Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics. Nano Today, 2020, 32: 100851
Wang S, Bai Y, Wang D, et al. Reversing tumor to “hot”: A NIR light-triggered carrier-free nanoplatform for enhanced tumor penetration and photo-induced immunotherapy. Chem Eng J, 2022, 442: 136322
Wang Y, Cui J, Chen J, et al. Novel bone tumor cell targeting nano-system for chemo-photothermal therapy of malignant bone tumors. Chem Eng J, 2022, 446: 136905
Xie M, Dai F, Li J, et al. Tailoring the electronic metal-support interactions in supported atomically dispersed gold catalysts for efficient Fenton-like reaction. Angew Chem Int Ed, 2021, 60: 14370–14375
Pellico J, Gawne PJ, T. M. de Rosales R. Radiolabelling of nanomaterials for medical imaging and therapy. Chem Soc Rev, 2021, 50: 3355–3423
Yang Y, Yang T, Chen F, et al. Degradable magnetic nanoplatform with hydroxide ions triggered photoacoustic, MR imaging, and photothermal conversion for precise cancer theranostic. Nano Lett, 2022, 22: 3228–3235
Yin SY, Song G, Yang Y, et al. Persistent regulation of tumor microenvironment via circulating catalysis of MnFe2O4@metal-organic frameworks for enhanced photodynamic therapy. Adv Funct Mater, 2019, 29: 1901417
Shi L, Wang Y, Zhang C, et al. An acidity-unlocked magnetic nanoplatform enables self-boosting ROS generation through upregulation of lactate for imaging-guided highly specific chemodynamic therapy. Angew Chem Int Ed, 2021, 60: 9562–9572
Teng L, Han X, Liu Y, et al. Smart nanozyme platform with activity-correlated ratiometric molecular imaging for predicting therapeutic effects. Angew Chem Int Ed, 2021, 60: 26142–26150
Lu C, Zhang C, Wang P, et al. Light-free generation of singlet oxygen through manganese-thiophene nanosystems for pH-responsive chemiluminescence imaging and tumor therapy. Chem, 2020, 6: 2314–2334
Liu Y, Ai K, Liu J, et al. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater, 2013, 25: 1353–1359
Tao W, Ji X, Xu X, et al. Antimonene quantum dots: Synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew Chem Int Ed, 2017, 56: 11896–11900
Ren W, Yan Y, Zeng L, et al. A near infrared light triggered hydrogenated black TiO2 for cancer photothermal therapy. Adv Healthcare Mater, 2015, 4: 1526–1536
Guo B, Sheng Z, Hu D, et al. Through scalp and skull NIR-II photothermal therapy of deep orthotopic brain tumors with precise photoacoustic imaging guidance. Adv Mater, 2018, 30: 1802591
Pu Y, Yin H, Dong C, et al. Sono-controllable and ROS-sensitive CRISPR-Cas9 genome editing for augmented/synergistic ultrasound tumor nanotherapy. Adv Mater, 2021, 33: 2104641
Yang G, Xu L, Chao Y, et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun, 2017, 8: 902
He T, Jiang C, He J, et al. Manganese-dioxide-coating-instructed plasmonic modulation of gold nanorods for activatable duplex-imaging-guided NIR-II photothermal-chemodynamic therapy. Adv Mater, 2021, 33: 2008540
Fu LH, Wan Y, Qi C, et al. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv Mater, 2021, 33: 2006892
Ma Z, Jia X, Bai J, et al. MnO2 gatekeeper: An intelligent and O2-evolving shell for preventing premature release of high cargo payload core, overcoming tumor hypoxia, and acidic H2O2-sensitive MRI. Adv Funct Mater, 2017, 27: 1604258
Feng L, Xie R, Wang C, et al. Magnetic targeting, tumor microenvironment-responsive intelligent nanocatalysts for enhanced tumor ablation. ACS Nano, 2018, 12: 11000–11012
Wang Y, Li Y, Zhang Z, et al. Triple-jump photodynamic theranostics: MnO2 combined upconversion nanoplatforms involving a type-I photosensitizer with aggregation-induced emission characteristics for potent cancer treatment. Adv Mater, 2021, 33: 2103748
Zhou J, Li M, Hou Y, et al. Engineering of a nanosized biocatalyst for combined tumor starvation and low-temperature photothermal therapy. ACS Nano, 2018, 12: 2858–2872
You Q, Zhang K, Liu J, et al. Persistent regulation of tumor hypoxia microenvironment via a bioinspired Pt-based oxygen nanogenerator for multimodal imaging-guided synergistic phototherapy. Adv Sci, 2020, 7: 1903341
Gao G, Sun X, Liang G. Nanoagent-promoted mild-temperature photothermal therapy for cancer treatment. Adv Funct Mater, 2021, 31: 2100738
Bao YW, Hua XW, Zeng J, et al. Bacterial template synthesis of multifunctional nanospindles for glutathione detection and enhanced cancer-specific chemo-chemodynamic therapy. Research, 2020, 2020: 2020/9301215
Lin X, Zhu R, Hong Z, et al. GSH-responsive radiosensitizers with deep penetration ability for multimodal imaging-guided synergistic radiochemodynamic cancer therapy. Adv Funct Mater, 2021, 31: 2101278
Meng X, Yang F, Dong H, et al. Recent advances in optical imaging of biomarkers in vivo. Nano Today, 2021, 38: 101156
Luo Z, Hu D, Gao D, et al. High-specificity in vivo tumor imaging using bioorthogonal NIR-IIb nanoparticles. Adv Mater, 2021, 33: 2102950
Dong S, Dong Y, Jia T, et al. GSH-depleted nanozymes with hyperthermia-enhanced dual enzyme-mimic activities for tumor nanocatalytic therapy. Adv Mater, 2020, 32: 2002439
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21705117, 21904095, 22001193, and 22174110), the Elite Scholar Program of Tianjin University (2019XRG-0065), the Program of Tianjin Science and Technology Major Project and Engineering (19ZXYXSY00090), the Program for Chang Jiang Scholars and Innovative Research Team, Ministry of Education, China (IRT-16R61), and the Special Fund Project for the Central Government to Guide Local Science and Technology Development (2020).
Author information
Authors and Affiliations
Contributions
Author contributions Lu X and Zhang Z initiated and supervised the project. Chen M performed the cell culture studies. Dai F designed the experiments, collected the data and wrote this paper. Xie M analyzed the experimental data. Du P commented on the manuscript. All authors contributed to the general discussion.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Experimental details and supporting data are available in the online version of the paper.
Fangfang Dai received her BSc degree from the College of Science, Tianjin Normal University. She obtained her MSc and PhD degrees at Tianjin University under the supervision of Prof. Lu. Her research focuses on the design and fabrication of functional nanomaterials and related applications.
Zhen Zhang obtained his PhD degree from the Institute of Chemistry, Chinese Academy of Sciences in 2017, after which he joined Tianjin University as a lecturer in 2017. He was a visiting scholar in the laboratory of Prof. Yadong Yin at the University of California (Riverside) in 2019–2020. His scientific interest is mainly focused on the design and fabrication of functional nanomaterials and related applications.
Meiling Chen graduated from Liaoning Normal University majoring in chemistry in 2008. She received her PhD degree in analytical chemistry from Northeastern University in 2013. Her scientific interest is carbon nanomaterials and related analytical applications in life sciences.
Xiaoquan Lu received his BSc and MSc degrees from the Department of Chemistry, Northwest Normal University in 1994, and his PhD degree from Sun Yat-sen University in 1997. He was a visiting scholar at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences in 2001. His research interests are bioelectrochemistry, visual sensing, and new energy development.
Supporting Information
40843_2023_2583_MOESM1_ESM.pdf
Mutually reinforcing nanofactory mimicking controllable “cluster bomb” for synergistic diagnosis and treatment of hypoxic cancer
Rights and permissions
About this article
Cite this article
Dai, F., Xie, M., Du, P. et al. Mutually reinforcing nanofactory mimicking controllable “cluster bomb” for synergistic diagnosis and treatment of hypoxic cancer. Sci. China Mater. 66, 4499–4511 (2023). https://doi.org/10.1007/s40843-023-2583-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2583-5