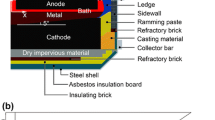
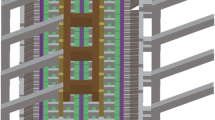
Abstract
The present study investigates the impact of erosion holes and subsequent repairs on the current distribution at the cathode-metal interface in aluminum reduction cells. The research focuses on examining the effects of erosion hole location, size, repair material properties, and the modification of cathode collector bars to optimize cathode repair strategies. The findings indicate that erosion holes lead to a localized concentration of current distribution in the metal at the erosion site. Notably, the maximum current density observed reaches 46125 A/m2, and the maximum horizontal current in the lateral cell direction at the cathode-metal interface increases with the depth of the erosion hole. Furthermore, the study reveals that the electrical conductivity of repair materials significantly influences current distribution. Materials with high resistivity behave similarly to insulators. Post-repair actions, including the cutting off of the collector bar, result in a noticeable reduction in current density, with a maximum horizontal current of 5860 A/m2. These results provide valuable insights into optimizing cathode repair processes, offering implications for enhancing aluminum reduction cells' efficiency, productivity, and cost-effectiveness.
Graphical Abstract














Similar content being viewed by others
Abbreviations
- Δρ :
-
Increase in resistivity, Ω·m.
- ρ repair :
-
Electrical resistivity of the repair material, Ω·m.
- ρ e :
-
Resistivity of the electrolyte, Ω·m.
- ρ cr :
-
Contact resistivity, Ω·m.
- σ :
-
Electrical conductivity, S/m.
- φ e :
-
Electric potential in the electrolyte, V.
- φ c :
-
Electric potential in the cathode, V.
- φ b :
-
Electric potential in the collector bar, V.
- φ h :
-
Electric potential in the repair material, V
- E :
-
Electric field intensity, V.
- E corr :
-
Potential of the cathode surface, V
- J :
-
Electric current density, A/m2.
- V :
-
Scalar potential, V.
- V1:
-
Surface 1 potential, V.
- V2:
-
Surface 2 potential, V.
- Vave:
-
Cell voltage, V.
- V bemf:
-
Reaction voltage, V.
- Va:
-
Anode voltage drop, V.
- Vb:
-
Electrolyte voltage drop, V.
- Vc:
-
Cathode voltage drop (CVD), V.
- Vbus:
-
Busbar voltage drop, V.
- Vg:
-
Gas voltage drop, V.
- Vrepair:
-
Erosion hole voltage drop, V.
- fbar:
-
Cathode collector bar that is cut off
- j :
-
Current density in the electrolyte, A/m2
References
Kvande K (1997) Retrofitting older aluminum reduction cell lines - A way to extend productive life. JOM 49:21–26. https://doi.org/10.1007/BF02915474
Zhou Y, Yang YC (2022) On causes and preventive measures for cathode damage in 500 kA aluminum reductions cells. Light Metals 7:39–43. https://doi.org/10.13662/j.cnki.qjs.2022.07.009
Youssif K, Star AEZAE (2018) Partial repair and restart of a damaged aluminium reduction cell. In: Martin O (ed) Light Metals. Springer, Cham, pp 501–506
Sørlie M, Hvistendahl J, Øye HA (2016) Early failure mechanisms in aluminium cell cathodes. In: Tomsett A, Johnson J (eds) Essential readings in light metals: electrode technology for aluminum production. The Minerals, Metals & Materials Society. https://doi.org/10.1002/9781118647745.ch122
Wang W, Sun K (2020) Influence of current density on the microstructure of carbon-based cathode materials during aluminum electrolysis. Appl Sci-Basel 10:1–9. https://doi.org/10.3390/app10072228
Lindsay SJ, Welch BJ (2021) A review: understanding the science and the impacts of impurities upon the electrolytic bath of hall–héroult reduction cells. JOM 73:1196–1209. https://doi.org/10.1007/s11837-021-04572-7
Siew EF, Ireland-Hay T, Stephens GT, Chen JJJ, Taylor MP (2005) A study of the fundamentals of pothole formation. Light metals. TMS, Warrendale, pp 763–769
Dell MB (2016) Potlining failure modes. In: Tomsett A, Johnson J (eds) Essential readings in light metals: electrode technology for aluminum production. The Minerals, Metals & Materials Society, pp 909–913
James BJ, Welch BJ, Hyland MM, Metson JB, Morrison CD (1995) Interfacial processes and the performance of cathode linings in aluminum smelters. JOM 47:22–25. https://doi.org/10.1007/BF03221401
Liu YX, Li J (2008) Modern aluminum electrolysis, modern aluminum electrolysis, (Metallurgical industry press, Beijing), pp 234–235.
Luo Y, Li SX, Chen DL, Liu XL, Ma CD, Li XB (2021) Evaluation of cathode quality and damage of aluminium electrolytic cell based on non-destructive technology. T Nonferr Metal Soc 31:3929–3942. https://doi.org/10.1016/S1003-6326(21)65775-8
Maity A, Thalagani V, Das D, Shankar B, Das A, Gupta K, Sahoo M, Rajgire S, Gupta A (2023) Achieving low pot failure rate at aditya aluminium. In: Broek S (ed) Light metals 2023. Springer, Cham, pp 782–790
Lalancette F, Desilets M, Pansiot B, LeBreux M, Bilodeau JF (2023) Dimensional reduction of a 3D thermoelectric model to create a reliable and time-efficient 2D model representing an aluminum electrolysis cell. Int J Heat Mass Tran 202:1–13. https://doi.org/10.1016/j.ijheatmasstransfer.2022.123777
Vachaparambil KJ, Johansen ST, Solheim A, Einarsrud KE (2023) A pragmatic model for bath temperature evolution during alumina feeding. In: Broek S (ed) Light metals. Springer, Cham, pp 113–120
Necheporenko I, Arkhipov A, Alzarouni A (2023) Simplified 3D MHD model for quick evaluation of aluminium electrolysis cell design. In: Broek S (ed) Light metals. Springer, Cham, pp 773–781
Dupuis M, Bojarevics V (2005) Weakly coupled thermo-electric and MHD mathematical models of an aluminium electrolysis cell. In: Kvande H (ed) Light metals, The Minerals, Metals & Materials Society, Warrendale, pp 449–454.
Dreyfus JM, Joncourt L (1999) Erosion mechanisms in smelters equipped with graphite blocks-a mathematical modeling approach. In: Eckert CE (ed) Light metals (Warrendale, PA: TMS, 1999), pp 199–206.
Mahran GMA, Ali MM (2020) Numerical simulation of cathodic voltage drop in Hall-Héroult cell. Miner Eng 157:1–14. https://doi.org/10.1016/j.mineng.2020.106534
Song Y, Peng JP, Di YZ, Wang YM, Li BK, Feng NX (2016) The impact of cathode material and shape on current density in an aluminum electrolysis cell. JOM 68:593–599. https://doi.org/10.1007/s11837-015-1719-7
Tao WJ, Li TF, Wang ZW, Gao BL, Shi ZN, Hu XW, Cui JZ (2016) A numerical assessment of anodic large bubble on horizontal current in metal pad of aluminum electrolytic cells. JOM 68:600–609
Li J, Liu W, Lai YQ, Wang ZG, Liu YX (2007) Analysis of cathode voltage drop in aluminum electrolysis cells with an electric contact model. In: Sorlie M (ed) Light metals, The Minerals, Metals & Materials Society, Warrendale, PA, p 465
Zhang HL, Li J (2019) Simulation and its practice for modern large-scale pre-baked aluminum reduction cell. Central South University Press, Changsha, pp 99–101
Sen Z, Diop MA, Gao BL, Wang ZW (2023) Controlled ledge profile of aluminum smelting cell using sidewalls heat exchangers supplied with molten salt. J Sustain Metall. https://doi.org/10.1007/s40831-023-00666-5
Richard D, Fafard M, Lacroix R, Clery P, Maltais Y (2001) Aluminum reduction cell anode stub hole design using weakly coupled thermo-electro-mechanical finite element models. Finite Elem Anal Des 37:287–304
Wang Q, Li BK, Fafard M (2016) Effect of anode change on heat transfer and magneto-hydrodynamic flow in aluminum reduction cell. JOM 68:610–622. https://doi.org/10.1007/s11837-015-1714-z
Tao WJ, Wang ZW, Gao BL, Shi ZN, Hu XW, Cui JZ (2014) Numerical simulation of sludge influence on horizontal electric in metal pad of aluminum reduction cell. Metalurgija 53:311–313
Di YZ, Tao SH, Peng JP, Feng NX, Wang ZG (2013) Thermal conductivity of precipitate on the bottom of aluminum reduction cells. Light Metals 9:30–33
Delcet J, Heus R, Egan JJ (1978) Electronic conductivity in solid CaF2 at high temperature. J Electrochem Soc 125:755–758. https://doi.org/10.1149/1.2131542
Qi YY, Zhang T, Cheng Y, Chen XR, Wei DQ, Cai LC (2016) Lattice dynamics and thermal conductivity of calcium fluoride via first-principles investigation. J Appl Phys. https://doi.org/10.1063/1.4942841
Liu PX (1998) Critical review of electrical conductivity measurements and charge distribution analysis of magnesium oxide. Foreign Refract 8:34–35
Saito Y, Kanematsu K, Matsui T (2009) Measurement of thermal conductivity of magnesia brick with straight brick specimens by hot wire method. Mater Trans 50:2623–2630. https://doi.org/10.2320/matertrans.M2009098
Arkhipov A, Jassim A, Jabri NA, Boraie MT (2022) EGA journey with different ramming pastes. In: Eskin D (ed) Light metals, The Minerals, Metals & Materials Society, Warrendale, pp 911–920
Liu HS (2006) Research on prolong the life of large pre-baked aluminium electrolysis cell, (Ph. D. thesis, Northeastern University, Shenyang, China).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51974081), Liaoning Natural Science Foundation (Grant No. 2022-MS-121), the Fundamental Research Funds for the Central Universities (Grant No. N2225045), and Ministry of Education-Weiqiao Industrial Program (Grant Nos. 2021021800101 and 2021021800102).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
The contributing editor for this article was Hongmin Zhu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, S., Diop, M.A., Gao, B. et al. Enhancing Sustainability in Aluminum Reduction Cells Through Cathode Repair Optimization and Numerical Simulations Study on Current Distribution and Erosion Hole Impact. J. Sustain. Metall. (2024). https://doi.org/10.1007/s40831-024-00803-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40831-024-00803-8