Abstract
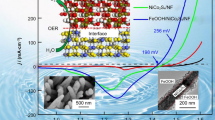
In this work, we demonstrated the enhanced oxygen evolution reaction (OER) activity of flower-shaped cobalt-nickel oxide (NiCo2O4) decorated with iridium-nickel bimetal as an electrode material. The samples were prepared by carefully depositing pre-synthesized IrNi nanoparticles on the surfaces of the NiCo2O4 nano-flowers. Compared with bare NiCo2O4, IrNi, and IrNi/Co3O4, the IrNi/NiCo2O4 exhibited significantly enhanced electrocatalytic activity in the OER, including a lower overpotential of 210 mV and a higher current density at an overpotential of 540 mV. We found that the IrNi/NiCo2O4 showed more efficient electron transport behavior and reduced polarization because of its bimetal IrNi modification by analyzing its Tafel slope and turnover frequency. Furthermore, the electrocatalytic mechanism of IrNi/NiCo2O4 in the OER was studied, and it was found that the combined active sites of the composite effectively improved the rate determining step. The synergic effect of the bimetal and metal oxide plays an important role in this reaction, enhancing the transmission efficiency of electrons and providing more active sites for the OER. The results reveal that IrNi/NiCo2O4 is an excellent electrocatalyst for OER.
摘要
本文制备了一种双金属IrNi修饰的花状NiCo2O4复合材料, 并研究了其对于氧析出反应的电化学活性, 结果显示其电化学活性明显提升. NiCo2O4和IrNi分别通过水热法和热分解法制备, 再通过超声复合, 使得双金属附着在复合氧化物表面. 通过与纯NiCo2O4, IrNi以及IrNi/Co3O4相比较, 所制备的IrNi/NiCo2O4对于氧析出反应的性能最为优异. 在各个参数指标中, 拥有最低的过电势210 mV, 在540 mV的过电势下具有最高的电流密度. 电子转移数和塔菲尔斜率分析表明该复合材料由于修饰上了双金属材料, 极大地降低了极化, 具有更高效的电子转移速率. 此外本文还对电催化机理进行了研究, 发现复合材料结合反应位点有效改善了反应速率决定步骤. 其中, 协同效应起着至关重要的作用, 这一效应明显提高电子传输效率的同时提供了更多的活性位点. IrNi/NiCo2O4是一种出色的氧析出反应电催化剂.
Similar content being viewed by others
References
Wang J, Cui W, Liu Q, et al. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv Mater, 2016, 28: 215–230
Ehteshami SMM, Vignesh S, Rasheed RKA, et al. Numerical investigations on ethanol electrolysis for production of pure hydrogen from renewable sources. Appl Energy, 2016, 170: 388–393
Koper MTM. Hydrogen electrocatalysis: a basic solution. Nat Chem, 2013, 5: 255–256
Norskov JK, Rossmeisl J, Logadottir A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B, 2004, 108: 17886–17892
Chen S, Zhao Y, Sun B, et al. Microwave-assisted synthesis ofmesoporous Co3O4 nanoflakes for applications in lithium ion batteries and oxygen evolution reactions. ACS Appl Mater Interfaces, 2015, 7: 3306–3313
Irshad A, Munichandraiah N. High catalytic activity of amorphous Ir-Pi for oxygen evolution reaction. ACS Appl Mater Interfaces, 2015, 7: 15765–15776
Jeon HS, Jee MS, Kim H, et al. Simple chemical solution deposition of Co3O4 thin film electrocatalyst for oxygen evolution reaction. ACS Appl Mater Interfaces, 2015, 7: 24550–24555
Masud J, Swesi AT, Liyanage WPR, et al. Cobalt selenide nanostructures: an efficient bifunctional catalyst with high current density at low coverage. ACS Appl Mater Interfaces, 2016, 8: 17292–17302
Sun W, Song Y, Gong XQ, et al. Hollandite structure K x≈0.25IrO2 catalyst with highly efficient oxygen evolution reaction. ACS Appl Mater Interfaces, 2016, 8: 820–826
Reier T, Oezaslan M, Strasser P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal, 2012, 2: 1765–1772
Lee Y, Suntivich J, May KJ, et al. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J Phys Chem Lett, 2012, 3: 399–404
Li Y, Zhang L, Xiang X, et al. Engineering of ZnCo-layered double hydroxide nanowalls toward high-efficiency electrochemical water oxidation. J Mater Chem A, 2014, 2: 13250–13258
Wang D, Chen X, Evans DG, et al. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale, 2013, 5: 5312–5315
Yeo BS, Bell AT. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. JAmChem Soc, 2011, 133: 5587–5593
Liang Y, Li Y, Wang H, et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat Mater, 2011, 10: 780–786
Elumalai P, Vasan HN, Munichandraiah N. Electrochemical studies of cobalt hydroxide—an additive for nickel electrodes. J Power Sources, 2001, 93: 201–208
Gong M, Dai H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res, 2015, 8: 23–39
Wang HY, Hsu YY, Chen R, et al. Ni3+-induced formation of active NiOOH on the spinel Ni-Co oxide surface for efficient oxygen evolution reaction. Adv Energy Mater, 2015, 5: 1500091
Friebel D, Louie MW, Bajdich M, et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting. J Am Chem Soc, 2015, 137: 1305–1313
Kinoshita K. Electrochemical Oxygen Technology. New York: John Wiley & Sons, 1992, 30
Lyons MEG, Brandon MP. A comparative study of the oxygen evolution reaction on oxidised nickel, cobalt and iron electrodes in base. J Electroanal Chem, 2010, 641: 119–130
Meng Y, Wang K, Zhang Y, et al. Hierarchical porous graphene/polyaniline composite film with superior rate performance for flexible supercapacitors. Adv Mater, 2013, 25: 6985–6990
Han F, Li WC, Li MR, et al. Fabrication of superior-performance SnO2@C composites for lithium-ion anodes using tubular mesoporous carbon with thin carbon walls and high pore volume. J Mater Chem, 2012, 22: 9645–9651
Wang X, Han X, Lim M, et al. Nickel cobalt oxide-single wall carbon nanotube composite material for superior cycling stability and high-performance supercapacitor application. J Phys Chem C, 2012, 116: 12448–12454
Xia XH, Tu JP, Zhang YQ, et al. Three-dimentional porous nano-Ni/Co(OH)2 nanoflake composite film: a pseudocapacitive material with superior performance. J Phys Chem C, 2011, 115: 22662–22668
Chatterjee S, Nafezarefi F, Tai NH, et al. Size and synergy effects of nanofiller hybrids including graphene nanoplatelets and carbon nanotubes in mechanical properties of epoxy composites. Carbon, 2012, 50: 5380–5386
Zeng Y, Liu P, Du J, et al. Increasing the electrical conductivity of carbon nanotube/polymer composites by using weak nanotube–polymer interactions. Carbon, 2010, 48: 3551–3558
Chen B, Zhu Z, Guo Y, et al. Facile synthesis ofmesoporous Ce–Fe bimetal oxide and its enhanced adsorption of arsenate from aqueous solutions. J Colloid Interface Sci, 2013, 398: 142–151
Lee JP, Chen D, Li X, et al. Well-organized raspberry-like Ag@Cu bimetal nanoparticles for highly reliable and reproducible surfaceenhanced Raman scattering. Nanoscale, 2013, 5: 11620–11624
Mondal AK, Su D, Chen S, et al. A microwave synthesis of mesoporous NiCo2O4 nanosheets as electrode materials for lithium-ion batteries and supercapacitors. Chem Phys Chem, 2015, 16: 169–175
Zhu Y, Cao C, Zhang J, et al. Two-dimensional ultrathin ZnCo2O4 nanosheets: general formation and lithium storage application. J Mater Chem A, 2015, 3: 9556–9564
Tong X, Chen S, Guo C, et al. Mesoporous NiCo2O4 nanoplates on three-dimensional graphene foamas an efficient electrocatalyst for the oxygen reduction reaction. ACS Appl Mater Interfaces, 2016, 8: 28274–28282
Lian KK. Investigation of a “two-state” Tafel phenomenon for the oxygen evolution reaction on an amorphous Ni-Co alloy. J Electrochem Soc, 1995, 142: 3704–3712
Mansour AN. Characterization of NiO by XPS. Surf Sci Spectra, 1994, 3: 231
Shalvoy R. Characterization of coprecipitated nickel on silica methanation catalysts by X-ray photoelectron spectroscopy. J Catalysis, 1979, 56: 336–348
Kim JG, Pugmire DL, Battaglia D, et al. Analysis of the NiCo2O4 spinel surface with Auger and X-ray photoelectron spectroscopy. Appl Surface Sci, 2000, 165: 70–84
Salvati L, Makovsky LE, Stencel JM, et al. Surface spectroscopic study of tungsten-alumina catalysts using X-ray photoelectron, ion scattering, and Raman spectroscopies. J Phys Chem, 1981, 85: 3700–3707
Marco JF, Gancedo JR, Gracia M, et al. Characterization of the nickel cobaltite, NiCo2O4, prepared by several methods: an XRD, XANES, EXAFS, and XPS study. J Solid State Chem, 2000, 153: 74–81
Tan BJ, Klabunde KJ, Sherwood PMA. XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J Am Chem Soc, 1991, 113: 855–861
Allen GC, Harris SJ, Jutson JA, et al. A study of a number of mixed transition metal oxide spinels using X-ray photoelectron spectroscopy. Appl Surface Sci, 1989, 37: 111–134
McIntyre NS, Cook MG. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal Chem, 1975, 47: 2208–2213
Crist BV. XPS Handbook: Elements & Native Oxides. New York: John Wiley & Sons, 2007
Mansour AN. Nickel monochromated Al Kα XPS spectra from the physical electronics model 5400 spectrometer. Surf Sci Spectra, 1994, 3: 221
Choudhury T, Saied SO, Sullivan JL, et al. Reduction of oxides of iron, cobalt, titanium and niobium by low-energy ion bombardment. J Phys D-Appl Phys, 1989, 22: 1185–1195
Roginskaya YE, Morozova OV, Lubnin EN, et al. Characterization of bulk and surface composition of CoxNi1-x Oy mixed oxides for electrocatalysis. Langmuir, 1997, 13: 4621–4627
Menezes PW, Indra A, González-Flores D, et al. High-performance oxygen redox catalysis with multifunctional cobalt oxide nanochains: morphology-dependent activity. ACS Catal, 2015, 5: 2017–2027
Boissel V, Tahir S, Koh CA. Catalytic decomposition of N2O over monolithic supported noble metal-transition metal oxides. Appl Catalysis B-Environ, 2006, 64: 234–242
Qun S, Landong L, Zhengping H, et al. Highly active and stable bimetallic Ir/Fe-USY catalysts for direct and NO-assisted N2O decomposition. Appl Catalysis B-Environ, 2008, 84: 734–741
Zou X, Goswami A, Asefa T. Efficient noble metal-free (electro) catalysis of water and alcohol oxidations by zinc–cobalt layered double hydroxide. J Am Chem Soc, 2013, 135: 17242–17245
Wu L, Li Q, Wu CH, et al. Stable cobalt nanoparticles and their monolayer array as an efficient electrocatalyst for oxygen evolution reaction. J Am Chem Soc, 2015, 137: 7071–7074
Lee DU, Kim BJ, Chen Z. One-pot synthesis of a mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J Mater Chem A, 2013, 1: 4754
Acknowledgments
This work was supported by the National Natural Science Foundation of China (61371021 and 61671284). The authors also acknowledge the support of Shanghai Education Commission (Peak Discipline Construction).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hongbin Zhao is an associate professor at Shanghai University. He received his MSc degree in environmental science and engineering from Dalian Jiaotong University in 2006. Then he pursued his PhD degree in applied chemistry at Shanghai Jiaotong University. He worked at the Department ofMaterial Science and Engineering, Shanghai University as a postdoctor from 2009 to 2011. He joined Shanghai University as an associate professor since 2011. From 2014 to 2015, he worked at the Department of Chemical Engineering, University ofWaterloo (Canada) as a visiting scholar. His research focuses on electrochemical energy storage/transfer and nanostructured energy materials, including noble metal (Pt, Pd, Ru) catalysts in PEMFA fuel cell, micro-nanoprous carbon (graphene, conducting polymer) on lithium-sulfur batteries, rechargeable aqueous hybrid batteries and supercapacitor.
Jiujun Zhang is the President of the College of Science in Shanghai Univserty and a Senior Research Officer and Catalysis Core Competency Leader at the National Research Council of Canada Institute for Fuel Cell Innovation (NRC-IFCI, now changed to Energy, Mining & Environment Portfolio (NRC-EME)). Dr. Zhang received his BSc and MSc degrees in electrochemistry from Peking University in 1982 and 1985, respectively, and his PhD degree in electrochemistry fromWuhan University in 1988.
Jiaqiang Xu obtained his BSc degree in chemistry from Zhengzhou University, MSc degree in inorganic chemistry from the University of Science and Technology of China (USTC) and PhD degree in material science from Shanghai University, respectively. He has been a professor in chemistry since 2001. Currently, he is the director of China Special Committee on Gas andHumidity Sensor Technologies. His research interests include the synthesis of nanomaterials and their applications in gas sensors and other fields.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chen, Z., Zhao, H., Zhang, J. et al. IrNi nanoparticle-decorated flower-shaped NiCo2O4 nanostructures: controllable synthesis and enhanced electrochemical activity for oxygen evolution reaction. Sci. China Mater. 60, 119–130 (2017). https://doi.org/10.1007/s40843-016-5134-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-016-5134-5