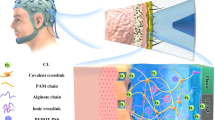
Abstract
Signal drift and performance instability of brain-computer interface devices induced by the interface failure between rigid metal electrodes and soft human skin hinder the precise data acquisition of electroencephalogram (EEG). Thus, it is desirable to achieve a robust interface for brain-computer interface devices. Here, a kind of polydopamine methacrylamide-polyacrylamide (PDMA-PAAM) hydrogel is developed. To improve the adhesion, dopamine is introduced into the polyacrylamide hydrogel, through the amino and catechol groups of dopamine in an organic-inorganic interface to build a covalent and non-covalent interaction. A strong attachment and an effective modulus transition system can be formed between the metal electrodes and human skin, so that the peeling force between the PDMA-PAAM hydrogel and the porcine skin can reach 22 N m−1. In addition, the stable conductivity and long-term operating life of the PDMA-PAAM hydrogel for more than 60 days at room temperature are achieved by adding sodium chloride (NaCl) and glycerol, respectively. The PDMA-PAAM hydrogel membrane fabricated in this work is integrated onto a flexible Au electrode applied in a brain-computer interface. In comparison, the collected EEG signal intensity and waveform are consistent with that of the commercial counterparts. And obviously, the flexible electrode with PDMA-PAAM hydrogel membrane is demonstrated to enable a more stable and user-friendly interface.

摘要
硬质金属电极与柔软人体皮肤之间的界面失效会导致脑机接口器件的信号漂移和性能不稳定, 影响脑电信号的精确采集. 本研究设计并制备了一种聚多巴胺甲基丙烯酰胺-聚丙烯酰胺(PDMA-PAAM)水凝胶. 将多巴胺引入到聚丙烯酰胺水凝胶中, 通过多巴胺的氨基与邻苯二酚基团在有机-无机界面上建立共价和非共价相互作用提高了界面粘结力. 金属电极与人体皮肤之间形成了强附着和有效模量过渡体系, PDMA-PAAM水凝胶与猪皮之间的剥离力达到22 N m−1. 此外, 通过添加氯化钠和甘油, PDMA-PAAM水凝胶具有稳定的电导率和室温下60天以上的长期使用寿命. 将本研究制备的PDMA-PAAM水凝胶集成在柔性金电极上用于脑机接口, 采集到的脑电信号强度和波形与同类商用产品基本一致, 且PDMA-PAAM水凝胶柔性电极具有更稳定的界面.
Similar content being viewed by others
References
Nelson PG, Brenneman DE. Electrical activity of neurons and development of the brain. Trends Neuroscis, 1982, 5: 229–232
Alivisatos AP, Andrews AM, Boyden ES, et al. Nanotools for neuroscience and brain activity mapping. ACS Nano, 2013, 7: 1850–1866
Ma Y, Zhang Y, Cai S, et al. Flexible hybrid electronics for digital healthcare. Adv Mater, 2020, 32: 1902062
Minev IR, Musienko P, Hirsch A, et al. Electronic dura mater for long-term multimodal neural interfaces. Science, 2015, 347: 159–163
Rios G, Lubenov EV, Chi D, et al. Nanofabricated neural probes for dense 3-D recordings of brain activity. Nano Lett, 2016, 16: 6857–6862
Wang J, Thow XY, Wang H, et al. A highly selective 3D spiked ultraflexible neural (SUN) interface for decoding peripheral nerve sensory information. Adv Healthcare Mater, 2017, 7: 1700987
Lee B, Koripalli MK, Jia Y, et al. An implantable peripheral nerve recording and stimulation system for experiments on freely moving animal subjects. Sci Rep, 2018, 8: 6115
Yang J, Liu L, Li T, et al. Array focal cortical stimulation enhances motor function recovery and brain remodeling in a rat model of ischemia. J Stroke Cerebrovasc Dis, 2017, 26: 658–665
Nam J, Lim HK, Kim NH, et al. Supramolecular peptide hydrogel-based soft neural interface augments brain signals through a three-dimensional electrical network. ACS Nano, 2020, 14: 664–675
Grill WM, Norman SE, Bellamkonda RV. Implanted neural interfaces: Biochallenges and engineered solutions. Annu Rev Biomed Eng, 2009, 11: 1–24
Bettinger CJ. Recent advances in materials and flexible electronics for peripheral nerve interfaces. Bioelectron Med, 2018, 4: 1
Won SM, Song E, Zhao J, et al. Recent advances in materials, devices, and systems for neural interfaces. Adv Mater, 2018, 30: 1800534
Lee M, Shim HJ, Choi C, et al. Soft high-resolution neural interfacing probes: Materials and design approaches. Nano Lett, 2019, 19: 2741–2749
Li H, Liu H, Sun M, et al. 3D interfacing between soft electronic tools and complex biological tissues. Adv Mater, 2021, 33: 2004425
Pedrosa P, Fiedler P, Schinaia L, et al. Alginate-based hydrogels as an alternative to electrolytic gels for rapid EEG monitoring and easy cleaning procedures. Sens Actuat B-Chem, 2017, 247: 273–283
Chen Y, Liu F, Lu B, et al. Skin-like hybrid integrated circuits conformal to face for continuous respiratory monitoring. Adv Electron Mater, 2020, 6: 2000145
Debener S, Emkes R, De Vos M, et al. Unobtrusive ambulatory EEG using a smartphone and flexible printed electrodes around the ear. Sci Rep, 2015, 5: 16743
Valentin O, Viallet G, Delnavaz A, et al. Custom-fitted in- and around-the-ear sensors for unobtrusive and on-the-go EEG acquisitions: Development and validation. Sensors, 2021, 21: 2953
Jiang X, Bian GB, Tian Z. Removal of artifacts from EEG signals: A review. Sensors, 2019, 19: 987
Frankel MA, Lehmkuhle MJ, Watson M, et al. Electrographic seizure monitoring with a novel, wireless, single-channel EEG sensor. Clin Neurophysiol Pract, 2021, 6: 172–178
Bleichner MG, Debener S. Concealed, unobtrusive ear-centered EEG acquisition: cEEGrids for transparent EEG. Front Hum Neurosci, 2017, 11: 163
Bleichner MG, Mirkovic B, Debener S. Identifying auditory attention with ear-EEG: cEEGrid versus high-density cap-EEG comparison. J Neural Eng, 2016, 13: 066004
Sterr A, Ebajemito JK, Mikkelsen KB, et al. Sleep EEG derived from behind-the-ear electrodes (cEEGrid) compared to standard polysomnography: A proof of concept study. Front Hum Neurosci, 2018, 12: 452
Sheng H, Wang X, Kong N, et al. Neural interfaces by hydrogels. Extreme Mech Lett, 2019, 30: 100510
Yuk H, Lu B, Lin S, et al. 3D printing of conducting polymers. Nat Commun, 2020, 11: 1604
Wong TH, Yiu CK, Zhou J, et al. Tattoo-like epidermal electronics as skin sensors for human machine interfaces. Soft Sci, 2021, 1: 10
Oribe S, Yoshida S, Kusama S, et al. Hydrogel-based organic subdural electrode with high conformability to brain surface. Sci Rep, 2019, 9: 13379
Wirthl D, Pichler R, Drack M, et al. Instant tough bonding of hydrogels for soft machines and electronics. Sci Adv, 2017, 3: e1700053
Kim SH, Jung S, Yoon IS, et al. Ultrastretchable conductor fabricated on skin-like hydrogel-elastomer hybrid substrates for skin electronics. Adv Mater, 2018, 30: 1800109
Zhu J, Zhou C, Zhang M. Recent progress in flexible tactile sensor systems: From design to application. SS, 2021, 1: 3
Lim C, Hong YJ, Jung J, et al. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci Adv, 2021, 7: eabd3716
Wellman SM, Eles JR, Ludwig KA, et al. A materials roadmap to functional neural interface design. Adv Funct Mater, 2017, 28: 1701269
Ge W, Cao S, Yang Y, et al. Nanocellulose/LiCl systems enable conductive and stretchable electrolyte hydrogels with tolerance to dehydration and extreme cold conditions. Chem Eng J, 2021, 408: 127306
Pan X, Wang Q, Ning D, et al. Ultraflexible self-healing guar gum-glycerol hydrogel with injectable, antifreeze, and strain-sensitive properties. ACS Biomater Sci Eng, 2018, 4: 3397–3404
Peng S, Liu S, Sun Y, et al. Facile preparation and characterization of poly(vinyl alcohol)-NaCl-glycerol supramolecular hydrogel electrolyte. Eur Polym J, 2018, 106: 206–213
Zhang Q, Liu X, Duan L, et al. Ultra-stretchable wearable strain sensors based on skin-inspired adhesive, tough and conductive hydrogels. Chem Eng J, 2019, 365: 10–19
Shao C, Wang M, Meng L, et al. Mussel-inspired cellulose nanocomposite tough hydrogels with synergistic self-healing, adhesive, and strain-sensitive properties. Chem Mater, 2018, 30: 3110–3121
He X, Liu L, Han H, et al. Bioinspired and microgel-tackified adhesive hydrogel with rapid self-healing and high stretchability. Macromolecules, 2018, 52: 72–80
Han L, Liu K, Wang M, et al. Mussel-inspired adhesive and conductive hydrogel with long-lasting moisture and extreme temperature tolerance. Adv Funct Mater, 2017, 28: 1704195
Wu L, Li L, Qu M, et al. Mussel-inspired self-adhesive, antidrying, and antifreezing poly(acrylic acid)/bentonite/polydopamine hybrid glycerol-hydrogel and the sensing application. ACS Appl Polym Mater, 2020, 2: 3094–3106
Yan Y, Huang J, Qiu X, et al. An ultra-stretchable glycerol-ionic hybrid hydrogel with reversible gelid adhesion. J Colloid Interface Sci, 2021, 582: 187–200
Zhu HW, Zhang JN, Su P, et al. Strong adhesion of poly(vinyl alcohol)-glycerol hydrogels onto metal substrates for marine antifouling applications. Soft Matter, 2020, 16: 709–717
Dreyer DR, Miller DJ, Freeman BD, et al. Elucidating the structure of poly(dopamine). Langmuir, 2012, 28: 6428–6435
Liebscher J, Mrówczyński R, Scheidt HA, et al. Structure of polydopamine: A never-ending story? Langmuir, 2013, 29: 10539–10548
Liu Y, Ai K, Lu L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev, 2014, 114: 5057–5115
Han L, Lu X, Wang M, et al. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small, 2017, 13: 1601916
Yu QH, Zhang CM, Jiang ZW, et al. Mussel-inspired adhesive polydopamine-functionalized hyaluronic acid hydrogel with potential bacterial inhibition. Glob Challenges, 2020, 4: 1900068
Han L, Wang M, Li P, et al. Mussel-inspired tissue-adhesive hydrogel based on the polydopamine-chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl Mater Interfaces, 2018, 10: 28015–28026
Yang Z, Huang R, Zheng B, et al. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv Sci, 2021, 8: 2003627
Han L, Lu X, Liu K, et al. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano, 2017, 11: 2561–2574
Wang C, Zhao N, Yuan W. NIR/thermoresponsive injectable self-healing hydrogels containing polydopamine nanoparticles for efficient synergistic cancer thermochemotherapy. ACS Appl Mater Interfaces, 2020, 12: 9118–9131
Kaveh R, Doong J, Zhou A, et al. Wireless user-generic ear EEG. IEEE Trans Biomed Circuits Syst, 2020, 14: 727–737
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U20A6001, 11921002, and 11902292), Zhejiang Province Key Research and Development Project (2021C01183, 2020C05004, and 2021C05007-4), and the Natural Science Foundation of Zhejiang Province of China (LQ19E030003).
Author information
Authors and Affiliations
Contributions
Author contributions Liu L and Liu Y designed the samples, performed the experiments and analyzed the data; Liu L wrote the paper with support from Tang R, Ai J and Ma Y; Chen Y and Feng X helped to analyze the data and conceived the framework of this paper. All authors contributed to the general discussion.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper.
Lanlan Liu received her PhD degree in chemistry from Beijing University of Chemical Technology in 2014, and then worked as a postdoc at the Institute of Chemistry, Chinese Academy of Sciences during 2014 to 2017. Currently, she is an associate researcher at the Institute of Flexible Electronics Technology of Tsinghua University (THU), Zhejiang. Her research focuses on flexible conductive materials, interfaces and devices, and their applications in the biomedical field.
Ying Chen received her PhD degree in solid mechanics from THU, Beijing in 2017. Currently, she is an associate researcher at the Institute of Flexible Electronics Technology of THU, Zhejiang and doing her postdoctoral research at Zhejiang University. She is working as the deputy director of Soft Matter Mechanics Committee of Zhejiang Society of Theoretical and Applied Mechanics. Her research interests include the mechanical design, MEMS fabrication and performance reliability analysis of flexible electronic devices.
Xue Feng received his PhD degree in mechanics from THU in 2003 and then worked as a postdoc at the University of Illinois at Urbana-Champaign during 2004 to 2007. From 2005 to 2006, he also worked as a postdoc at California Institute of Technology (Caltech). He joined THU in 2007. He is now a tenure professor of the School of Aerospace Engineering, THU, and he also serves as the director of the Ministry of Education Key Laboratory of Applied Mechanics of THU. His research focuses on the fundamental and applied science in flexible electronic technology, including the mechanics design and inorganic materials enabled integrated flexible devices.
Rights and permissions
About this article
Cite this article
Liu, L., Liu, Y., Tang, R. et al. Stable and low-resistance polydopamine methacrylamide-polyacrylamide hydrogel for brain-computer interface. Sci. China Mater. 65, 2298–2308 (2022). https://doi.org/10.1007/s40843-022-2145-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2145-3