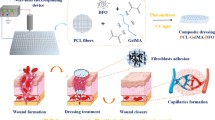
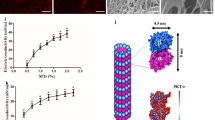
Abstract
Diabetic wounds are hard-healing chronic wounds, mainly caused by wound infection, excessive inflammation, diabetic neuropathy, and peripheral vascular disease. Hence, comprehensive improvement of diabetic wound healing is of great significance in clinical practice. However, the current research on diabetic wounds mainly focuses on wound infection and angiogenesis, lacking the exploration of neuroregeneration and immunomodulation. In this study, we develop a multifunctional conductive hydrogel (PACPH) based on polydopamine-modified silver nanoparticles (AgNPs@PDA), cellulose nanocrystals (CNC)/polypyrrole (PPy) composites, and polyvinyl alcohol as an efficient wound dressing. The PACPH scaffold features multiple functions, including desirable mechanical properties, tissue adhesion, conductivity, and broad-spectrum antibacterial activity. Inspired by the endogenous electric field, the strategy of combining hydrogel with electrical stimulation (ES) is also proposed to accelerate the healing of diabetic wounds. Notably, the participation of ES can effectively promote nerve repair, realize the polarization of macrophages toward the M2 phenotype, and rapid angiogenesis, comprehensively improving the healing of diabetic wounds. This advanced collaborative strategy opens a new avenue in treating diabetic wounds.

摘要
糖尿病创面是难以愈合的慢性创面, 主要由创面感染、 过度炎症、 糖尿病神经病变、 外周血管病变等引起. 因此, 全面改善糖尿病创面愈合在临床上具有重要意义. 然而, 目前对糖尿病创面的研究主要集中在创面感染和血管生成方面, 缺乏对神经再生和免疫调节的探索. 在这项研究中, 我们开发了多功能导电水凝胶作为一种有效的伤口敷料. 该水凝胶具有多种功能, 包括优异的机械性能、 组织粘附性、 导电性和广谱抗菌活性. 受内源性电场的启发, 我们提出了将水凝胶与电刺激相结合的策略来加速糖尿病伤口的愈合. 值得注意的是, 电刺激结合导电水凝胶的治疗手段可有效促进神经修复, 实现巨噬细胞向M2表型的极化以及快速血管生成, 从而全面改善糖尿病创面的愈合. 这种先进的协作策略为治疗糖尿病伤口开辟了一种新途径.
Similar content being viewed by others
References
Clark RAF, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. J Investig Dermatol, 2007, 127: 1018–1029
Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv Drug Deliver Rev, 2019, 146: 344–365
Han G, Ceilley R. Chronic wound healing: A review of current management and treatments. Adv Ther, 2017, 34: 599–610
Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care, 2015, 4: 560–582
Wong SL, Demers M, Martinod K, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med, 2015, 21: 815–819
Falanga V. Wound healing and its impairment in the diabetic foot. Lancet, 2005, 366: 1736–1743
Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med, 2006, 23: 594–608
Patel S, Srivastava S, Singh MR, et al. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother, 2019, 112: 108615
Zhao X, Liang Y, Huang Y, et al. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv Funct Mater, 2020, 30: 1910748
Qu J, Zhao X, Liang Y, et al. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J, 2019, 362: 548–560
Wang M, Wang C, Chen M, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano, 2019, 13: 10279–10293
Zhang J, Zheng Y, Lee J, et al. A pulsatile release platform based on photo-induced imine-crosslinking hydrogel promotes scarless wound healing. Nat Commun, 2021, 12: 1670
Zhao Y, Song S, Ren X, et al. Supramolecular adhesive hydrogels for tissue engineering applications. Chem Rev, 2022, 122: 5604–5640
Chen J, He J, Yang Y, et al. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater, 2022, 146: 119–130
Wang K, Dong R, Tang J, et al. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem Eng J, 2022, 430: 132664
Liang Y, Li M, Yang Y, et al. pH/glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot wound healing. ACS Nano, 2022, 16: 3194–3207
Gao G, Jiang YW, Jia HR, et al. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials, 2019, 188: 83–95
Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med, 2014, 6: 265sr6
Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature, 2008, 453: 314–321
Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity, 2016, 44: 450–462
Song B, Gu Y, Pu J, et al. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat Protoc, 2007, 2: 1479–1489
Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature, 2006, 442: 457–460
Wang J, Lin J, Chen L, et al. Endogenous electric-field-coupled electrospun short fiber via collecting wound exudation. Adv Mater, 2022, 34: 2108325
Thrivikraman G, Boda SK, Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials, 2018, 150: 60–86
Long Y, Wei H, Li J, et al. Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators. ACS Nano, 2018, 12: 12533–12540
Luo R, Dai J, Zhang J, et al. Accelerated skin wound healing by electrical stimulation. Adv Healthc Mater, 2021, 10: 2100557
Yao G, Mo X, Yin C, et al. A programmable and skin temperature-activated electromechanical synergistic dressing for effective wound healing. Sci Adv, 2022, 8: eabl8379
Kai H, Yamauchi T, Ogawa Y, et al. Accelerated wound healing on skin by electrical stimulation with a bioelectric plaster. Adv Healthc Mater, 2017, 6: 1700465
Kargarzadeh H, Mariano M, Gopakumar D, et al. Advances in cellulose nanomaterials. Cellulose, 2018, 25: 2151–2189
Tang J, Sisler J, Grishkewich N, et al. Functionalization of cellulose nanocrystals for advanced applications. J Colloid Interface Sci, 2017, 494: 397–409
Yang X, Biswas SK, Han J, et al. Surface and interface engineering for nanocellulosic advanced materials. Adv Mater, 2021, 33: 2002264
Lagerwall JPF, Schütz C, Salajkova M, et al. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater, 2014, 6: e80
Shi Z, Phillips GO, Yang G. Nanocellulose electroconductive composites. Nanoscale, 2013, 5: 3194–3201
Wang S, Zheng H, Zhou L, et al. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett, 2020, 20: 5149–5158
Zhou L, Zheng H, Liu Z, et al. Conductive antibacterial hemostatic multifunctional scaffolds based on Ti3C2Tx MXene nanosheets for promoting multidrug-resistant bacteria-infected wound healing. ACS Nano, 2021, 15: 2468–2480
Mao C, Xiang Y, Liu X, et al. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ ZnO nanostructures. ACS Nano, 2017, 11: 9010–9021
Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol, 2015, 173: 370–378
Cuttle L, Nataatmadja M, Fraser JF, et al. Collagen in the scarless fetal skin wound: Detection with picrosirius-polarization. Wound Repair Regen, 2005, 13: 198–204
Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Transl Med, 2021, 13: eabe4839
Alavi A, Sibbald RG, Mayer D, et al. Diabetic foot ulcers. J Am Acad Dermatol, 2014, 70: 1.e1–1.e18
Sawaya AP, Stone RC, Brooks SR, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun, 2020, 11: 4678
Nowak NC, Menichella DM, Miller R, et al. Cutaneous innervation in impaired diabetic wound healing. Transl Res, 2021, 236: 87–108
Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol, 2014, 5: 614
Nassiri S, Zakeri I, Weingarten MS, et al. Relative expression of proinflammatory and antiinflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. J Investig Dermatol, 2015, 135: 1700–1703
Okizaki S, Ito Y, Hosono K, et al. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed Pharmac-other, 2015, 70: 317–325
Siqueira MF, Li J, Chehab L, et al. Impaired wound healing in mouse models of diabetes is mediated by TNF-α dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetologia, 2010, 53: 378–388
Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev, 2003, 23: 117–145
Qian Z, Wang H, Bai Y, et al. Improving chronic diabetic wound healing through an injectable and self-healing hydrogel with platelet-rich plasma release. ACS Appl Mater Interfaces, 2020, 12: 55659–55674
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81901365 and 82003284), the Department of Finance of Jilin Province (2019SRCJ005 and 2020SCZT051), Jilin Science and Technology Agency Funds in China (20210402001GH), the Key Research and Development Project of Jilin Provincial Science and Technology Department (20210204142YY), and the Norman Bethune Program of Jilin University (2022B44).
Author information
Authors and Affiliations
Contributions
Author contributions Guan L designed the research ideas and wrote the article. Ou X performed the animal experiment. Wang Z, Li X, Feng Y, and Yang X participated in the data analysis. Qu W, Yang B, and Lin Q conceived and guided the program. All authors contributed to the general discussion.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Experimental details and supporting data are available in the online version of the paper.
Lin Guan is currently a PhD candidate at the State Key Laboratory of Supramolecules, Jilin University. Her current research focuses on the design of adhesive hydrogel materials as well as their applications for diabetic wound dressing.
Wenrui Qu is a deputy chief physician at the Department of Hand Surgery, the Second Hospital of Jilin University, a doctor of medicine, and a postgraduate tutor. He received his PhD degree from Jilin University and worked as a visiting scholar at the School of Medicine, Indiana University-Purdue University Indianapolis, from 2013 to 2016. His research focuses on mechanisms of neuroprotection, nerve regeneration and diabetic neuropathy and unique repair strategies to promote healing of diabetic wound.
Quan Lin is a professor at the State Key Laboratory of Supramolecules, Jilin University. His current research focuses on the application of polymer hydrogels in medical dressings, flexible electronics, and energy storage.
Supporting information
40843_2022_2242_MOESM1_ESM.pdf
Electrical stimulation-based conductive hydrogel for immunoregulation, neuroregeneration and rapid angiogenesis in diabetic wound repair
Rights and permissions
About this article
Cite this article
Guan, L., Ou, X., Wang, Z. et al. Electrical stimulation-based conductive hydrogel for immunoregulation, neuroregeneration and rapid angiogenesis in diabetic wound repair. Sci. China Mater. 66, 1237–1248 (2023). https://doi.org/10.1007/s40843-022-2242-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2242-y