Abstract
This study investigated a novel approach of using Al and Al–Mg scrap as heat providers and reductants that do not cause direct carbon-containing emissions in pyrometallurgical copper processing. Aluminum and magnesium are typical elements in metal wastes, such as WEEE, and they oxide easily under copper smelting conditions. In the reduction experiments, a copper- and nickel-rich industrial slag was equilibrated under Ar gas atmosphere at 1300 °C, after which a reductant metal piece was dropped on top of the slag. The slag-reductant samples were drop quenched in brine after 2–128 min of reduction. Thermodynamic calculations were executed with MTDATA to evaluate the phase equilibria and thermochemistry of the copper slag in metallothermic reduction. All the results proved that Al and Al-5wt% Mg alloys can be used as reductants in copper processes to enhance the recoveries of nickel and copper in metal/matte. Cu concentration in slag decreased from 2 to 1.2 wt% and Ni from 1.7 to 1.2 wt% in 30 min in aluminothermic reduction experiments, despite an immediate formation of a solid alumina layer on the surface of the reductant, hindering the reduction kinetics. The heat produced was calculated as 31 kWh/ton slag or 2.1 kWh/kg added Al or Al–Mg.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper and many other valuable metals contained in electronic scrap can be recycled efficiently in pyrometallurgical copper processes. However, the composition of the feed materials will influence the process chemistry, energy balance, and total metal recovery efficiency. If metals that burn, i.e., form oxides easily, such as Al and Mg, are present in the feed, they can behave as reductants for more noble metals and produce additional heat for the process. The Ellingham diagram in Fig. 1a includes typical copper and nickel smelting slag compounds (in liquid state) and partial pressures of oxygen between 10–10 and 10–6 atm (standard Gibbs energies of formation of the compounds ∆Gof = RTlnpO2). Figure 1 is drawn based on HSC 9.4.1 software and its database [1].
a The Ellingham diagram of the main compounds in copper smelting conditions. The black-dotted trend lines represent the partial pressures of oxygen from 10−10 atm (lowest line) to 10−6 atm (highest line). b The Ellingham diagram for aluminothermic reduction reactions for Ni, Cu, Si, and Fe oxides as a function of temperature
Figure 1a shows that all the metals in the system, except Ni and Cu, have a tendency to form oxides in the copper smelting conditions (1300 °C, pO2 ≈ 10–8 atm). Especially MgO, Al2O3, and SiO2 are very stable oxides, and thus, the oxidation of these metals (Mg, Al, Si) will occur quickly after feeding into the furnace. Simultaneously, more noble metals will reduce based on the oxidation–reduction interaction reactions. On the other hand, an addition of Al and Mg can also reduce SiO2 to metallic silicon unintentionally.
When metals are purposefully used as reductants in a reduction process, the process can be called metallothermic reduction. The most typical reductant metals are Al, Si, FeSi, Mg, Ca, and Na, which have low standard Gibbs energies of formation for their oxidic compounds. Additionally, for instance, iron is used as reductant for NiO and MoO3 in arc furnaces. [2] Obviously, metallothermic reactions and interactions occur ‘naturally’ in all pyrometallurgical processes. The main metallothermic reaction in the reduction is presented as simple anion exchange reaction as follows:
where Me is the reductant metal and M the desired recoverable metal. When using aluminum or magnesium as reductants, the processes are called aluminothermic or magnesiothermic reduction, respectively. The Ellingham diagram for the main aluminothermic reductions of Cu2O, NiO, FeO, and SiO2 is presented in Fig. 1b and shows that all oxides can be reduced by aluminum. In metallothermic reduction, Al is added in metal form, but also Al2O3, CaO, and MgO additions in feed will influence the slag chemistry and metals behavior as shown in multiple previous studies [3,4,5].
When scrap metals are used as reductants, no direct carbon dioxide emissions are produced during the reduction process, contrary to the more typical carbon-based reductants, including metallurgical coke, used in several metallurgical processes. Additionally, Al-containing non-ferrous scrap used as a raw material and reductant would promote a sustainable use of waste materials and carbon-free processing with lowered external energy need in smelting. Moreover, energy and electricity savings on mechanical recycling plants can be achieved if less pre-treated material feed can be used in the smelting process. This less pre-treated secondary feed can, furthermore, improve recoveries of valuable metals that would otherwise mistakenly end in the wrong scrap fractions or ‘disappear’ in the mechanical recycling circuit [6].
The mechanically treated copper-rich scrap fraction contains multiple elements, especially non-ferrous precious metals, Al, Zn, Sn, and Pb, but also for example iron that will be fed in the copper smelter as secondary raw material. The elements influence and behave differently in the prevailing process conditions, depending on their chemical, physical, and thermodynamic properties. The concept of employing certain metals, such as base metals Al and Fe, for pre-reduction and heat production in copper smelting is rather new. Study by Heo et al. [7] focused on the aluminothermic process for copper smelting slag to remove hazardous elements (As, Bi, Pb, and Sb) and to recover iron. The experiments were conducted employing a rod-dipping technique from iron metal–copper smelting slag system with an addition of different ratios of Al/FeO shots under an inert atmosphere at 1500 °C. The presented reaction mechanisms showed how the oxides in the slag, especially FeO, firstly reacted with Al and produced an irregular Al2O3 oxide layer on the interface of an Al particle, simultaneously reducing Fe droplets. Spinel phase was precipitated due to crucible corrosion and interaction with alumina layer, whereas further from the Al particle, olivine was shown to be decomposed, at least, at the crucible wall. This can simultaneously re-oxidize some of the Fe particles back into FeO. The time interval for all this to occur was extremely short, within 5 min according to their observations. Rinne et al. [8] studied aluminum-rich battery scrap as a reducing agent for industrial copper smelting slag at 1300 °C under Ar. They found that Cu concentration in slag decreased 0.3 wt% after 60 min reduction period and that many hazardous metals were removed efficiently from the slag.
Other studies on the aluminothermic reduction related to steel/iron production can be found quite extensively [e.g., 9,10,11], as well as studies on the recovery of different metals from wastes, such as red mud and chromite concentrate, by Al and various ferroalloys (FeAlSi, FeMo etc.) [e.g., 11, 12]. Heo et al. have quite extensively investigated the metallothermic reduction in the sense of Fe recovery from electric arc furnace slag in steelmaking by Al [9], Al dross [13] or Al-C composite pellets [14] at 1500–1550 °C in magnesia crucibles and the influence of Al/FeO ratio and fluxes (CaO, MnO) on Fe recovery. Sun and Mori [15] investigated the oxidation rate of aluminum in molten Fe(–Al)–CaO–SiO2–Al2O3–FeO–MnO system in alumina crucibles at 1600 °C under argon atmosphere.
This study employed experimental and computational techniques to investigate the influence of typical non-ferrous metals present in WEEE, Al, and Mg, on the thermochemistry and valuable metal behavior in copper flash smelting/direct-to-blister/converting process conditions. Kinetic experiments and thermodynamic calculations using MTDATA software [16] were employed to investigate the metallothermic reduction produced by pure Al and Al–Mg 5056 alloy in copper smelting process at 1300 °C.
Material and Methods
The experimental technique included sample melting with added Al or Al–Mg reductant piece, rapid quenching in brine, SEM-BSE (scanning electron microscope – back-scattered electrons) imaging and direct-phase analyses with EPMA (electron probe micro-analyzer). This drop-quenching technique has been used widely for phase equilibrium [e.g., 17,18,19] and minor element distribution studies [e.g., 20,21,22], and recently also in few kinetic studies [8, 23,24,25,26,27]. In the studies by Ruismäki et al. [24,25,26] and Avarmaa et al. [27], reduction kinetics were investigated for DON slag (Direct-Outokumpu-Nickel) with different reductants under nickel slag cleaning conditions.
Materials
The slag investigated was industrial ‘non-cleaned’ copper smelting slag with an addition of copper and nickel oxides. The initial industrial slag composition is shown in Table 1a. Copper and nickel were noticed to exist as mechanical segregations (matte droplets in slag) instead of being chemically dissolved in the industrial slag. Thus, additional Cu2O and NiO were mixed with the slag in order to follow the reduction kinetics and efficiency. The added amounts corresponded to typical Cu and Ni concentrations in slag before slag cleaning. The cuprite (Cu2O) employed was prepared by oxidizing pure A-grade cathode copper (Boliden Harjavalta Oy) pellets in air at 1025 °C for 120 h in a chamber furnace. An estimated purity of 99.99% was achieved [28]. The nickel monoxide (NiO) employed was commercial grade with purity of 99.99% supplied by Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The industrial slag was grinded, further mixed, and homogenized with the NiO and Cu2O reagents in an agate mortar. NiO and Cu2O additions in the slag were 2.6 wt% and 2.3 wt% (2 wt% Cu and Ni), respectively. The details of the reductant metals employed in the experiments are presented in Table 1b. The inert gas used in the experiments was argon supplied by Linde plc (Germany, 99.999 vol% purity).
Cone-shaped silica crucibles employed in the experiments were produced from Heraeus HSQ®300 glass (fused quartz with purity of > 99.998%) supplied by Finnish Special Glass (Espoo, Finland). The crucibles were 25 mm wide and 15 mm high. The slag weight in the experiments was 2.0 g and the added reductant material 0.03–0.033 g (~ 1.5 wt% of sample weight) per experiment. This was calculated with MTDATA to be adequate Al-reductant addition to reduce all solid magnetite in the industrial slag.
Experimental Equipment
The experimental furnace type was LTF 16/50/450 supplied by Lenton (Hope Valley, UK) using Eurotherm controller 3216 PID (Virginia, USA) and silicon carbide heating elements. Temperature was measured with a calibrated S-type (Pt-PtRh) thermocouple (Johnson-Matthey Noble Metals, UK) located close to the sample and the cold-junction compensation was measured with a Pt100 resistance thermometer (SKS Group, Finland). The thermocouple was connected to a 2010 DMM multimeter (Keithley, USA) and the cold junction to a 2000 DMM multimeter (Keithley, USA). Both temperatures were followed and recorded online with a LabVIEW program. The experimental furnace and setup during the experiments are presented elsewhere [23].
Experimental Procedure and Analytical Techniques
Metallothermic reduction experiments were executed by dropping an Al or Al–Mg piece on top of equilibrated slag. This was considered to simulate flash smelting furnace conditions when Al-containing scrap material is fed via concentrate burner or alternatively directly dropped to the furnace and it hits on top of the slag surface. The equilibration time for the slag was investigated with a time series between 15 and 240 min by melting 2.0 g of slag under inert Ar atmosphere at 1300 °C. The phase compositions were measured with SEM–EDS and the results showed that the slag was fully equilibrated in 120 min. Thus, for the metallothermic reduction experiments, the slag was equilibrated for 120 min before adding the reductant metal to investigate the reduction kinetics. In all experiments, 2.0 g of industrial slag with Cu2O and NiO additions were weighed in silica crucibles and equilibrated. The reductant metal dropped on top of the slag weighed 0.03–0.033 g (1.5% of the sample weight). All the reduction experiments are listed in Table 2.
The samples were prepared for analyses employing wet metallographic techniques. The samples were imaged, and preliminary phase compositions were analyzed by employing SEM–EDS (Tescan MIRA 3; Brno, Czech Republic) equipped with an UltraDry Silicon Drift Energy Dispersive X-ray Spectrometer (EDS) supplied by Thermo Fisher Scientific (Waltham, MA, USA) at Aalto University. The imaging was executed by BSE detector employing a systematic approach, meaning that the same imaging settings were used for all the samples. These settings were WD: 20.00 mm, SEM HV: 15 kV, SEM Mag: × 113. Also, the brightness and contrast were kept same for all the samples.
Different phases in the specimen display different brightness in the black and white BSE images based on the atomic number of the elements present. This means that higher brightness indicates a higher average atomic number, i.e., heavier elements/phases and lower brightness lighter elements/phases. This provides an opportunity to follow the progress of the reduction when systematically imaging the samples.
SEM–EDS phase composition analyses were executed as the preliminary analysis technique for the aluminum and aluminum–magnesium reduction experiments, and primary analyses were executed with EPMA at the Geological Survey of Finland (GTK). The EPMA analyses were conducted from the polished cross sections by an SX100 (Cameca SAS, France) microprobe equipped with five wavelength dispersive spectrometers (WDS). Systematical approach also with EPMA was necessary in order to achieve comparable results and to understand the reduction kinetics and progress. The acceleration voltage used for the EPMA analysis was 20 kV and the emission current was 60 nA. The slag was analyzed mainly as area analyses with a 100 µm defocused beam, except for the slag composition profile measurements, where focused beam settings were used. For the matte, area analyses with 50–100 µm beam were employed and for the reductant material, the beam size was varied from 1 to 100 µm with the aim to analyze all different phases present. 6 analyses were taken from the slag phase, 3–5 analyses were taken from the matte and speiss phases. The standard materials used were natural minerals and synthetic metals: O Kα (hematite), Na Kα (tugtupite), Mg Kα and Ca Kα (diopside), Al Kα (Al2O3), Si Kα (quartz), S Kα (pentlandite), K Kα (sanidine), Cr Kα (chromite), Fe Kα (hematite), Co Kα ja As Kβ (cobaltite), Ni Kα (Ni), Cu Kα (Cu), Zn Kα (ZnS), Sb Lα (SbTe), Pb Lβ (PbS), Bi Lα (BiSe), and Mo Lα (Mo) ja Ti Kα (rutile). The calculated averages and standard deviations of the EPMA totals were for matte 99.1 ± 0.7, for slag 98.3 ± 1.1 and for reductant metal pieces 99.3 ± 3.3. The WDS analytical results were corrected employing the PAP-ZAF matrix online correction program [29] and normalized to 100%. The detection limits achieved for each element in each phase are presented in the supplementary material (Table S1).
Heat Balance Calculations
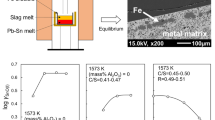
The energy balance calculations were executed with MTDATA and its TCFE, MTOX, and MTOXGAS databases [16] to evaluate the heat produced and the adiabatic temperature by metallothermic reduction of Al and Al–Mg in copper smelting conditions. The pure Al–Mg alloy included FCC Al-rich phase with intermetallic Al–Mg solid phase at 25 °C. The system was defined to correspond to the experimental compositions, despite simplifying it to a pure iron-silicate slag containing copper and nickel oxides. The ‘start’ composition for slag was Al/Al–Mg-free slag and ‘end’ composition contained 1.45 wt% Al/Al–Mg. The oxygen partial pressure in the system without the addition of Al or Al–Mg was 10–7.1 atm (start composition), whereas the addition of Al or Al–Mg decreased the pO2 down to 10–9.1 atm (end composition). No differences in the phase compositions existed between different reductants, except small MgO concentration increase in slag (max 0.15 wt% MgO) with Al–Mg reductant. The liquid metal phase started to form when 0.25 wt% of reductant was added in the slag system. Although alumina concentration in slag was maximum 3 wt% and MgO 0.15 wt%, nickel and copper oxide concentrations decreased significantly from their original values (2 wt% Cu and Ni each in slag). With the highest Al and Al–Mg additions (1.45 wt% in the system), Ni and Cu concentrations in slag decreased to 1 and 0.6 wt%, respectively.
The heat produced at constant total pressure and temperature by the addition of Al or Al–Mg in the copper smelting slag was calculated as follows:
where the enthalpy change for slag at 1300 °C is
and the heating enthalpy of the reductant metal is
Table 3 collects the numerical data used for the heat calculations and provides the heat results. The results show that the addition of Al or Al–Mg in the copper smelting slag is an exothermic process, and it produces heat of 31 kWh/ton slag or 2.1 kWh/kg added Al or Al–Mg. The adiabatic temperature was calculated and for 1.45 wt% Al concentration in slag, the temperature increased to 1385 °C (see Fig. 2). Heo et al. [7] computationally calculated (Factsage) temperature increase of 350–800 °C produced by addition of Al/FeO shots in iron–copper smelting slag system.
Results
The sample imaging and phase composition analyses were carried out and examined systematically in order to investigate the reduction progress. Normalized results were employed, and uncertainties were calculated as standard deviations (± 1σ). The time interval for aluminum-reduction experiments was from 2 to 128 min (7 experiments) whereas for Al–Mg reductions from 15 to 60 min (3 experiments).
The sample micrographs of both series Al and Al–Mg are presented with × 20 magnification in Figs. 3 and 4, respectively. The visual evolution of the metallothermic reduction can be seen in the figures, and dramatical changes clearly occurred between 30 and 60 min.
As aluminum and Al-magnesium alloy are less dense (densities for liquid Al 2.21 g/cm3 and Al-5wt% Mg 2.15 g/cm3 at 1300 °C calculated by Factsage© FTLite database) than slag (3.3–4.1 g/cm3 [30]) and matte (3.9–5.2 g/cm3 [30]), the reductant metal pieces dropped on top of the slag remained floating on top of the slag layer. Even after 120 min, the highly reacted Al and Al–Mg pieces showed a tendency to stay close to the upper edge of the slag, which might indicate high viscosity and/or influence of surface and interfacial tensions of the system. Instead, the more dense and heavier matte phase was found at the bottom of the crucible. The following chapters examine more profoundly the compositional results of matte/speiss (“Matte and Speiss Phases”), slag (“Slag”), as well as the reductant metal and its surroundings (“Reductants”).
Matte and Speiss Phases
The microstructures image series for matte, taken by SEM-BSE with × 113 magnification, are shown in Figs. 5 and 6. One main matte droplet was settled to the bottom of the crucible in all experiments, except OT19 (4 min reduction by Al) which had two separate matte droplets (the bigger one included in the figure).
The formed matte clearly included two phase compositions and structures, as shown in Figs. 5 and 6. In the cross section of sample OT20 (8 min reduction by Al), only one phase structure was visible (speiss). Both phase compositions in matte were measured with EPMA (3–5 area analysis on both phases), and the results showed that the brighter phase is indicated to speiss type of composition and the gray phase to white metal (Cu2S) composition. The relatively high arsenic and antimony concentrations revealed that the heavier matte phase was a speiss type of intermetallic phase. Speiss in non-ferrous metal production is a complex mixture of copper, nickel, iron, and/or silver as arsenides and antimonides that can also contain some sulfur and lead [31,32,33,34,35]. According to Figs. 5 and 6, it seemed that the overall matte droplet size increased, and the amount of brighter phase (speiss) increased as the reduction time increased. Note that the cross sections presented were chosen randomly and will most likely vary to some extent throughout the samples, indicating that especially matte droplet size and the white metal/speiss ratio will in reality vary in the samples.
Figure 7 presents the main element results (Cu, S, and Ni) for the matte phase with both reductants. The uncertainties (± 1σ) are included for the Al-reduction results, but not for the Al–Mg-reduction results as they were smaller than the used symbol for every sample. The zero-time interval results (OT17 sample) were achieved by equilibrating the slag for 120 min in inert gas atmosphere without metal reductant addition.
The main elements in the matte phase as a function of time for both metal reductants at 1300 °C. Black-filled symbols indicate the results for Al-reduction experiments and the gray cross symbols for the corresponding results of Al–Mg-reduction experiments. The error bars in Al-reductant series show the standard deviations of EPMA analyses
The composition of the matte phase stayed very constant as a function of time and was independent on the reductant used. The copper-nickel matte formed was high-grade Cu–Ni matte with 70–72 wt% Cu, 19–20 wt% S, 6–8 wt% Ni, and 1 wt% Fe that was relatively close to white metal (Cu,Ni)2S composition. Additionally, traces of impurity metals As (0.2–1.2 wt%), Sb (0.1–0.2 wt%), Pb (0–0.1 wt%), and Bi (0.01–0.05 wt%) were measured.
The analytical results for the speiss phase are presented in Fig. 8. Only uncertainties for nickel and copper were included in the figure, because the uncertainties of other elements (S, As and Sb) were smaller than their symbols. Traces of Bi (0.03–0.3 wt%) and Pb (˜0.1 wt%) were measured in the samples. Cobalt and zinc concentrations were close to their detection limits, around 100–300 ppmw.
Although the white metal composition stayed constant as a function of time, the composition of the speiss phase changed considerably as a function of time. Figure 8 shows that nickel concentration in speiss first increased close to 60 wt% and after 5 min started to decrease and stabilized at around 30 wt% in 60 min. Copper instead first decreased down to 25 wt% followed by an increase to 50 wt%. Arsenic showed small increase for the first 5 min, after which it started to decrease as a function of time and stabilized at 5 wt%. Sulfur and antimony concentrations remained relatively constant throughout the investigated time period. The speiss phase did not have homogeneous phase structure, but more as a net-type pattern with different mixtures of phases. Thus, as the analyses were conducted with a defocused beam (50–100 µm), the speiss composition results are more suggestive and contain more variation than the white metal results (Fig. 7), probably due to the low melting point of the As-Fe system [36].
In addition to the literature dealing with speiss in non-ferrous metal processing [31,32,33,34,35], studies on phase equilibrium and phase diagrams on Cu–Ni–As/Sb systems exist. Uhland et al. [37] calculated phase diagrams for Cu–As–Ni alloys and showed that these three elements have great tendencies to form multiple different phase assemblies depending on temperature and their concentrations in the system. Itakagi et al. [36] studied ternaries of the As–Cu/Fe-S system at 1150 °C including phase boundaries with different Cu/Fe ratios. Their results show how a miscibility gap appears into the system producing copper matte and low-sulfur speiss in the presence of As. The diagram also presents how the Cu/Fe ratio influences the miscibility gap and the compositions of the matte and speiss. In the present study, the experiments were conducted for white metal smelting slag where iron concentration in matte was very low (around 1 wt%), see Fig. 7.
In general, arsenic has a high tendency to volatilize in the copper smelting and converting conditions, and its distribution behavior and activities have been broadly studied the in matte–metal–slag–gas(-dust) systems, e.g., [38,39,40,41,42,43]. High arsenic concentration is not permitted in the blister copper and it has maximum limit determined; thus, its removal is critical and its accumulation in the flue dust that is recycled into smelting circuit should be avoided. Additionally, speiss formation in, e.g., flash smelting furnace can have detrimental effects on furnace lining and bottom integrity due to its infiltration tendency in the refractories [44, 45].
Slag
Slag was homogeneous, i.e., well quenched in all the samples, as can be seen from the micrographs in Figs. 3, 4, 5, and 6. Overall, only some small brighter dispersions were visible locally in the samples, and in some samples close to the reductant metal, slag seemed visually somewhat different (darker in BSE figures). The slag was systematically analyzed from different parts of the sample (3 separate locations) in order to investigate the overall reduction of the slag and to determine the interaction area of the reductant metal. The slag results in Figs. 9 and 10 are the calculated averages and standard deviations from the analyses of two different locations: close to the matte (3 analysis) and opposite corner from the Al piece and matte (3 analyses). Only alumina concentration was varying between these locations and had greater standard deviation when compared to other components and elements, see Fig. 9. Third analysis location for slags was close to the reductant and its interaction volume, but these results were not included in the result Figs. 9 and 10.
Iron and the stable oxide results for slag are presented in Fig. 9. The uncertainties (± 1σ) were added only for iron, silica, and alumina as the standard deviations for the other components were smaller than their symbols. In the Al-reductant experiments, most of the elements/oxide concentrations remained constant as a function of time, only alumina and silica increased as time increased, whereas Na2O seemed to decrease slightly. For the Al–Mg experimental series, all the components in Fig. 9 stayed constant as a function of time. In general, the reductant metal did not influence the slag composition, even the MgO concentration was equal for both series. Alumina concentration in slag was dependent on the location at 64 and 128 min experiments, and thus, presents larger uncertainties in Fig. 9.
Copper, nickel, zinc, and other minor metal results are presented in Fig. 10. The uncertainties (± 1σ) are shown for Ni, Cu, and Zn. The presented ‘other metals’ include sum of As, Sb, Pb, and Bi concentrations. Sulfur and chromium were also detected in the slags in the levels of 100–300 ppmw each, as well as traces of molybdenum were measured in some samples (0.1–0.3 wt%).
Until 30 min, the slag composition remained relatively constant for Al-reductant experiments, after which Cu concentration dropped from 2 to 1.2 wt%, Ni from 1.7 to 1.2 wt%, and Zn from 1 to 0.5 wt%. For Al–Mg-reduction experiments, zinc concentration seemed to stay constant throughout the entire time range investigated, and (possibly) the decrease for Ni and Cu was not as radical as for Al-reductant experiments.
Clear reaction zones in the slag close to the reductant piece, where the slag was visually and compositionally different, existed in the samples. This zone enlarged as a function of increasing reduction time. Slag composition profiles of the slags were measured, and they showed that aluminum concentration was higher close to the reductant and decreased as the distance increased, whereas nickel and copper concentrations were almost zero close to the reductant, indicating that they had been effectively reduced from the reaction zone.
The distribution coefficients of copper and nickel between different phases (speiss, matte, and slag) as a function of reduction time are presented in the supplementary material (Fig. S1). The distribution coefficients between matte and slag (Lm/s) increased as a function of time from 35 to 60 and from 3.5 to 5–7 for copper and nickel, respectively. Nickel was enriched in the speiss with the distribution coefficient Lspeiss/m values between 4 and 8, whereas copper was enriched in the matte phase with the distribution coefficient Lspeiss/m values between 0.2 and 0.7. The distribution coefficient of As between speiss and matte was between 10 and 40 without dependency on reduction time. If compared to the reduction studies of DON slag by gas reductant (methane), battery scrap rich in graphite, biochar, and coke [24, 25, 27], the metallothermic reduction used in this study seems to have slower reduction kinetics and achieve lower distribution coefficients between matte and slag.
Reductants
Figures 11 and 12 show SEM-BSE micrographs of the reductant metal pieces with × 113 magnification for aluminum and aluminum–magnesium alloy series, respectively. The analysis points measured from the different phases formed and reduced are numbered in the figures, and their compositions are presented in the supplementary material (Tables S2 and S3). The composition (Al, Fe, Si, Cu, and Ni concentrations) of the reductants and the darker crust formed around the reductant are shown in Fig. 13 up to 32 min, after which the reductant metal pieces were decomposed to entirely different compositions and phases.
As seen in the micrograph series and composition results (Fig. 13 and supplementary material), several phases: solids and liquids formed, reduced, and decomposed during the reduction. The reaction interface appeared very irregular and metal alloys reduced (brighter, i.e., heavier phases) started to appear immediately after the reductant metal addition and their amounts increased as the reduction proceeded. The reductant compositions stayed rather constant up to 32 min, see Fig. 13a and b. Immediately at 2 min, 4–5 wt% Fe and Si were dissolved in the Al reductant, as well as 0.6 wt% Ni and 2 wt% Cu. Ni and Cu concentrations doubled in four minutes in the Al reductant. The Al–Mg reductant dissolved two or three times more Fe, Cu, and Ni than pure Al reductant, as shown in Fig. 13b. At 64 min Al-reductant experiment, the reductant material had approximately a composition of Ni(Al,Fe)4(Cu,O)4.5 (22.5 mol% O, 22.5 mol% Cu, 20 mol% Fe, 20 mol% Al, and 10 mol% Ni). Similar phase composition was measured at one analysis spot for 60 min Al–Mg-reduction experiment. Nevertheless, at 128 min Al reduction and 60 min Al–Mg-reduction experiment, the reductant composition was reacted close to mullite structure. Magnesium was dissolved from the reductant quickly and was not detected at any of analyses conducted from the Al–Mg reductant pieces.
If Al and Al–Mg reductants are compared visually, it seems that Mg-containing reductant reduced and reacted faster with the slag than the pure Al reductant. Nevertheless, both reductants were present up to 30 min experiments and were severely degraded at 60 min experiments.
The crust formed around the reductant, typically between 20 and 60 µm thick, was of relatively pure Al2O3 with traces of other metals, as shown in Fig. 13c. In the first 4 min, the concentrations of Si, Fe, Cu, and Ni increased in the crust, after which they decreased, and the crust composition stayed relatively unchanged between 8 and 32 min.
The compositions of the other phases decomposing and reducing in the reaction interface are shown in the supplementary material. Al- and Fe-rich metal alloys with varying concentrations of Ni and Cu started to form immediately after reductant metal addition. As the reduction proceeded, the Al-rich metal phase decomposed and Fe–Ni–Cu phases became dominant metal alloys with only small concentrations of Al, Si, and other elements. Several other solid solutions and liquid phases were observed, including an Al2O3-rich slag and mullite type of structures. SEM–EDS analyses also measured spinel type of structures close to the reductant. The phase compositions varied and were dependent on the exact analysis location. For the longest experiments at 60 min and longer, no pure alumina crust was found but recrystallized Al–Si–Fe oxides with varying concentrations of Ni and Cu. Magnesium was measured at low concentrations (1.2–1.6 wt%) in the Si-enriched mullite phase (10–20 mol% Al, 15–20 mol% Si, and 65 mol% O) for the 30 and 60 min Al–Mg experiments.
Discussion
Phase Assembly
Phase equilibrium of a Al–SiO2–FeO–Cu2O–NiO–O2 system was computationally examined to confirm and clarify all the possible phases present during the aluminothermic at 1300 °C and pO2 = 10–8 atm. Figure 14 presents the ternary diagram of Al-SiO2–FeO system with constant Cu2O = 2.3 wt% and NiO = 2.6 wt% calculated employing MTDATA software and its MTOX and SGTE_SOL databases [16]. The oxygen amount in the system was defined to show the phase equilibrium with gas phase from the SP + COR + MUL + LIQ phase assembly onwards to the SiO2–FeO quasibinary, but without the gas towards the Al corner. This enabled to predict all the possible phases, from metallic aluminum to the slag, during the reduction.
Al–SiO2–FeO ternary diagram at 1300 °C and pO2 10–8 atm with constant Cu2O and NiO concentrations calculated by MTDATA with MTOX and SGTE_SOL databases [16]
The phase diagram shows that liquid metal alloy and metal solids with FCC and BCC structures (Fe(s)) can form during the aluminothermic reduction. Additionally, typical solid oxides present according to Fig. 14 are corundum ((Al,Fe)2O3), mullite ((Al)2(Al,Si)(O)5), and spinel (magnetite, (Cu,Fe,Mg,Ni)(Al,Cu,Fe,Ni)2(Fe,Mg)2(O)4) phases. These are in agreement with the measured compositions of the reduced phases in the experiments shown in the supplementary material (Tables S2 and S3).
Kinetic Analysis
Various rate-controlling steps in an aluminothermic reduction have been considered: a chemical reaction, a penetration of molten aluminum into slag phase, and a diffusional transfer in slag phase [7, 8]. The reductant–slag interface was irregular and unstable, in addition to spontaneous exothermic reactions at the interface causing dynamic condensed phase flow and temperature imbalance (Marangoni flow). As shown, multiple different phases were visible and measured in the reaction interface, this made it impossible to determine the reaction area and volume. Additionally, the analyzing results of slag focused on investigating the overall aluminothermic reduction of the slag further from the interaction area. Moreover, the combination of matte and speiss as the metallic phase(s) as well as the various phases in the aluminum reductant made it difficult to analyze the composition change of the metallic phase(s) as a function of time. Due to these characteristics, the apparent reaction rate equation based on mass transfer was employed for kinetic analysis for the slag phase, as in previous study [7]:
where the function f(t) was determined by finding the best fitting lines for the experimental results of CMO (wt% of NiO and Cu2O calculated from EPMA results) as a function of time, (CMO)0 and (CMO)t were metal oxide concentrations in slag initially and at time t, respectively, and \({k}_{\mathrm{MO}}^{\mathrm{App}}\) was the apparent mass transfer coefficient of metal oxide. The fitted lines and their details are presented in Fig. 15.
These fitted lines were employed to evaluate the apparent mass transfer coefficients of copper and nickel oxides. The natural logarithm of NiO and Cu2O concentration changes as a function of time are presented in Fig. 16, from where the apparent mass transfer coefficients can be determined from the slopes of the curves. The blue lines are calculated purely based on the values of the fitted lines in Fig. 15, the black lines employ the measured C0 values with fitted Ct values and the orange symbols present the results based on the experimental results measured by EPMA. The concentration values of Ni and Cu in slag based on EPMA analyses stayed constant until 32 min, indicating no mass transfer occurred into these measured slag areas (further from the slag–reductant interface) at these stages. In the first stage(s), it is expected that the reduction of ferric iron to ferrous (Fe3+ → Fe2+) occurs in the industrial copper and nickel smelting slags. Thus, the apparent mass transfer coefficients were possible to evaluate only between 32 and 64 min from the experimental results, being 0.10 and 0.06 min−1 for Cu2O and NiO, respectively.
The apparent mass transfer coefficients of nickel oxide defined from the slopes of the curves set for − ln(CNiO0 − CNiOt) fitted and − ln(CNiO0 measured − CNiOt fitted) between 16–40 min and 25–45 min were 0.10 min−1. For copper oxide, the apparent mass transfer coefficients defined from the slopes of the curves set for − ln(CCu2O0–CCu2Ot) fitted and − ln(CCu2O0 measured–CCu2Ot fitted) between 16–40 min and 30–40 min were 0.22 and 0.26 min−1, respectively. More data from different times (especially between 30 and 60 min) and from the interfacial area are needed to determine properly the mass transfer coefficients and other kinetic parameters. Additionally, longer time experiments were equilibrium with the reductant has been reached, with and without stirring, would bring valuable information for aluminothermic reduction in copper smelting processes.
Conclusions
WEEE is the fastest growing waste stream in the world with the annual growth rate of 3–6%, and the evaluated amount is already over 50 Mtons/year. In addition of being valuable secondary metal resource, WEEE fractions can be used for other functional purposes, such as fuel or metallothermic reductants. This work investigated experimentally and computationally the pre-reduction potential of Al and Al–Mg alloy in pyrometallurgical copper processing. Metallothermic reduction experiments were executed as a function of time (2–128 min) under inert Ar gas atmosphere at 1300 °C employing drop-quenching technique followed by SEM-BSE imaging and EPMA. Additionally, MTDATA software with MTOX, TCFE and SGTE_SOL databases was employed to calculate the phase equilibrium and heat content data of the investigated system and forecast the phases present during the aluminothermic reduction. Based on the computational and experimental results, 1.5 wt% addition (or even less) of Al or Al–Mg is enough to improve the recoveries of Cu and Ni in the matte and metal phases, i.e., to use for metallothermic reduction of Ni, Cu, and other more noble metals in copper smelting processes. For efficient process, the reductant metals will need proper stirring and turbulent conditions in the furnace, as a solid corundum crust is rapidly formed around the reductant pieces on the slag surface, and process monitoring to control possible accretions or other solid formations.
References
Roine A (2017) HSC chemistry® [9.4.1]. Metso Outotec, Pori
Hall F (2000) Aluminothermic processes. Ullmann’s encyclopedia of industrial chemistry. Wiley, Weinheim
Chen M, Avarmaa K, Klemettinen L, O’Brien H, Sukhomlinov D, Shi J, Taskinen P, Jokilaakso A (2020) Recovery of precious metals (Au, Ag, Pt, and Pd) from urban mining through copper smelting. Metall Mater Trans B 51(4):1495–1508
Dańczak A, Klemettinen L, Kurhila M, Taskinen P, Lindberg D, Jokilaakso A (2020) Behavior of battery metals lithium, cobalt, manganese and lanthanum in black copper smelting. Batteries 6(1):16
Avarmaa K, Klemettinen L, O’Brien H, Taskinen P (2019) Urban mining of precious metals via oxidizing copper smelting. Miner Eng 133:95–102
Hagelüken C (2006) Improving metal returns and eco-efficiency in electronics recycling-a holistic approach for interface optimisation between pre-processing and integrated metals smelting and refining. In: IEEE international symposium on electronics and the environment. IEEE, Scottsdale (AZ), USA, pp 218–223. https://doi.org/10.1109/ISEE.2006.1650064
Heo J, Chung Y, Park J (2016) Recovery of iron and removal of hazardous elements from waste copper slag via a novel aluminothermic smelting reduction (ASR) process. J Clean Prod 137:777–787
Rinne T, Klemettinen A, Klemettinen L, Ruismäki R, O’Brien H, Jokilaakso A, Serna-Guerrero R (2021) Recovering value from end-of-life batteries by integrating froth flotation and pyrometallurgical copper-slag cleaning. Metals 12(1):15
Heo J, Park J (2017) Thermochemical analysis for the reduction behavior of FeO in EAF slag via Aluminothermic Smelting Reduction (ASR) process: Part I. Effect of aluminum on Fe & Mn recovery. Calphad 58:219–228
Park J, Song H, Min D (2004) Reduction behavior of EAF slags containing Cr2O3 using aluminum at 1 793 K. ISIJ Int 44(5):790–794
Shibata E, Egawa S, Nakamura T (2002) Reduction behavior of chromium oxide in molten slag using aluminum, ferrosilicon and graphite. ISIJ Int 42(6):609–613
Atasoy A (2011) Reduction of ferric oxides in the red mud by the aluminothermic process. In: 6th international advanced technologies symposium (IATS‘11), Elazığ, Turkey, pp 16–18
Heo J, Park J (2017) Thermochemical analysis for the reduction behavior of FeO in EAF slag via Aluminothermic Smelting Reduction (ASR) process: Part II. Effect of aluminum dross and lime fluxing on Fe and Mn recovery. Calphad 58:229–238
Heo J, Yoo J, Chung Y, Park J (2019) Influence of aluminum-carbon composite pellets on FeO reduction and iron recovery from electric arc furnace slag. Metall Mater Trans B 50(2):903–913
Sun H, Mori K (1996) Oxidation rate of aluminum in molten iron by CaO-SiO2-Al2O3-FeO-MnO slag. ISIJ Int 36:S34–S37
Gisby J, Taskinen P, Pihlasalo J, Li Z, Tyrer M et al (2017) MTDATA and the prediction of phase equilibria in oxide systems: 30 years of industrial collaboration. Metall Mater Trans B 48(1):91–98
Zhang R, Mao H, Halli P, Taskinen P (2016) Experimental phase stability investigation of compounds and thermodynamic assessment of the BaO–SiO2 binary system. J Mater Sci 51:4984–4995
Hamuyuni J, Hellstén N, Akdogan G, Taskinen P (2015) The liquidus in Cu-O-CaO system at metallic copper saturation up to 1698 K. J Am Ceram Soc 98(1):320–323
Jak E, Hayes P, Lee H (1995) Improved methodologies for the determination of high temperature phase equilibria. Met Mater 1:1–8
Avarmaa K, O’Brien H, Johto H, Taskinen P (2015) Equilibrium distribution of precious metals between slag and copper matte at 1250–1350 ˚C. J Sustain Metall 1(3):216–228
Klemettinen L, Avarmaa K, O’Brien H, Taskinen P, Jokilaakso A (2019) Behavior of tin and antimony in secondary copper smelting process. Minerals 9(1):39–55
Hidayat T, Chen J, Hayes P, Jak E (2019) Distributions of Ag, Bi, and Sb as minor elements between iron-silicate slag and copper in equilibrium with tridymite in the Cu-Fe-O-Si system at T= 1250 ˚C and 1300 ˚C (1523 K and 1573 K). Metall Mater Trans B 50(1):229–241
Wan X, Fellman J, Jokilaakso A, Klemettinen L, Marjakoski M (2018) Behavior of Waste Printed Circuit Board (WPCB) materials in the copper matte smelting process. Metals 8(11):887
Ruismäki R, Dańczak A, Klemettinen L, Taskinen P, Lindberg D, Jokilaakso A (2020) Integrated battery scrap recycling and nickel slag cleaning with methane reduction. Minerals 10(5):435
Ruismäki R, Rinne T, Dańczak A, Taskinen P, Serna-Guerrero R, Jokilaakso A (2020) Integrating flotation and pyrometallurgy for recovering graphite and valuable metals from battery scrap. Metals 10(5):680
Dańczak A, Ruismäki R, Rinne T, Klemettinen L, O’Brien H, Taskinen P, Jokilaakso A, Serna-Guerrero R (2021) Worth from waste: utilizing a graphite-rich fraction from spent lithium-ion batteries as alternative reductant in nickel slag cleaning. Minerals 11(7):784
Avarmaa K, Järvenpää M, Klemettinen L, Marjakoski M, Taskinen P, Lindberg D, Jokilaakso A (2020) Battery scrap and biochar utilization for improved metal recoveries in nickel slag cleaning conditions. Batteries 6(4):58
Hamuyuni J, Taskinen P (2015) Liquidus in system Cu2O-CaO-Al2O3 at 1250°C. In: 6th international symposium on high-temperature metallurgical processing. Springer, Cham, pp 123-130
Pouchou J, Pichoir F (1986) Basic expression of “PAP” computation for quantitative EPMA. In: Brown JD, Packwood RH (eds) 11th international congress on X-ray optics and microanalysis (ICXOM). University of Ontario, Canada, pp 249–256
Schlesinger M, Sole K, Davenport W, Alvear G (2021) Extractive metallurgy of copper, 6th edn. Elsevier, Amsterdam, p 574
Azakami T, Hino M, Yazawa A (1979) The liquid miscibility gap and the distribution of silver between speiss and metallic lead in the Pb-Fe-As, Pb-Cu-As and Pb-Fe-Cu-As systems at 1200 °C. Can Metall Q 18(4):389–394
Voisin L (2012) Distribution of precious metals during the reducing pyrometallurgical processes of complex copper materials. In Noble metals. InTech, Rijeka, Croatia, pp 47–70
Thornton C, Rehren T, Pigott V (2009) The production of speiss (iron arsenide) during the Early Bronze Age in Iran. J Archaeol Sci 36(2):308–316
Peterson M, Twidwell L (1985) Removal of arsenic from lead smelter speiss. J Hazard Mater 12(3):225–229
Czerny C, Holliday R, Floyd J (1986) The distribution of gold between bullion, speiss, and slag during zinc-lead smelting. In: 13th CMMI Congress, vol 4, pp 133–142
Itagaki K, Hino M, Yazawa A (1983) Phase relations and activity of arsenic in liquid Cu-Fe-S-As systems. Erzmetall 36(2):59–64
Uhland S, Lechtman H, Kaufman L (2001) Assessment of the As-Cu-Ni system: an example from archaeology. Calphad 25(1):109–124
Montenegro V, Sano H, Fujisawa T (2013) Recirculation of high arsenic content copper smelting dust to smelting and converting processes. Miner Eng 49:184–189
Itagaki K, Yazawa A (1982) Thermodynamic evaluation of distribution behaviour of arsenic in copper smelting. Trans Jpn Inst Met 23(12):759–767
Hino M, Toguri J (1986) Arsenic activities in molten copper and copper sulfide melts. Metall Trans B 17(4):755–761
Nagamori M, Mackey P, Tarassoff P (1975) The distribution of As, Sb, Bi, Se, and Te between molten copper and white metal. Metall Trans B 6(1):197–198
Lau K, Lamoreaux R, Hildenbrand D (1983) Vapor pressure determination of arsenic activity in a molten Cu-Fe-S matte. Metall Trans B 14(2):253–258
Chaubal P, Nagamori M (1983) Volatilization of arsenic and antimony in copper matte converting. Metall Trans B 14(2):303–306
Warzok A, Font J, Montenegro V, Caballero C, Moyano A (2009) Mechanism of buildup formation in an electric furnace for copper slag cleaning. In: Sanches M, Parra R, Riveros G, Diaz C (eds) MOLTEN 2009 conference. Gecamin Ltd., Santiago (Chile), Ch IMM, Santiago, pp 1211–1219
Sadri A, Henstock M, Szyplinski P, Ying W (2018) Progress towards furnace modernisation by utilizing comparative analysis of acusto ultrasonic-echo (AU-E) monitoring: case studies. In: Davis B, Moats M, Wang S (eds) Extraction 2018. TMS, Warrendal, pp 549–559
Acknowledgements
This work was financially supported by the Business Finland financed SYMMET project [Grant Number 3891/31/2018]. Katri Avarmaa also received funding from the Finnish Steel Producer’s Fund via Prof. Daniel Lindberg’s research group grant [Application Number 1497]. Lassi Klemettinen is grateful for the doctoral study grant provided by the Finnish Steel and Metal Producers' Fund and the funding provided by Aalto University School of Chemical Engineering. This study utilized the Academy of Finland’s RawMatTERS Finland Infrastructure (RAMI) based jointly at Aalto University, GTK, and VTT in Espoo. The authors are also grateful for Professor Akbar Rhamdhani on his support and feedback when finalizing the manuscript.
Funding
Open Access funding provided by Aalto University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Hiromichi Takebe.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Avarmaa, K., Klemettinen, L., Taskinen, P. et al. Utilization of Scrap Metals as Reductants for Improved Ni and Cu Recoveries in Copper Smelting. J. Sustain. Metall. 8, 1915–1931 (2022). https://doi.org/10.1007/s40831-022-00614-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00614-9