Abstract
This study explores the isothermal hydrogen reduction of sintered pellets made of a mixture of bauxite residue and calcite with varying compositions at different reduction temperatures. Sintered pellets with varying compositions show three primary iron-containing oxide phases including brownmillerite, srebrodolskite, and fayalite; however, brownmillerite is the major phase in all the sintered pellets. The sintered pellets were reduced in a thermogravimetry furnace to establish instantaneous weight reduction with respect to time. Phases and microstructural analysis were carried out using X-ray diffraction and scanning electron microscopy, respectively. Mercury intrusion porosimeter and pycnometer were utilized to assess the porosity and density of the reduced pellets. Thermochemistry calculations were performed using the thermodynamics software FactSage 8.2. The reduction rate is most pronounced at a temperature of 1000 °C for all pellet compositions. It is intriguing to note that the rate of reduction shows minimal variance across pellets with different compositions; however, the higher calcite pellets exhibit a higher initial rate of reduction. Various kinetic models were examined to determine the activation energies for three different composition pellets, and the three-dimensional diffusion model has been well suited for this process. Close activation energies in the range of 84.6 to 94.8 kJ were obtained. A slightly higher activation energy was obtained for lower CaCO3 added pellets, and it was attributed to their reduced porosity and increased sintering, impeding the reaction kinetics. There were no significant differences in the formation of mayenite with varying the calcite amount; however, higher calcite pellets indicated more mayenite formation.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bauxite residue is a waste generated in alumina refineries during the processing of bauxite ore by the Bayer process to produce alumina. During the process, bauxite ore is digested in caustic solution at high temperatures and pressures for effective extraction of alumina [1,2,3,4]. After the leaching process remaining undigested oxides are discarded. Because of high Fe2O3 content, it appears in a reddish color, that is why it is also known as as red mud [3]. Bauxite residue is dewatered red mud, it is filtered to recover the residual soda, which is recirculated to the plant. Moreover, the filtering makes it possible to dry stack residue in a landfill [5]. Even after the filtration, bauxite residue has a pH of at least 11. The global stockpiled amount of bauxite residue exceeds more than 4.6 billion tons and the annual generation is exceeding 150 million tons [6], which increases the concern for storage and utilization for the alumina industry. Despite of many R&D projects, the large-scale utilization of bauxite residue has not been implemented yet, although, it is a polymetallic source of several metals [7]. Based on the origin and processing parameters of the bauxite ore, the composition of bauxite residue is varies a lot [8]. The major components in bauxite residue are iron and aluminum oxides, which are about 50 to 70 wt.% [9]. Recovering iron and alumina from the bauxite residue will decrease the bauxite residue volume and may be accompanied by the retrieval of other valuable metals. Most of the previous pyrometallurgical work for iron recovery from the bauxite residue has been through carbothermic reduction process. However, due to increasing environmental concern about greenhouse gases, the use of hydrogen based reduction process has been given more priority over carbothermic reduction process. Substitution of carbon with hydrogen as reductant will emit water vapor during reduction process as compared to CO2 emission in the carbothermic reduction processes [10, 11].
Research related to iron and alumina recovery based on hydrogen reduction as a key step is now quite limited. Pilla et al. [12] studied the BR-20% NaOH added pellets reduction in the presence of 5 vol% hydrogen in Ar for 120 min at 500 °C [12]. Their finding revealed that the maximum conversion of hematite to magnetite was 96% and the rest converted to metallic iron. The alumina present in the residue underwent conversion into a water-soluble sodium aluminate solution. Kapelari et al. [13] explored three distinct methods to recover iron and alumina from BR-NaOH pellets through 100% H2 reduction at 600 °C, to convert hematite to magnetite (Fe3+). In the first process, a combination of water leaching and magnetic separation was employed. Following dry magnetic separation, the iron content increased from 31.57 wt.% to 38.5 wt.%. The second process involved water leaching followed by smelting of the leach residue to produce sponge iron containing Al and minor Na, aiming to maximize iron recovery. In the third process, wet magnetic separation, a simultaneous leaching, and magnetic separation approach resulted in a slight increase in iron content from 31.57 wt.% to 31.85 wt.% in the separated magnetic fraction. Notably, this process achieved a high alumina recovery of 91%.
Skibelid et al. studied the iron and alumina recovery from the BR-lime sintered pellets through hydrogen reduction at elevated temperature [14]. They conducted the hydrogen reduction at a temperature range of 1000 °C to 1200 °C and observed that the reduction of sintered pellets was faster at 1000 °C as compared to 1100 °C and 1200 °C. The decrease in reduction rate at higher temperatures was claimed to be due to porosity losses at higher temperatures via sintering and the reduction mechanism changed to a diffusion-controlled step. Moreover, the alumina present in the sintered pellets as gehlenite was converted to an alkali leachable phase mayenite, and the alumina recovery above 87 wt.% was achieved. Hoseinpur et al. [15] studied the hydrogen reduction of BR-calcite sintered pellets and observed that iron oxide complexes are converted to metallic iron while alumina transferred to leachable calcium-aluminate phase. At 1170 °C, reduction temperature even after one hour of reduction certain amount of iron was still present in the form of wüstite, which indicates that more time was needed for the complete reduction. The reduced iron grains were adjacent to mayenite phase and iron was present in form of clusters due to high temperature reduction.
The present study focuses on the formation of calcium-aluminate phase (adjacent to iron particles) by varying the CaO ratio in the reduced BR-calcite pellets. The CaO:Al2O3 ratio was varied 0.85, 1, and 1.15 to examine the effect of CaO addition on the formation of calcium-aluminate phases during hydrogen reduction. Moreover, the different pellet compositions are reduced at different temperatures to investigate the effect of reduction temperature and determine the process kinetic data. In our previous work, we focused on the phases formation and reduction kinetics for similar pellets with CaO:Al2O3 = 1 (C1A) pellets [16] and the optimization of sintering parameter of different pellets were previously presented [9]. In this work, however, the mechanisms of phases formation, reduction kinetics, and the mechanism involving in gas–solid reduction reactions have been studied. X-ray diffraction, scanning electron microscopy, mercury porosimeter, pycnometer have been used for the phase, microstructural, porosity, and density analysis, respectively. Thermodynamics software (FactSage 8.2) was used to evaluate the thermodynamics results with experimental results. It is worth to emphasize that extracting iron and alumina from the reduced pellets is the main objective. The non-metallic part of the reduced pellets has slag-type phases, and the production of leachable calcium-aluminate phase for further alumina recovery is the main target.
Experimental Procedure
Materials and Pelletizing
BR was provided by Mytilineos of Greece (Aluminum of Greece) and the calcite (CaCO3) was from Omya S.A. Norway. These raw materials were received in the form of agglomerates containing moisture. The chemical composition and phase analysis of BR and calcite are presented here [9]. The primary composition of bauxite residue comprises Al2O3 (22.0 wt.%), CaO (8.8 wt.%), Fe2O3 (42.0 wt.%), Na2O (3.1 wt.%), SiO2 (6.1 wt.%), and TiO2 (5.0 wt.%). Calcite, on the other hand, is predominantly composed of CaCO3, with a minor fraction of MgO (0.95 wt.%) and SiO2 (2.07 wt.%). Raw materials were dried in an oven overnight at 80 ± 5 °C and deagglomerated in a ball mill and then sieved to below 500 µm. BR and calcite were mixed in three different ratios, which are named as C0.85A, C1A, and C1.15A hereafter. The incorporation of CaCO3 (CaO) into bauxite residue in C1A is determined via theoretical calculations, with the aim of promoting the formation of three phases: CaO·TiO2, 2CaO·SiO2, and CaO·Al2O3. Of these phases, CaO·TiO2 and 2CaO·SiO2 always appear due to high reactivity of TiO2 and SiO2 with CaO, while any excess CaO reacts with the adjacent Al2O3 to form calcium-aluminate phases such as CaO·Al2O3 [9]. In C1A, the composition entails one mole of CaO combined with one mole of Al2O3, two moles of CaO combined with one mole of SiO2, and one mole of CaO combined with one mole of TiO2. However, alterations in CaO content are implemented in C0.85A and C1.15A, with a reduction and addition of 0.15 mol of CaO to the bauxite residue. In C0.85A and C1.15A, the sole disparity lies in the ratio of one mole of Al2O3 with 0.85 mol of CaO and 1.15 mol of CaO, respectively. In the C0.85A and C1.15A pellets, the ratios of 2CaO.SiO2 and CaO.TiO2 remain akin to those in C1A, with the sole discrepancy residing in the CaO:Al2O3 ratio.
The primary objective is to investigate phase formation with a range of compositions, leading to experimentation with three different types of pellets. These different compositions of BR and calcite were mixed in a ball mill for 30 min to ensure homogenization. The prepared mixtures were pelletized in a lab scale disk pelletizer via the addition of approximately 10 wt.% water. The pellet size ranged from 4 to 10 mm. The prepared green pellets were dried in an oven at 80 ± 5 °C to remove the moisture content. After drying, pellets were sintered in a muffle furnace at 1150 ± 10 °C for 120 min. The optimized parameters for sintering was presented in our previous study [9]. The experimental methodology is shown in Fig. 1.
Hydrogen Reduction
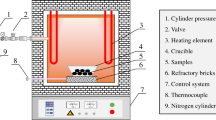
Sintered pellets were reduced in a thermogravimetric furnace for in-situ weight reduction measurement with respect to time. The schematic view and operational procedure of the furnace is presented here [16]. Heating was done by purging Ar to the targeted temperature at a heating rate of 10 °C/min and a flow rate of 1 Nl/min. Once the targeted temperature was reached, the gas switched to hydrogen with a flow rate of 4 Nl/min. After reduction, these reduced pellets were cooled down to room temperature by blowing Ar (flow rate 1 Nl/min) to avoid reoxidation of the obtained metallic iron upon cooling. The reduction was carried out across a range of temperatures from 500 °C to 1000 °C, with interval of 100 °C. The reduction time was 90 min for higher temperature such as 1000 °C, 900 °C, 800 °C, and 120 min for lower temperatures of 700 °C, 600 °C, 500 °C to compensate the slower reduction kinetics.
Characterization of Materials
The particle size distribution of dried BR and calcite was measured by a laser particle size analyzer, Horiba Partica LA-950. For the analysis of particle size, water was used as a medium. For mineralogical analysis of the materials X-ray diffraction method was employed, using Bruker D8 A25 DAVINCI_, with CuKα (λ = 1.54 Å) radiation, in the range of 15 to 75 diffraction degree with 0.03° step size. The raw data were analyzed by EVA diffraction software with mineralogical database. For the X-ray diffraction analysis, these materials were powdered in a ring mill for 60 s at a rate of 800 revolution per minute. The microstructural analysis of the reduced pellets was acquired by using scanning electron microscope (Zeiss ultra 55LE, Carl Zeiss, Jena Germany), and elemental analysis and mapping were acquired by Energy-dispersive spectroscopy (Bruker, AXS, microanalysis GmbH, Berlin, Germany). Porosities of reduced pellets were quantified using mercury intrusion porosimeter (Autopore IV 9520, micropolitics). Density of the reduced pellets was determined using Pycnometer, micromeritics, Accupyc 1350) with helium gas of 99.99% purity.
Results
Mass Changes of Different Pellets
The weight reduction with respect to time at different temperatures for C1A, C1.15A, and C0.85A pellets are shown in Figs. 2, 3, and 4, respectively. For the C1.15A pellet the total weight reduction is less at a given temperature in comparison with C1A and C0.85A Pellets. The weight reduction curves show similar reduction behavior for all the pellets at a given temperature. For the reduction temperature 1000 °C, the weight reduction curve is leveled off after 30 min. However, at the lower temperatures (below 900 °C), even after 90 min of reduction/weight loss is observed, indicating that the reduction has not been completed. The theoretical mass loss for complete iron oxide complexes reduction is 11.42 wt.%, 11.64 wt.%, and 12.2 wt.% for C1.15A, C1A, and C0.85A, respectively. The maximum weight reduction for C0.85A at 1000 °C is around 12.0 wt.%. The weight reduction for 500 °C is in the range of 2–3% for all pellets. At reduction temperatures of 1000, 900, and 800 °C, there was an initial rapid weight loss, which was then followed by a slower rate of reduction. There are three reduction regimes of weight reduction; however, for temperature exceeding 800 °C there is weight reduction with smaller transition regime, and for lower reduction temperatures (below 800 °C) larger transition regime is observed.
Physical Properties
Porosity and density measurement results for the BR-calcite pellets are shown in Figs. 5 and 6, respectively. There exhibits an inverse correlation between porosity and their densities of the reduced pellets. The measured density is the highest for C0.85A pellets and lowest for C1.15A pellets at a given reduction temperature. Correspondingly, porosity is the highest for C1.15A and lowest for C0.85A. The difference between the measured porosities for C0.85A and C1A reduced pellets is low, within a range of 45 to 50 vol%. However, the porosity is exceeded above 65 vol% for C1.15A. In the context of a gas–solid reaction, the degree of reduction is primarily contingent upon the reducibility of the oxides and the porosity of the materials involved. The porosity of the reduced pellets serves as a direct indicator of the material’s reducibility. It is important to note that porosity and density exhibit an inverse relationship. To reaffirm the connection between the porosity of various reduced pellets, density measurements were conducted.
The mechanical properties and physical properties of sintered pellets with different bauxite residue and calcite composition have been presented in our previous work [9].
Phase Analysis
X-ray diffraction spectra of C1A, C0.85A, and C1.15A reduced pellets are shown in Figs. 7, 8, and 9, respectively. Metallic iron (Fe), mayenite (Al14Ca12O33), perovskite (CaTiO3), gehlenite (Ca2Al2SiO7), lime (CaO), larnite (Ca2SiO4), brownmillerite (Ca2Fe1.634Al0.356O5), and srebrodolskite (Ca2Fe2O5) are the major peaks identified. The phases exhibited for all the pellet compositions are comparable, however, with different intensities. As shown with increases in reduction temperature, the iron oxide complexes (brownmillerite and srebrodolskite) peak intensities are decreased, while metallic iron intensity is increases. At 1000 °C reduction temperature all the iron oxide complexes reduced to metallic iron, which signifies their complete reduction. Perovskite and gehlenite are observed in all compositions of the pellets as these phases were formed during sintering. However, the intensity of CaO peak increases with rise in reduction temperature for all the different compositions. The intensity of mayenite varies with different compositions, and it is more intense for C1A and C1.15A pellets as compared to C0.85A. Above 900 °C, the mayenite peak is becoming more intense, while gehlenite intensity decreases. The relative intensity of gehlenite is decreased with increasing the reduction temperature, and the larnite peak becomes more intense above 900 °C. The iron present in the sintered pellets in form of brownmillerite (Ca2(Fe,Al)2O5), serbrodolskite (Ca2Fe2O5), and fayalite (Fe2SiO4) however are majorly in the form of brownmillerite. The phase analysis of the sintered pellets of different compositions is presented here [9].
Microstructural Analysis
The major phases detected in the scanning electron microscopy by imaging and energy-dispersive spectroscopy analysis are metallic iron, perovskite, gehlenite, and mayenite, which are correlated with the above X-ray diffraction results. Iron was distributed everywhere in the oxide matrix; however, in some areas iron particles were clustered. The elemental analysis of different phases is presented in Fig. 10. The gray color phase is gehlenite, and the light gray color is perovskite. Some of the impurities are most likely from surrounding signals as the size of the particles/phases is small; the darkest gray area around gehlenite might be mayenite; however, determining the composition through the EDS analysis is challenging due to the small thickness of this phase. Mayenite was observed in the X-ray diffraction spectra of the reduced sample, hence we may consider this phase here as mayenite as the other major phases were analyzed well by EDS. The elemental mapping of that area is shown in Appendix Fig. 25. It is observed that the identified phase as gehlenite and mayenite here have Al, Ca, Si, and O. As the thickness of the dark gray phase around gehlenite is about 0.4 microns, the energy-dispersive spectroscopy mapping does not provide much information as it detects Si from the gehlenite, and in addition the other components have Al, Ca, and O.
As shown in Fig. 11, calcium and titanium have overlapping with oxygen, confirming the presence of perovskite, which is correlated with X-ray diffraction results. Iron is present separately without overlapping with oxygen, indicating metallic iron. The microstructural analysis and elemental analysis of C0.85A pellets reduced at 500 °C are shown in Fig. 12. As depicted in Fig. 12, the bright phase is majorly composed of Fe, Ca, Al, and O suggesting it may be the brownmillerite phase. Sodium is present in all the area may be due to insignificant sodium losses at 500 °C reduction temperature. EDS is not particular for sensitive elements with lower concentrations, the emitted X-rays after heating the target may not be sufficient for adequate measurement. As a result, the measurement of sodium concentration is less precise due to lighter element and lower concentration. Regarding our observations, Na is most likely in amorphous phases that were not detected by X-ray diffraction, or it is dissolved in some crystalline phases. For example, Na can be dissolved in small portions in mayenite structure. However, it is not possible to illustrate it by EDS analysis as it is a light element.
The elemental mapping of the reduced C0.85A pellets at 500 °C is shown in Fig. 13. The bright phases are majorly Fe, Ca, and O with minor presence of Al, which is also correlated to the energy-dispersive spectroscopy analysis. The light dark areas are majorly composed of Al, Ca, Na, and O.
The microstructural analysis indicated that there is inhomogeneity in the microstructures of the reduced pellets regarding the distribution of the elements. In addition, the analysis of the phases is correlated with the X-ray diffraction results; however, the microstructural analysis indicated fine metallic iron particles in the pellets reduced over 900 °C, while at lowest temperature of 500 °C, reduction did not proceed completely to metallic iron, and hence both metallic iron and iron oxide complexes coexist. Appendix 1 contains the microstructural analysis, specifically elemental mapping, of the C1.15A and C0.85A reduced at 1000 °C. Upon examining the elemental mapping results for C0.85A, C1.15A, and C1A reduced at 1000 °C, minimal differences were observed in the microstructure. However, variations were noted in phase analysis.
Discussion
Equilibrium in Fe2O3–CaO–Al2O3, and CaO–Al2O3–SiO2 Slag Systems
As Fe2O3, CaO, and Al2O3 are the major oxides in the mixture of BR and calcite, studying the equilibrium in this system is important. Figure 14 shows the calculated equilibrium phase diagram for Fe2O3–CaO–Al2O3 system at 1150 °C, which was the sintering temperature. Obviously, during sintering in air of bauxite residue, the Fe2O3, CaAl4O7, and liquid slag are transformed to the stable Ca2(Fe,Al)2O5 and monoxide after addition of CaO to the system. Monoxide is a complex with Fe,Ca,Mg in the rock salt structure and diluted with Al, Ti, and Na. As the CaO percentage rises, the composition of the pellets is shifted toward the CaO. The dominated phase detected in sintered pellets was dicalcium ferrite (Ca2(Fe,Al)2O5 as per X-ray diffraction, which is correlated with thermodynamics result [9].
Figure 15 shows the equilibrium phase diagram of CaO–SiO2–Al2O3 at 1000 °C, an oxide system excluding the produced metallic iron after reduction. Liquid slag comprises a mixture of various oxides, including Al, Fe(II), Fe(III), Mg, Mn(II), Mn(III), Na, Si, Ti(III), Ti(IV), with S and F present in dilute solutions (< 50%). Following the process of hydrogen reduction at 1000 °C, iron complexes are reduced to metallic iron and the major oxides present in the matrix are mostly the CaO, SiO2, Al2O3, and TiO2. A reaction between titanium dioxide (TiO2) and calcium oxide occurs to form calcium titanate (CaTiO3), and this phase is predominantly stable at 500 °C (∆G500 °C = − 85.65 kJ) and higher temperatures. Experimental finding suggests that the formation of alkali leachable calcium-aluminate phase, mayenite (Ca12Al14O33), during reduction may be attributed to the interaction of alumina from BR and calcite. Referring to the phase diagram in Fig. 15, without calcite addition the BR main components (excluding Fe2O3) yield CaAl2Si2O8, Ca2Al2SiO7, and SiO2 phases during reduction. However, with the introduction of CaO, Ca2SiO4, Ca3Al2O6, and monoxides become more stable. It was confirmed based on results obtained from X-ray diffraction and SEM, the major phases are identified in reduced pellets are Ca12Al14O33, Ca2SiO4, and CaO. In accordance with thermodynamics calculation illustrated in Fig. 15, to the left of this experimental stochiometric composition, mayenite phase (10) is present (Phases named 10, 11, and 12). Azof et al. [17] found that mayenite maintains stability at room temperature, a characteristic reinforced by impurities like SiO2. The presence of SiO2 is crucial for sustaining the 12CaO·7Al2O3 structure; silicon serves to stabilize the mayenite phase, and it is formed in both reducing and oxidizing atmospheres.
Thermochemistry of the Oxide Pellets
The phase equilibrium calculation was carried out to study the sintering and reduction process steps with composition and temperature changes using the equilibrium module of FactSage 8.2. To simplify hereafter, MeO is a monoxide solid solution composed mainly of CaO and low levels of FeO (below 1 wt.%). Olivine is a solid solution composed mainly of Ca2SiO4. CaTi is a solution with Ca3Ti2O7–Ca3Ti2O6 endmembers. C3A is a solid solution of Ca3Al2O6 and Ca3Fe2O6. NCA is a solid solution mainly composed of Na2CaAl4O8. Upon sintering at 1150 °C of BR (varying CaCO3 addition), major phases such as Ca2Fe2O5, 5CaO·4TiO2, 2CaO·SiO2, and 2CaO·Al2O3·SiO2 were detected previously [9]. Figure 16 shows the formation of phases at different reduction temperatures at constant hydrogen amount (32 g).
As shown in Fig. 16, the reduction of iron oxides complexes to metallic iron started at 500 °C; however, during actual process the reduction reaction was not completed. The reduction reaction is thermodynamically feasible; however, in reality the reaction is not completed due to kinetic barriers. The thermodynamic calculations show that the whole amount of hydrogen directly reacts with all iron oxides complexes and yields metallic iron. The formation of metallic Fe at 500 °C that was detected by X-ray diffraction analysis (Figs. 7, 8, and 9) confirms the FactSage calculations. However, in actual practice complete reduction was not occurred due to kinetic barriers and slow chemical reactions. The calcium titanate and calcium silicate are stable phases at all reduction temperatures as observed in the experimental trials by both X-ray diffraction and SEM analysis, and this is predicted by FactSage simulations. The sodium losses was observed at temperatures surpassing 870 °C. Despite the thermodynamic calculations indicating the presence of sodium calcium-aluminate (NCA), X-ray diffraction (X-ray diffraction), and scanning electron microscopy (SEM) analyses failed to detect this phase. It is plausible that the NCA phase is amorphous, rendering it undetectable by X-ray diffraction. However, the absence of its detection in the SEM analysis suggests some sodium loss within the system. It was included the reference for the X-ray fluorescence analysis conducted on the sintered pellets before and after reduction, specifically highlighting the chemical composition of C1A [16]. Notably, sodium losses were not observed during the sintering phase of the dried bauxite residue pellets. However, such losses became apparent during the subsequent hydrogen reduction of the sintered pellets. Given the initial sample size of 50 g and the relatively low sodium content in the bauxite residue, quantifying losses during hydrogen reduction poses a challenge due to their minimal magnitude. This challenge is further compounded by the difficulty in precise measurement owing to the low sodium levels involved in the process. The possibility of sodium reduction from compounds, coupled with sodium evaporation, emerges as potential contributors to these losses. Consistent results were obtained across various pellet compositions, each exhibiting distinct phase quantities. Figures 17 and 18 illustrate comparable outcomes for compositions C1A and C1.15A, respectively.
Despite the assumptions made for the thermodynamic calculations and their differences with the experimental results, the main phase distribution and the reducibility of iron oxides complexes are well predicted the mayenite formation, which was observed at higher reduction temperatures, is not predicted by the FactSage thermodynamic calculations, and this might be due to lack of fundamental data in the thermodynamics databases.
Effect of Hydrogen Amount
FactSage calculations indicates that the equilibrium phases formed during hydrogen reduction of sintered pellets at 1000 °C are Fe, Na2Ca3Al16O28, Na2Ca8Al6O18, Ca3Al2O6, Ca2SiO4, and Ca3Ti2O7. According to X-ray diffraction analysis, Ca, Al, and O present in form of mayenite (Ca12Al14O33) in the reduced pellets; however, in thermodynamics calculation Na2Ca3Al16O28, Na2Ca8Al6O18, and Ca3Al2O6 are present. The activity of Na might be overestimated in the calculations due to the assumption of a closed system. Nevertheless, in the experimental setup, sodium undergoes evaporation, and X-ray diffraction (X-ray diffraction) analyses did not reveal the presence of any sodium-containing phases. The elevated vapor pressure of sodium oxide leads to mass loss at higher temperature reductions, a phenomenon previously observed in our prior research [16]. As depicted in Fig. 19, the escalation in hydrogen quantity corresponds to an augmentation in sodium losses. This observed trend provides supporting evidence for the formation of sodium (Na) and its subsequent loss through vaporization, aligning with the explanation provided earlier.
With the introduction of hydrogen, the amount of calcium ferrite decreases and metallic iron increases as illustrated in Fig. 19. All iron oxide complexes reduced to metallic iron when more than 4 g of hydrogen introduced. The calcium silicate and calcium titanate are the stable phase at 1000 °C, independent of hydrogen addition. The Ca2(Al,Fe)2O5 solid solution forms at lower hydrogen additions, and it transforms to Ca3(Al,Fe)2O6 at higher H2 additions with less amount of Ca3Fe2O6 in the solution as H2 increases. Melilite (a mixture of phases of calcium iron silicate, calcium iron aluminate, and calcium alumino silicate) solid solution forms at low H2 additions, and upon more H2 availability melilite solution is decomposed to calcium-aluminate, calcium silicate, and metallic iron phases. The formation of phases is similar for all different composition pellets shown in Figs. 19, 20, and 21. Mayenite is not predicted thermodynamically because in FactSage 8.2 it is recognized as a metastable phase.
Physical Properties Changes During Reduction
The density and porosity of the reduced pellets exhibit an inverse behavior, which is in principle correct for many materials behavior upon heating. With temperature increase from 500 °C to 800 °C, porosity increases minimally, and this increase is higher when more Ca exists in the pellets. However, the porosity is significantly decreased at higher temperatures reduction from 800 °C to 1000 °C. This observed behavior of porosity in the reduced pellets may be attributed to the sintering phenomena in matrix as well as clustering of metallic iron that takes place most at 1000 °C reduction temperature. At reduction temperatures 800 °C and 500 °C, there is greater weight reduction at 800 °C with minimal change in volume of the pellets. Due to the volume of pellets remains constant at 800 °C and 500 °C reduction, the pronounced weight reduction at 800 °C leads to greater density at 500 °C as compared to 800 °C. The absence of insignificant volume change that are reduced at 800 °C and 500 °C may be attributed to insignificant sintering during the reduction process. It is noteworthy that the slightly elevated porosity observed in the reduced pellets C1.15A compared to the other two is due to the increased CaCO3 content present in the green pellets. Additionally, the lower density observed in the C1.15A reduced pellets, at constant reduction temperature, can be attributed to the higher mass loss during sintering, a consequence of the higher percentage of CaCO3 content.
Phase Changes During Reduction
In the sintered pellets iron was detected in form of brownmillerite, srebrodolskite, and fayalite; however, it was predominantly in the form of brownmillerite. Those iron complexes completely reduced to metallic iron at 1000 °C. As shown in Eq. (1), brownmillerite is reduced to metallic iron, calcium oxide, mayenite, and water vapor. The observation is consistent with X-ray diffraction analysis as with increases in reduction temperature corresponds to decrease in intensity of brownmillerite and increasing intensity of metallic iron, calcium oxide, mayenite. The srebrodolskite undergoes reduction to the formation of metallic iron, calcium oxide, and water vapor. Similarly, fayalite is reduced to metallic iron, silica, and water vapor. The reduction of brownmillerite, serbrodolskite, and fayalite are described in Eqs. 1, 2, and 3. The free energy of formation values of Eqs. 4, 5, 6, and 7 were calculated by using FactSage 8.2:
Stable oxide phases formed during sintering (and reduction) are as follows:
As shown in the equation Gibbs free energy of formation (\(\Delta G{^\circ }_{1000^\circ{\rm C} })\) in Ca2Al2SiO7 is more favorable than the formation of Ca2SiO4; however, in hydrogen reduction at 1000 °C the Ca2SiO4 phase formed. The formation of Ca2SiO4 is detected both in thermodynamics calculation and actual calculation correlated with X-ray diffraction. The formation of Ca2Al2SiO7 is associated with alumina involvement; however, the formation of mayenite (12CaO·7Al2O3) is more favorable in hydrogen reduction. The Gibbs free energy of formation of mayenite is more negative than Ca2Al2SiO7. Thermodynamically it was seen that Melilite (with gehlenite as endmember) is forming at low H2 additions, which indicates that the reduction of iron oxides and the dissolution of melilite solution shifts the system closer to mayenite formation. Both the experimental result and the thermodynamics calculation is related with the formation of Ca2SiO4. The dissociation reaction of gehlenite to larnite and alumina is given in Eq. 8:
The dissociation of Ca2Al2SiO7 results in the formation of Ca2SiO4 and Al2O3. The formed Al2O3 may react with adjacent CaO to form mayenite. As the reaction occurs in the solid state, the mass transport of the species may be a rate controlling step.
Effect of Temperature and Composition on the Reduction Rate
The results of thermogravimetry experiments for hydrogen reduction of sintered pellets were interpreted by assuming some kinetic models [18]. The BR-calcite sintered pellets exhibit significant porosity and iron oxide complexes distributed randomly adjacent to the unreduced oxide sintered matrix. Various diffusion, nucleation, and interfacial chemical reaction models have been employed to analyze the reduction behavior of sintered pellets at various reduction temperatures. To determine the most suitable model for reduction behavior of sintered pellets, average R2 (Average value for all reduction temperature) value were compared, assuming linear approximation. As shown in Table 1, the diffusion-controlled model is more suited for the reduction process as compared to nucleation model and interfacial chemical reaction model. The R2 value for one-, two-, and three-dimensional diffusion models are above 0.84, and it was well suited for three-dimensional (3D) diffusion model. The three-dimension diffusion model may be more favorable due to the following reasons.
-
1.
The iron oxide complexes distributed all over the pellets.
-
2.
The diffusion of reactant gas and product gas through the product layer and sintered matrix to outer surface.
-
3.
Due to elevated temperature sintering, the predominate diffusion phenomena is in the oxide sintered matrix.
These results are shown in Figs. 22, 23, and 24. As per calculation(curve fitting) it was found that 3D diffusion model is the best suited for the reduction of sintered pellets and the equation is expressed as follows:
where, g(X) is the integral form of kinetic model, X is the fraction reduced, k is the rate constant, and t is the time:
where X is the fraction reduced, ∆w is the weight loss during reduction (actual oxygen removed from iron complexes), and win is the theoretical amount of oxygen present in Fe2O3 of the initial mass. Given that the principal oxides present in the bauxite residue include CaO, Al2O3, SiO2, TiO2, and Fe2O3, it is noteworthy that, thermodynamically, only iron oxides are susceptible to reduction. The remaining oxides maintain their unreduced state under these conditions. The reduction degrees for different pellets with varying temperature are summarized in Table 2.
3D diffusion model equation was applied to various reduction temperatures for pellets with different compositions. These curves are shown in Figs. 22, 23, and 24 for C1A, C1.15A, and C0.85A, respectively.
By using Arrhenius equation, activation energy was calculated for distinct pellet composition at different temperatures for the initial rate of reduction. It was found that, the activation energy is 94.80 kJ, 93.30 kJ, and 84.64 kJ for C0.85A, C1A, and C1.15A, respectively. The lower calcite added pellets exhibited slightly higher activation energy. However, for C1.15A pellets due to the higher porosity the activation energy is lower than other two pellets. Reduced calcite content in the pellets (C0.85A) leads to lower porosity, primarily because of calcite decomposition and an increased presence of bauxite residue, contributing to higher alkali oxide. At relative similar amount of initial mass, lower calcite added pellet has higher amount of BR which attributed more alkali oxides. So, during high temperature heat treatment process (sintering as well as reduction) higher alkali oxides result in more semi-molten phase in the solid matrix and more compacted than higher calcite added pellets (C1.15A). Lower porosity/high dense structure of lower calcite pellets results in increases in the activation energy.
Conclusions
The hydrogen reduction of various bauxite residue and calcite sintered pellets was studied using thermogravimetry methods under isothermal conditions at various reduction temperatures. The main conclusions of this work are summarized as follows:
-
From the experimental observation it was found that with increasing the CaCO3 percentage in the green pellets, the final porosity increases, and the density and porosity have the inverse relationship.
-
The density is highest and porosity lowest for 1000 °C reduced pellets due to more metallic iron in the matrix and some porosity loss.
-
At a reduction temperature 1000 °C for fixed flow rate of hydrogen, the iron oxide complexes completely reduced to metallic iron after 30 min in all compositions of pellets.
-
The formation of the calcium-aluminate phase (mayenite) takes place at temperatures exceeding 900 °C during hydrogen reduction, with a greater quantity formed in pellets with higher amounts of added calcite.
-
Among the different kinetic models, three-dimensional diffusion model was best suited to experimental data.
-
The activation energy for the pellets reduction by hydrogen is higher for lower calcite added pellets due to lower porosity and more sintering. The activation energies as 94.80 kJ, 93.30 kJ, and 84.64 kJ were determined for C0.85A, C1A, and C1.15A pellets, respectively.
References
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T (2016) Smelting of bauxite residue and selective rare Earths recovery. J Sustain Metall 2:28–37. https://doi.org/10.1007/s40831-015-0026-4
Khairul MA, Zanganeh J, Moghtaderi B (2019) The composition, recycling and utilisation of bayer red mud. Resour Conserv Recycl 141:483–498
Verma AS, Suri NM, Kant S (2017) Applications of bauxite residue: a mini-review. Waste Manag Res 35:999–1012. https://doi.org/10.1177/0734242X17720290
Evans K, Nordheim E, Tsesmelis K (2012) Bauxite residue management. Light Metals. https://doi.org/10.1007/978-3-319-48179-1_11
Evans K (2016) The history, challenges, and new developments in the management and use of bauxite residue. J Sustain Metall 2:316–331. https://doi.org/10.1007/S40831-016-0060-X
Xue S, Liu Z, Fan J, Xue R, Guo Y, Chen W, Hartley W, Zhu F (2022) Insights into variations on dissolved organic matter of bauxite residue during soil-formation processes following 2-year column simulation. Environ Pollut 292:118326
Smirnov DI, Molchanova TV (1997) The investigation of sulphuric acid sorption recovery of Scandium and Uranium from the red mud of alumina production. Hydrometallurgy 45:249–259
Gräfe M, Power G, Klauber C (2011) Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 108:60–79
Kar MK, Safarian J (2023) Characteristics of bauxite residue-limestone pellets as feedstock for Fe and Al2O3 recovery. Processes 11:137
Heidari A, Niknahad N, Iljana M, Fabritius T (2021) A review on the kinetics of iron ore reduction by hydrogen. Materials (Basel) 14:7540
Spreitzer D, Schenk J (2019) Reduction of iron oxides with hydrogen—a review. Steel Res Int 90:1900108. https://doi.org/10.1002/srin.201900108
Pilla G, Kapelari SV, Hertel T, Blanpain B, Pontikes Y (2022) Hydrogen reduction of bauxite residue and selective metal recovery. Mater Today Proc 57:705–710
Kapelari S, Gamaletsos PN, Pilla G, Pontikes Y, Blanpain B (2023) Developing a low-temperature, carbon-lean hybrid valorisation process for bauxite residue (red mud) towards metallic Fe and Al recovery. J Sustain Metall 9:578–587. https://doi.org/10.1007/s40831-023-00648-7
Skibelid OB, Velle SO, Vollan F, Van der Eijk C, Hoseinpur-Kermani A, Safarian J (2022) Isothermal hydrogen reduction of a lime-added Bauxite residue agglomerate at elevated temperatures for iron and alumina recovery. Materials (Basel) 15:6012. https://www.mdpi.com/1996-1944/15/17/6012
Hoseinpur A, Friborg PI, Van Der Eijk C, Safarian J (2023) High-temperature hydrogen reduction of bauxite residue for iron recovery. In: Proceedings of the 61st conference of metallurgists, COM 2022. Springer, pp 119–127. https://doi.org/10.1007/978-3-031-17425-4_19
Kar MK, van der Eijk C, Safarian J (2023) Kinetics study on the hydrogen reduction of bauxite residue-calcite sintered pellets at elevated temperature. Metals (Basel) 13:644
Azof FI, Tang K, You J, Safarian J (2020) Synthesis and characterization of 12CaO·7Al2O3 slags: the effects of impurities and atmospheres on the phase relations. Metall Mater Trans B 51(6):2689–2710. https://link.springer.com/article/10.1007/s11663-020-01969-8
Szekely J, Lin CI, Sohn HY (1973) A structural model for gas—solid reactions with a moving boundary—V an experimental study of the reduction of porous nickel-oxide pellets with hydrogen. Chem Eng Sci 28:1975–1989
Khawam A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B 110(35):17315–17328
Vasilopoulos Y, Skořepová E, Šoóš M (2020) Comf: comprehensive model-fitting method for simulating isothermal and single-step solid-state reactions. Crystals 10(2):139
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 958307 (HARARE project). We are genially thankful to Adamantia Lazou for her support on the thermodynamics FactSage analysis.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was M. Akbar Rhamdhani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kar, M.K., van der Eijk, C. & Safarian, J. The Effect of Composition and Temperature on the Hydrogen Reduction Behavior of Sintered Pellets of Bauxite Residue-Lime Mixtures. J. Sustain. Metall. 10, 1393–1414 (2024). https://doi.org/10.1007/s40831-024-00849-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-024-00849-8