Abstract
Iridium (Ir) is widely used in electrochemical, electrical, and chemical applications. Because of its low production volume, Ir recycling is crucial. Dissolution is necessary for recycling, and the conventional dissolution method involves using a highly hazardous strong acid. In this study, we investigated a method to dissolve Ir in hydrochloric acid (HCl) by employing the principle that acid solubility improves when a platinum group metal becomes a complex oxide. The conditions for the formation of Ir and CaO complex oxides were investigated via X-ray diffraction (XRD) analysis of Ir and CaCO3 powders that were mixed and heated. The solubility of Ir was also investigated by dissolving the sample with HCl after heating and analyzing it via inductively coupled plasma-optical emission spectroscopy (ICP-OES). The formation of Ca2IrO4 and Ca4IrO6 was confirmed, and both compounds exhibited high solubility in HCl. The solubilities of Ca2IrO4 and Ca4IrO6 in hydrochloric acid were also compared. The decomposition temperatures of the experimental complex oxides were verified thermodynamically. The dissolution mechanism of Ir via complex oxides is also discussed.
Graphical Abstract
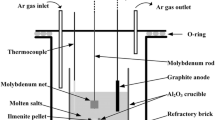
In this study, in order to develop a new recycling method for Ir, the conditions for the formation of a complex oxide of Ir and CaO and its solubility in hydrochloric acid (HCl) were investigated. In the experiment, Ir and CaCO3 powders were mixed and heated, and analyzed by X-ray diffraction (XRD) to investigate the conditions for the formation of complex oxides. In addition, after heating, the samples were dissolved in hydrochloric acid and analyzed by inductively coupled plasma-optical emission spectroscopy (ICP-OES) to investigate the solubility of Ir. Figure 1 shows the results of dissolving a sample heated for one hour at a given temperature with 20 mL of concentrated HCl. The dissolution conditions were 3 h at 353 K. The acid solubility of Ir was shown to be enhanced by heating at temperatures above 973 K. In particular, the dissolution rate was higher when heated at 1473 K and 1573 K. The XRD profiles for the heating temperatures of 873K-1073K, in which changes in solubility appeared, are shown in Fig. 2. At 873 K, the metal remains metallic Ir after heating. At 973 K, the formation of Ca2IrO4, a complex oxide of Ir and CaO, was observed, but metallic Ir was also observed to remain. At 1073 K, metallic Ir was no longer identified, indicating that most of the metal was Ca2IrO4. As described above, the acid solubility of Ir is improved by forming a complex oxide with CaO, and it can be dissolved in oxidant-free HCl. In this paper, we also provide a thermodynamic verification of the experimental decomposition temperatures for a complex oxide of Ir and CaO. The dissolution mechanism of Ir by complex oxidation was also discussed.










Similar content being viewed by others
References
Yamabe-Mitarai Y, Zebin B, Murakami H, Abe H, Matsumoto T (2011) Effective use of platinum group metals. J Jpn Inst Metals 75:10–20. https://doi.org/10.2320/jinstmet.75.10
Yamabe-Mitarai Y, Murakami H (2013) High-temperature oxidation resistance of ir-based alloys. Mater Jpn 52:440–444. https://doi.org/10.2320/materia.52.440
Yamabe-Mitarai Y (2012) Effective use of platinum group metals and alloys. J Life Cycl Assess Jpn 2:143–150. https://doi.org/10.3370/lca.8.143
Okabe TH (2007) Current status of platinum group metals and recycling technologies. Mater Jpn 46:522–529. https://doi.org/10.2320/materia.46.522
Matthey J (2022) PGM market report, May 2022
Masuko N (1991) Oxygen evolving anode of titanium substrate. Tetsu-to-Hagane 7:871–877. https://doi.org/10.2355/tetsutohagane1955.77.7_871
Morimitsu M (2014) Oxide coated titanium electrode for oxygen evolution. J MMIJ 130:415–420. https://doi.org/10.2473/journalofmmij.130.415
Higobashi H (2015) Highly-durable iridium oxide anode. J Surf Finish Soc Jpn 66:3–5. https://doi.org/10.4139/sfj.66.3
Shibata J, Okuda A (2002) Recycling technology of precious metals. Shigen-to-Sozai 118:1–8. https://doi.org/10.2473/shigentosozai.118.1
Horike C, Morita K, Okabe TH (2013) Dissolution of platinum by hydrochloric acid: development of environmentally sound new recycling process. Mater Jpn 52:71–73. https://doi.org/10.2320/materia.52.71
Ohriner EK (2008) Platinum metals review. Process Iridium Iridium Alloys 52:186–197. https://doi.org/10.1595/147106708X333827
Kasuya R, Miki T, Morikawa H, Tai Y (2014) Development of new dissolution process of platinum via double oxides. J Jpn Inst Met Mater 78:242–249. https://doi.org/10.2320/jinstmet.JA201403
Kasuya R, Miki T, Tai Y (2013) Preparation of Li2PtO3 and its dissolution properties in hydrochloric acid. J Ceram Soc Jpn 121:261–264. https://doi.org/10.2109/jcersj2.121.261
Kasuya R, Miki T, Morikawa H, Tai Y (2013) Synthesis of alkali metal platinates and their dissolution behavior in hydrochloric acid. J Ceram Soc Jpn 121:884–890. https://doi.org/10.2109/jcersj2.121.884
Kasuya R, Miki T, Morikawa H, Tai Y (2014) Synthesis of sodium platinates and their dissolution behaviors in hydrochloric acid: effects of lithium carbonate addition on platinate formation. Int J Miner Process 128:33–39. https://doi.org/10.1016/J.MINPRO.2014.02.005
Kasuya R, Miki T, Morikawa H, Tai Y (2015) Enhanced dissolution of alkali metal platinates in dilute hydrochloric acid by addition of calcium chloride. Miner Eng 76:135–140. https://doi.org/10.1016/j.mineng.2014.10.011
Kasuya R, Miki T, Morikawa H, Tai Y (2015) Dissolution process of palladium in hydrochloric acid: a route via alkali metal palladates. Metall Mater Trans B 46:2476–2483. https://doi.org/10.1007/s11663-015-0431-x
Kasuya R, Nomura K, Narita H (2020) Solubilization of rhodium in hydrochloric acid using an alkali metal salt method. Metall Mater Trans B 51:377–385. https://doi.org/10.1007/s11663-019-01740-8
Nomura K, Kageyama H (2013) Recycling technology for platinum group metals by using perovskite-type oxides. Materia Japan 52:58–63. https://doi.org/10.2320/materia.52.58
Date M, Nomura K, Kageyama H, Fujitani T (2011) Noble metal collection through air: perovskite oxide as a novel collector. ChemPhysChem 12:109–111. https://doi.org/10.1002/cphc.201000618
Okabe TH, Kayanuma Y, Yamamoto S, Maeda M (2003) Platinum recovery using calcium vapor treatment. Mater Trans 44:1386–1393. https://doi.org/10.2320/matertrans.44.1386
Okabe TH, Yamamoto S, Kayanuma Y, Maeda M (2003) Recovery of platinum using magnesium vapor. J Mater Res 18:1960–1967. https://doi.org/10.1557/JMR.2003.0272
Kayanuma Y, Okabe TH, Maeda M (2004) Metal vapor treatment for enhancing the dissolution of platinum group metals from automotive catalyst scrap. Metall Mater Trans B 35:817–824. https://doi.org/10.1007/s11663-004-0075-8
Kayanuma Y, Okabe TH, Mitsuda Y, Maeda M (2004) New recovery process for rhodium using metal vapor. J Alloys Compd 365:211–220. https://doi.org/10.1016/S0925-8388(03)00666-2
Okabe TH, Nakata H, Morita K (2008) Recovery technology of platinum group metals. J Surf Finish Soc Jpn 29:592–600. https://doi.org/10.1380/jsssj.29.592
Sasaki H, Maeda M (2014) Zn-vapor pretreatment for acid leaching of platinum group metals from automotive catalytic converters. Hydrometallurgy 147–148:59–67. https://doi.org/10.1016/j.hydromet.2014.04.019
Horike C, Morita K, Okabe TH (2012) Effective dissolution of platinum by using chloride salts in recovery process. Metall Mater Trans B 43:1300–1307. https://doi.org/10.1007/s11663-012-9746-z
Kobayashi Y, Yamada S, Nagai T (2019) New dissolution process of iridium to hydrochloric acid. In: Azimi G, Kim H, Alam S, Ouchi T, Neelameggham NR, Baba AA (eds) Rare metal technology. Springer, Cham, pp 197–200. https://doi.org/10.1007/978-3-030-05740-4_19
Jacob KT, Okabe TH, Uda T, Waseda Y (1999) Solid-state cells with buffer electrodes for the measurement of thermodynamic properties of IrO2, CaIrO3, Ca2IrO4, and Ca4IrO6. J Electrochem Soc 146:1854–1861. https://doi.org/10.1149/1.1391855
Keawprak N, Tu R, Goto T (2009) Thermoelectric properties of Ca-Ir-O compounds prepared by spark plasma sintering. Mater Trans 50:853–858. https://doi.org/10.2320/matertrans.MRA2008377
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276. https://doi.org/10.1107/S0021889811038970
Acknowledgements
We would like to thank Editage (www.editage.com) for its English language editing services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Additional information
The contributing editor for this article was Yongxiang Yang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sanekata, R., Kobayashi, Y. & Nagai, T. Synthesis of Ir–CaO Complex Oxides and Their Solubilities in Hydrochloric Acid. J. Sustain. Metall. 9, 240–248 (2023). https://doi.org/10.1007/s40831-022-00643-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00643-4