Abstract
The use of lithium in manufacturing of lithium-ion batteries for hybrid and electric vehicles, along with stringent environmental regulations, have strongly increased the need for its sustainable production and recycling. The required purity of lithium compounds used for the production of battery components is very high (> 99.5%). In this work, a solvometallurgical process that exploits the differences in solubility between LiCl and other alkali and alkaline-earth chlorides and hydroxides in ethanolic solutions has been investigated for the purification of LiCl to battery grade at room temperature. A closed-loop flowsheet based on the green solvent ethanol is proposed for purification of LiCl, a precursor for battery-grade LiOH·H2O. High-purity LiCl solution (> 99.5% Li) could be obtained in a single-process step comprising the simultaneous selective dissolution of LiCl and the precipitation of Mg(OH)2 and Ca(OH)2 using LiOH·H2O in 95 vol% ethanol. However, the analogous process in aqueous solution resulted in impure LiCl (typically less than about 75%).
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium is a crucial raw material for lithium-ion batteries, where it is used as a constituent of the electrolyte and electrode materials [1,2,3]. The main lithium sources are brines from salt lakes (salars) and hard-rock lithium ores (mainly spodumene) [4,5,6]. Lithium is extracted through a series of processing steps (including heating, precipitation, carbonation) as lithium carbonate (Li2CO3), which presently is the most important lithium compound for lithium-ion battery applications [7,8,9,10]. However, the importance of lithium hydroxide, in the form of its monohydrate LiOH·H2O, is increasing sharply, mainly because it can be used as a starting compound for nickel-rich NMC cathode materials, which are desirable for their high reversible capacity, high energy density, good rate capability, and relatively low cost [11,12,13]. LiOH allows for fast and complete synthesis of the cathode materials at lower temperatures than when using Li2CO3 [14, 15]. The production of LiOH typically includes the reaction between Li2CO3 and Ca(OH)2, with formation of CaCO3 as a residue [14, 16]. This process is characterized by poor production yields and losses of Li2CO3 with CaCO3, due to the reduced solubility of Li2CO3 in the presence of LiOH, and inefficiencies during washing and settling operations of the CaCO3 precipitate [14]. Alternatively, a process for direct conversion of lithium chloride (LiCl) into LiOH·H2O, bypassing the Li2CO3 intermediate, would be advantageous to reduce the number of processing steps, including lower consumption of chemicals and generation of less waste. However, such process implies the purification of technical-grade LiCl from common impurities such as sodium, potassium, calcium, and magnesium, into a battery-grade LiCl, with a purity of at least 99.5% trace metal basis.
The use of organic solvents instead of aqueous solvents in extractive metallurgy is referred to as “solvometallurgy” [17]. The organic solvents can significantly increase the selectivity of dissolution processes. Several organic solvent-based processes for LiCl purification have been reported, including processes based on differences in solubilities of metal chloride salts in alcohols [18,19,20]. However, these methods are not suitable for removal of high concentrations of calcium and magnesium impurities by a simple dissolution process, because magnesium chloride (MgCl2) and calcium chloride (CaCl2) are quite soluble in more polar alcoholic solvents.
In this paper, we present a simple and efficient solvometallurgical process for the purification of technical-grade LiCl to a high-purity final solution product (> 99.5% of Li) that is suitable for further conversion into a battery-grade LiOH·H2O, for instance by an electrodialysis process [16, 21].
Experimental
Chemicals
Nitric acid (65%), hydrochloric acid (37%), standard solutions (1000 µg mL‒1) of sodium, potassium, magnesium, calcium, lithium, scandium and yttrium, and potassium chloride (99.5%) were purchased from Chem-Lab NV (Zedelgem, Belgium). Ethanol (99.8%), sodium hydroxide pearls (≥ 98%), and sodium chloride (≥ 99.5%) were purchased from Fisher Chemicals (Geel, Belgium). Anhydrous calcium chloride (96%) was purchased from Acros Organics (Geel, Belgium). Lithium chloride (≥ 99.5%) was obtained from Carl ROTH (Karlsruhe,Germany). Lithium hydroxide monohydrate (≥ 98%) and anhydrous magnesium chloride (99%) were purchased from Alfa Aesar (Steinheim and Kandel, Germany). The ultrapure water with a resistivity of > 18.2 MΩ·cm at 25 °C, and TOC ≤ 5 μg L‒1 was obtained from a Milli-Q™ Reference Ultrapure Water Purification System (Millipore).
Instrumentation
The dissolution and precipitation experiments were performed using a Thermo Fisher shaker (Type 462‒0355). The simultaneous dissolution/precipitation process on a large scale was studied using a customized 1-L jacketed laboratory reactor (HiTec Zang GmbH, Herzogenrath, Germany) with an overhead stirrer and an automatic filtration unit (LabKit 36167) (Figure S1 in the electronic supplementary material). The anchor stirrer of the reactor was made of stainless steel covered with PTFE. The length of the stirrer was 450 mm, and the diameter was 80 mm. The ratio between the diameter of the stirrer and the diameter of the reactor vessel was 0.8. The concentrations of elements in solutions were measured by an inductively coupled plasma‒optical emission spectrometer (ICP‒OES) (Perkin Elmer Avio 500) equipped with an axial/radial dual plasma view, a GemCone High Solids nebulizer, a baffled cyclonic spray chamber, and a demountable quartz torch with a 2.0 mm internal diameter alumina injector. The lines at 279.077, 317.900, 589.592, 610.362, and 766.490 nm are typically used for the determination of magnesium, calcium, sodium, lithium, and potassium, respectively. Yttrium (5 mg L‒1) measured at 324.227 nm and 371.029 nm was used as an internal standard. The concentrations of sodium (Na-23) and potassium (K-39) in ethanolic solutions were also measured by inductively coupled plasma‒mass spectrometer (ICP‒MS) (Perkin Elmer Nexion 5000) equipped with four quadrupoles. The elements were quantified with matrix-matched calibration standards containing LiCl, and scandium (Sc-45) was added as an internal standard (50 μg L‒1). The calibration standards, blanks, and all samples were prepared with 2 wt% HNO3. The samples were diluted at least 500 times for ICP‒OES analysis and 1000 times for ICP‒MS analysis. The high dilution factors reduced the impact of matrix effects on measurement with these techniques, originating either from high salt concentrations or from ethanol.
Methodology
The dissolution tests were performed by adding 10 mL of 95 vol% ethanol or ultrapure water to various solid mixtures of MgCl2, CaCl2, NaCl, KCl, and LiCl, as shown in the Table S1. The mixtures were shaken for 30 min at room temperature and 300 rpm and then filtered through a syringe filter with 0.45 µm pore size. In an analogous way, the one-step dissolution/precipitation tests were performed by adding 10 mL of 0.105 mol L‒1 LiOH·H2O in 95 vol% ethanol or in ultrapure water.
The continuous, upscaled one-step dissolution/precipitation process in the 1-L glass reactor (HiTec Zang) was performed with the solid salt mixture: 3.5 g MgCl2, 3.5 g CaCl2, 10 g KCl, 13 g NaCl, and 186 g LiCl (i.e., 2.08% Mg, 2.94% Ca, 12.2% K, 11.9% Na, and 70.9% Li, calculated based on masses of metal ions without the chloride content for easier comparison of Li metal purity before and after the purification process). A 1 L solution of 0.128 mol L−1 LiOH·H2O in 95 vol% ethanol was added, and the solid mixture was stirred at the speed of 400 rpm, at room temperature for 30 min. The mixture was filtered through a glass filter (pore size of 160–250 µm) which was covered with Manchery-Nagel MN 615 cellulose filter paper, with 4‒12 µm retention capacity.
The one-step dissolution/precipitation of mixtures mimicking brine compositions was performed with NaOH and LiOH solutions. A sample of 10 mL of 0.282 mol L−1 NaOH in 95 vol% ethanol or in water was added to a mixture of MgCl2, CaCl2, NaCl, KCl, and LiCl. After shaking the mixture at 300 rpm and room temperature for 30 min, 3 mL of 0.210 mol L‒1 LiOH·H2O in 95 vol% ethanol was added to the ethanolic mixture, and 0.210 mol L‒1 LiOH·H2O in water was added to the aqueous mixture. The mixtures were shaken for additional 5 min, and filtered through a syringe filter with 0.45 µm pore size. Samples without the addition of LiOH·H2O were prepared in the same way. The conceptual flowsheet proposed in the manuscript is based on the experimental conditions for the one-step dissolution/precipitation either by NaOH or LiOH·H2O in 95 vol% ethanol.
The experiments with a solution volume of 10 mL were performed in triplicate. The purity of lithium-rich solution was calculated based on the average concentrations of the elements in the filtrates, by applying the following equation:
where mLi is the mass of lithium and m is the total mass of all metal ions (calcium, magnesium, sodium, potassium and lithium) in the filtrate. The masses were calculated based on the concentrations of elements in the filtrate, measured by ICP-OES and/or ICP-MS. Therefore, it should be noted that the calculated purity of lithium refers only to the trace metal purity of the solution, without considering the concentrations of anions (chlorides or hydroxides).
Solvents of 25 vol% to 95 vol% of ethanol, prepared by adding a certain amount of water, were used to investigate the influence of the water content on the precipitation of magnesium and calcium from the mixtures of MgCl2, CaCl2, and LiCl. A volume of 0.250 mL of 4 mol L‒1 LiOH·H2O in water was added to a 5 mL sample of the salt mixture in ethanol of different concentrations. The mixture was shaken at a speed of 300 rpm for 15 min at room temperature and then filtered through the syringe filter with 0.45 µm pore size.
The precipitation efficiency of magnesium and calcium ions was calculated as follows:
where c1 (mmol L‒1) is the initial metal ion concentration and c2 (mmol L‒1) is the final concentration of metal ions in the solution corrected by the dilution factor due to the addition of 4 mol L‒1 LiOH·H2O in water.
Results and Discussion
Dissolution of Salts in Ethanol
Dissolution tests were performed in 95 vol% ethanol with different solid mixtures of LiCl and other alkali and alkaline-earth chloride salts, which are similar to lithium hard-rock concentrates (Table 1 and S1, Feed 1‒3) [22]. Besides, solid mixtures containing a similar composition of various lithium brines with low concentrations of LiCl and high concentrations of the other chloride salts were also investigated (Table 1 and S1, Brine 1‒2) [23].
NaCl and KCl have a limited solubility of 0.064 g and 0.07 g in 100 g of ethanol, respectively, at 25 °C (Table S2) [24]. This property allows them to be separated from LiCl, which has a high solubility of 24.28 g in 100 g of ethanol, at 20 °C [24]. Therefore, only trace amounts of sodium and potassium ions could be detected in the ethanolic solutions after removal of the solid residue by filtration (Table 2). The solubility of NaCl and KCl was slightly influenced by the initial composition of the salts (Tables 1, 2). The findings render ethanol a potential solvent for the recovery of highly pure LiCl from a wide variety of sources with different compositions, i.e., from lithium-containing hard rocks to brines. Note that the concentration of sodium and potassium in solution increased as a function of increasing water content in ethanol (Tables 2, 3). Therefore, ethanol with less than 5 vol% of water is the most suitable solvent to keep the solubility of NaCl and KCl low. On the other hand, there is no need to work with absolute ethanol, which largely improves the economics of the process.
One-Step Dissolution/Precipitation Process for Purification from Minerals
MgCl2 and CaCl2 co-dissolved along with LiCl, unlike NaCl and KCl (Table 2). Therefore, the removal of these ions by a combined dissolution/precipitation process was investigated in order to obtain a Li-rich solution in one step. When dissolving the solid mixtures of chloride salts (Table 1 and S1, Feed 2‒3) in 10 mL of a 0.105 mol L‒1 solution of LiOH·H2O in 95 vol% ethanol or in ultrapure water, the following reactions can occur:
Unlike their corresponding chloride salts, the hydroxides of Mg and Ca have very low solubility in ethanol (Table S2). Therefore, simultaneous dissolution of LiCl, MgCl2, and CaCl2 and precipitation of magnesium and calcium as Mg(OH)2 and Ca(OH)2 were performed by LiOH·H2O in 95 vol% ethanol. NaCl and KCl remained undissolved (Fig. 1, Table S3), thus, could not further react to form their hydroxides which are soluble in ethanol. LiCl solutions with > 99.5% of Li were obtained after a single-processing step (Fig. 1; Table S3). Similar results were obtained from a low-grade LiCl feed, which was prepared with 50% less amount of LiCl than typically used in this study (Table S4). In the same one-step dissolution/precipitation process, but with an aqueous solution of LiOH·H2O rather than an ethanolic solution, the vast majority of magnesium ions were also removed from the feed by precipitation (Fig. 1, Table S3). However, the precipitation of calcium was not efficient under these experimental conditions, due to a relatively high solubility of Ca(OH)2 in water: 0.129 g of CaO per 100 g of solution saturated with fine Ca(OH)2 at 25 °C (Table S2) [24]. Moreover, large amounts of NaCl and KCl co-dissolved along with LiCl, resulting in a LiCl solution with a purity of only 76.5% of Li (Fig. 1; Table S3).
Purity of lithium chloride solution after one-step processing of the chloride salt mixtures given in the Table 1 (Feeds 1–3) by 0.105 mol L‒1 LiOH⋅H2O in 95 vol% ethanol
The one-step dissolution/precipitation process was also performed in an upscaled glass reactor with 1 L of LiOH·H2O in 95 vol% ethanol (Fig. S1). A slight excess of LiOH·H2O (0.128 mol L‒1) compared to the small-scale batch experiments (0.105 mol L‒1) was used to ensure a complete precipitation of calcium and magnesium in the upscaled experiment. The temperature of the reaction mixture sharply increased up to 60 °C upon the addition of the ethanolic solution to the solid mixture due the exothermic character of the reactions, but it gradually decreased when mixing was complete. The concentration of lithium in the filtrate was 27.2 ± 1.3 g L‒1, and that of potassium was 0.10 ± 0.01 g L‒1. The concentrations of the magnesium, calcium, or sodium impurities were below the detection limit of ICP-OES. The upscaling of the process also resulted in a LiCl solution with > 99.5% trace metal purity.
One-Step Dissolution/Precipitation Process for Purification from Brines
The one-step dissolution/precipitation process with LiOH·H2O in 95 vol% ethanol for obtaining a high-purity LiCl solution can be considered suitable for the removal of various amounts of NaCl and KCl, and relatively low concentrations of MgCl2 and CaCl2 (e.g., Table 1, Feed 2–3). LiOH·H2O is not very soluble in ethanol: 2.18 g of this compound dissolves in 100 g of ethanol at 20 °C, after 48 h of mixing (Table S2) [25], and using LiOH·H2O to precipitate large amounts of magnesium or calcium would be neither practical nor economical. Therefore, the precipitation of magnesium and calcium from their concentrated feed was investigated by step-wise dissolution and precipitation, first in a NaOH solution, and then in a LiOH·H2O solution. The chloride salts (Table 1 and S1, Brine 1) were added in a 0.282 mol L‒1 solution of NaOH in 95 vol% ethanol or in water. Magnesium and the vast majority of calcium ions were precipitated by NaOH in the ethanolic feed (Table 4). In this step, NaCl was formed according to the reactions:
The formed NaCl does not dissolve well in ethanol (Table S2), so that the solution was simultaneously purified from magnesium, sodium, and the majority of calcium ions (Table 4). Less than the stoichiometric amount of NaOH required for precipitation of magnesium and calcium was added to avoid contamination of the LiCl ethanolic solution with sodium ions due to unreacted NaOH. The traces of calcium ions that remained in the ethanolic solution were then precipitated by addition of a solution of LiOH·H2O in ethanol. LiOH·H2O was added in a slight excess to ensure complete precipitation of the remaining calcium. It should be noted that the final purity of LiCl solution in this experiment was < 99.5%, due to the low concentration of lithium in the tested solid mixtures. However, when compared to the initial composition of the solid mixture (Table 1, Brine 1), it is evident that only traces of sodium and potassium ions were left along with lithium ions in the ethanolic feed (Table 4). The ethanolic solution of NaOH can, therefore, be used to simultaneously precipitate large amounts of magnesium and calcium, while still allowing dissolution of LiCl. Once again, the precipitation of calcium from the aqueous feed was not efficient, neither with NaOH or LiOH solutions (Table 4). Moreover, large amounts of sodium and potassium salts were dissolved in the aqueous solution.
Influence of the Water Content on the Precipitation Efficiency
Obviously, the water content in the organic solvents can greatly affect the precipitation efficiency of calcium (Tables 2, 3, 4). To get more insight into the role of water, the removal of magnesium and calcium from LiCl solution (27.7 mg L‒1 of lithium) in ethanol–water mixtures (from 95 to 25 vol% of ethanol) was studied by the addition of 4 mol L‒1 LiOH·H2O in water. Dilute solutions with an average of 0.739 mg L‒1 of magnesium and 1.14 mg L‒1 of calcium were studied (Table S5). High precipitation efficiencies of impurity metals and LiCl solutions with > 99.5% metal purity were obtained from feed solutions with up to 50 vol% of water (Fig. 2; Table S5). The precipitation efficiency of calcium decreased with increasing water content in the organic solutions (Fig. 2; Table S5), due to the higher solubility of Ca(OH)2 in water (Table S2).
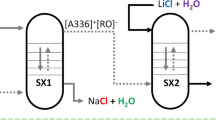
Conceptual Flowsheet
Based on the observations on the difference in the solubility of lithium and of other alkali and alkaline-earth metal salts and hydroxides in ethanol, a conceptual flowsheet for obtaining battery-grade LiCl in one-step solvometallurgical process is proposed (Fig. 3). The single-step process combines the dissolution of technical LiCl by LiOH·H2O solution in ethanol at room temperature for 30 min and simultaneous precipitation of magnesium and calcium as their insoluble hydroxides. NaCl and KCl are virtually insoluble in such a solution. After the selective dissolution/precipitation step, the ethanol solvent can be recovered by distillation and reused in the process, and the remaining solid LiCl can be further processed to obtain battery-grade LiOH·H2O. For this, an aqueous LiCl solution can be transformed first into a LiOH solution, for instance by membrane electrodialysis, followed by crystallization of LiOH·H2O [14, 26]. A demonstration of this conversion of LiCl into LiOH·H2O was outside the scope of the present study. A small part of the end-product, LiOH·H2O, can be used to prepare the LiOH solution for the selective dissolution/precipitation process, thus, rendering this a closed-loop process. The solution of NaOH in ethanol can be used for the removal of larger amounts of magnesium and calcium ions, as found in lithium brines. It is obvious that consumption of too much LiOH solution must be avoided.
The fundaments of the proposed solvometallurgical process are based on the intrinsically lower solubility of alkali and alkaline-earth compounds in less polar solvent such as ethanol than in water [27, 28]. Small ions of high charge density, like lithium ions, bind tightly polar molecules, such as water or ethanol molecules, whereas large monovalent ions of low charge density, like sodium or potassium ions, bind polar molecules weakly when compared to the strength of solvent–solvent interactions in bulk solution [29]. Small ions are strongly solvated because their point charge is close to the point charge of opposite sign on the solvent molecule, whereas large ions are weakly solvated because their point charge is distant from the point charge of opposite sign on the solvent molecule. Small-large ion pairs (LiCl) tend not to form inner sphere ions, they remain apart in solution, thus, are soluble in ethanol, whereas small-sized, medium-sized, large-sized ions of opposite charge (comparable-sized or comparable charge density) will tend to pair and result in low solubility in ethanol (NaCl, KCl, Mg(OH)2, Ca(OH)2).
Conclusions
A solvometallurgical process that produces a battery-grade (trace metal basis) LiCl precursor for LiOH·H2O by using a green solvent such as ethanol was developed. To the best of our knowledge, this is the first example of a process that produces a battery-grade LiCl precursor in a single-processing step. The inherent difference in solubility of LiCl and other alkali and alkaline-earth chlorides and hydroxides in a solution of LiOH·H2O in 95 vol% ethanol enabled the production of the high-purity LiCl feed (> 99.5% of Li) at room temperature. The developed process is feasible with feeds that contain less than 50 vol% of water. The presented process is versatile and suitable for purification of a wide range of solid mixtures of LiCl and other alkali and alkaline-earth metals, especially for lithium hard-rock ores (e.g., spodumene). The samples with large concentrations of magnesium and calcium can also be pre-purified with NaOH in ethanol, instead of LiOH·H2O in ethanol, to remove the vast majority of these ions.
References
Liu K, Liu L, Tan Q, Li J (2021) Selective extraction of lithium from a spent lithium iron phosphate battery by mechanochemical solid-phase oxidation. Green Chem 23:1344–1352. https://doi.org/10.1039/d0gc03683h
Di Lecce D, Verrelli R, Hassoun J (2017) Lithium-ion batteries for sustainable energy storage: recent advances towards new cell configurations. Green Chem 19:3442–3467. https://doi.org/10.1039/c7gc01328k
Bae H, Kim Y (2021) Technologies of lithium recycling from waste lithium ion batteries: a review. Mater Adv 2:3234–3250. https://doi.org/10.1039/d1ma00216c
Swain B (2017) Recovery and recycling of lithium: a review. Sep Purif Technol 172:388–403. https://doi.org/10.1016/j.seppur.2016.08.031
Salakjani NK, Singh P, Nikoloski AN (2021) Production of lithium: a literature review. Part 2. Extraction from Spodumene. Miner Process Extr Metall Rev 42:268–283. https://doi.org/10.1080/08827508.2019.1700984
Salakjani NK, Singh P, Nikoloski AN (2020) Production of lithium: a literature review part 1: pretreatment of spodumene. Miner Process Extr Metall Rev 41:335–348. https://doi.org/10.1080/08827508.2019.1643343
Li H, Eksteen J, Kuang G (2019) Recovery of lithium from mineral resources: state-of-the-art and perspectives—A review. Hydrometallurgy 189:105129. https://doi.org/10.1016/j.hydromet.2019.105129
Meshram P, Pandey BD, Mankhand TR (2014) Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: a comprehensive review. Hydrometallurgy 150:192–208. https://doi.org/10.1016/j.hydromet.2014.10.012
Yang S, Zhang F, Ding H et al (2018) Lithium metal extraction from seawater. Joule 2:1648–1651. https://doi.org/10.1016/j.joule.2018.07.006
Zhang X, Han A, Yang Y (2020) Review on the production of high-purity lithium metal. J Mater Chem A 8:22455–22466. https://doi.org/10.1039/d0ta07611b
Ye Z, Qiu L, Yang W et al (2021) Nickel-rich layered cathode materials for lithium-ion batteries. Chem A Eur J 27:4249–4269. https://doi.org/10.1002/chem.202003987
Pham HQ, Hwang EH, Kwon YG, Song SW (2019) Approaching the maximum capacity of nickel-rich LiNi0.8Co0.1Mn0.1O2 cathodes by charging to high-voltage in a non-flammable electrolyte of propylene carbonate and fluorinated linear carbonates. Chem Commun 55:1256–1258. https://doi.org/10.1039/c8cc10017a
Lai J, Zhang J, Li Z et al (2020) Structural elucidation of the degradation mechanism of nickel-rich layered cathodes during high-voltage cycling. Chem Commun 56:4886–4889. https://doi.org/10.1039/d0cc00327a
Ryabtsev AD, Nemkov NM, Kotsupalo NP, Serikova LA (2004) Preparation of high-purity lithium hydroxide monohydrate from technical-grade lithium carbonate by membrane electrolysis. Russ J Appl Chem 77:1108–1116. https://doi.org/10.1023/B:RJAC.0000044158.61704.93
Dong H, Koenig GM (2020) A review on synthesis and engineering of crystal precursors produced: via coprecipitation for multicomponent lithium-ion battery cathode materials. CrystEngComm 22:1514–1530. https://doi.org/10.1039/c9ce00679f
Ryabtsev AD, Nemkov NM, Kotsupalo NP et al (2019) Production of lithium hydroxide monohydrate from natural brine. Theor Found Chem Eng 53:626–631. https://doi.org/10.1134/S0040579519040079
Binnemans K, Jones PT (2017) Solvometallurgy: an emerging branch of extractive metallurgy. J Sustain Metall 3:570–600. https://doi.org/10.1007/s40831-017-0128-2
Gabra GG, Torma AE (1978) Lithium chloride extraction by n-butanol. Hydrometallurgy 3:23–33. https://doi.org/10.1016/0304-386X(78)90004-X
Hermann JA (1966) Preparation and purification of lithium chloride. US Pat US3278260
Brown PM, Beckerman SJ (1990) Production of lithium metal grade lithium chloride from lithium-containing brine. US Pat 4980136A
Melnikov S, Sheldeshov N, Zabolotsky V et al (2017) Pilot scale complex electrodialysis technology for processing a solution of lithium chloride containing organic solvents. Sep Purif Technol 189:74–81. https://doi.org/10.1016/j.seppur.2017.07.085
Braga P, França S, Neumann R, Rodriguez M, Rosales G (2019) Alkaline process for extracting lithium from spodumene. In: Proceedings of the 11th international seminar on process hydrometallurgy, hydroprocess. Santiago, Chile. https://www.cetem.gov.br/antigo/images/congressos/2019/ALKALINE-PROCESS-2019.pdf. Accessed 8 Mar 2022
An JW, Kang DJ, Tran KT et al (2012) Recovery of lithium from Uyuni salar brine. Hydrometallurgy 117–118:64–70. https://doi.org/10.1016/j.hydromet.2012.02.008
Seidell A, Linke WF (1958) Solubilities, inorganic and metal-organic compounds, 4th edn. American Chemical Society, Washington, D.C.
Khosravi J (2007) Production of lithium peroxide and lithium oxide in an alcohol medium. Doctoral dissertation, McGill University, Montreal, Canada. https://escholarship.mcgill.ca/concern/theses/6108vg687. Accessed 8 Mar 2022
Grageda M, Gonzalez A, Quispe A, Ushak S (2020) Analysis of a process for producing battery grade lithium hydroxide by membrane electrodialysis. Membranes 198(10):1–21. https://doi.org/10.3390/membranes10090198
Hassan S, Adam F, Abu Bakar MR, Abdul Mudalip SK (2019) Evaluation of solvents’ effect on solubility, intermolecular interaction energies and habit of ascorbic acid crystals. J Saudi Chem Soc 23:239–248. https://doi.org/10.1016/j.jscs.2018.07.002
Li M, Constantinescu D, Wang L, Mohs A, Gmehling J (2010) Solubilities of NaCl, KCl, LiCl, and LiBr in methanol, ethanol, acetone, and mixed solvents and correlation using the LIQUAC model. Ind Eng Chem Res 49:4981–4988. https://doi.org/10.1021/ie100027c
Collins KD (1997) Charge density-dependent strength. Biophys J 72:65–76. https://doi.org/10.1016/S0006-3495(97)78647-8
Acknowledgements
This work has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme: Grant Agreement 963281 (PoC grant SOLVOLi), and from KU Leuven (project C3/20/066) The authors acknowledge Dr. Clio Deferm and Jakob Bussé (KU Leuven, Belgium) for their support during the upscaling experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
The contributing editor for this article was Grace Ofori-Sarpong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Avdibegović, D., Nguyen, V.T. & Binnemans, K. One-Step Solvometallurgical Process for Purification of Lithium Chloride to Battery Grade. J. Sustain. Metall. 8, 893–899 (2022). https://doi.org/10.1007/s40831-022-00540-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00540-w