Abstract
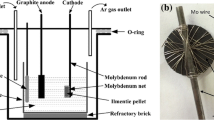
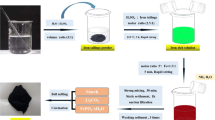
Electro-deoxidation of ilmenite (FeTiO3) is an economical production method of FeTi, particularly, if the end use is hydrogen storage. In this study, we show that electro-deoxidation of impure FeTiO3 with Ti content lower than Fe, as in the case of low-grade FeTiO3 ore, results in the formation of a two-phase material consisting of FeTi and Fe2Ti. The presence of Fe2Ti is detrimental to the hydrogen storage efficacy. We show for the first time that it is possible to avoid the formation of Fe2Ti or β-Ti as a second phase under similar operating conditions only by tailoring the composition of the cathode precursor, i.e., the addition of TiO2 to low-grade FeTiO3 so that the atomic ratio of Fe:Ti in the precursor is ~ 1:1. Low-grade FeTiO3 with 10 wt% TiO2 resulted in single-phase FeTi with the atomic ratio of Fe:Ti ~ 1:1 in the precursor and in the final reduced alloy. The hydrogen storage capacity of the single-phase FeTi is nearly 36% higher as compared to the two-phase alloy consisting of FeTi–Fe2Ti.
Graphical Abstract










Similar content being viewed by others
References
IEA (2019) Global Energy and CO2 status report—renewables, pp 1–8
Schiebahn S, Grube T, Robinius M et al (2015) Power to gas: technological overview, systems analysis and economic assessment for a case study in Germany. Int J Hydrogen Energy 40:4285–4294. https://doi.org/10.1016/j.ijhydene.2015.01.123
Zhu Y, Ouyang L, Zhong H et al (2020) Closing the loop for hydrogen storage: facile regeneration of NaBH4 from its hydrolytic product. Angew Chem Int Ed 59:8623–8629. https://doi.org/10.1002/anie.201915988
Yao L, Lyu X, Zhang J et al (2020) Remarkable synergistic effects of Mg2NiH4 and transition metal carbides (TiC, ZrC, WC) on enhancing the hydrogen storage properties of MgH2. Int J Hydrogen Energy 45:6765–6779. https://doi.org/10.1016/j.ijhydene.2019.12.139
Singh S, Bhatnagar A, Shukla V et al (2020) Ternary transition metal alloy FeCoNi nanoparticles on graphene as new catalyst for hydrogen sorption in MgH2. Int J Hydrogen Energy 45:774–786. https://doi.org/10.1016/j.ijhydene.2019.10.204
Zadorozhnyy V, Sarac B, Berdonosova E et al (2020) Evaluation of hydrogen storage performance of ZrTiVNiCrFe in electrochemical and gas-solid reactions. Int J Hydrogen Energy 45:5347–5355. https://doi.org/10.1016/j.ijhydene.2019.06.157
Wallace WE, Karllcek RF, Imamura H (1979) Mechanism of hydrogen absorption by LaNi5. J Phys Chem 83:1708–1712
Reilly JJ, Wiswall RH (1974) Formation and properties of iron titanium hydride. Inorg Chem 13:218–222
Jain IP, Lal C, Jain A (2010) Hydrogen storage in Mg: a most promising material. Int J Hydrogen Energy 35:5133–5144. https://doi.org/10.1016/j.ijhydene.2009.08.088
Zhang W, Zhu Z, Cheng CY (2011) A literature review of titanium metallurgical processes. Hydrometallurgy 108:177–188. https://doi.org/10.1016/j.hydromet.2011.04.005
Chen G, Fray D, Farthing T (2000) Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 407:361–364. https://doi.org/10.1038/35030069
Suzuki RO, Koh T, Ono K (2003) Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2. Metall Mater Trans B 34B:287–295
Pal UB, Powell AC (2007) The use of solid-oxide-membrane technology for electrometallurgy. JOM 59:44–49
Ma M, Wang D, Hu X et al (2006) A direct electrochemical route from ilmenite to hydrogen-storage ferrotitanium alloys. Chemistry 12:5075–5081. https://doi.org/10.1002/chem.200500697
Panigrahi M, Shibata E, Iizuka A, Nakamura T (2013) Production of Fe-Ti alloy from mixed ilmenite and titanium dioxide by direct electrochemical reduction in molten calcium chloride. Electrochim Acta 93:143–151. https://doi.org/10.1016/j.electacta.2013.01.089
Xiong L, Hua Y, Xu C et al (2016) Effect of CaO addition on preparation of ferrotitanium from ilmenite by electrochemical reduction in CaCl2-NaCl molten salt. J Alloys Compd 676:383–389. https://doi.org/10.1016/j.jallcom.2016.03.195
Tan S, Örs T, Aydınol MK, Öztürk T (2009) Synthesis of FeTi from mixed oxide precursors. J Alloys Compds 475:368–372. https://doi.org/10.1016/j.jallcom.2008.07.018
Panigrahi M, Iizuka A, Shibata E, Nakamura T (2013) Electrolytic reduction of mixed ( Fe, Ti ) oxide using molten calcium chloride electrolyte. J Alloys Compds 550:545–552. https://doi.org/10.1016/j.jallcom.2012.09.029
Ye XS, Lu XG, Li CH et al (2010) Preparation of Ti-Fe based hydrogen storage alloy by SOM method. Int J Hydrogen Energy 36:4573–4579. https://doi.org/10.1016/j.ijhydene.2010.04.098
Zou XL, Lu XG, Xiao W, Lu CY (2014) Reaction routes for the electro-deoxidation of ilmenite in molten salt. Adv Mater Res 937:58–63
Zhou Z, Zhang Y, Hua Y et al (2018) Preparation of ferrotitanium alloys by electrolysis-assisted calciothermic reduction of ilmenite in equimolar CaCl2-NaCl electrolyte: effect of calcium oxide. JOM 70:575–580. https://doi.org/10.1007/s11837-018-2743-1
Rizo-Acosta P, Cuevas F, Latroche M (2018) Optimization of TiH2 content for fast and efficient hydrogen cycling of MgH2-TiH2 nanocomposites. Int J Hydrogen Energy 43:16774–16781. https://doi.org/10.1016/j.ijhydene.2018.04.169
Padhee SP, Roy A, Pati S (2021) Mechanistic insights into efficient reversible hydrogen storage in ferrotitanium. Int J Hydrogen Energy 46:906–921. https://doi.org/10.1016/j.ijhydene.2020.09.221
Lutterotti L, Vasin R, Wenk HR (2014) Rietveld texture analysis from synchrotron diffraction images. I Calibration and basic analysis. Powder Differ 29:76–84. https://doi.org/10.1017/S0885715613001346
Lutterotti L, Pillière H, Fontugne C et al (2019) Full-profile search–match by the Rietveld method. J Appl Crystallogr 52:587–598. https://doi.org/10.1107/S160057671900342X
Lutterotti L, Matthies S, Wenk H (1999) MAUD: a friendly Java program for material analysis using diffraction
Hosni B, Fenineche N, Elkedim O et al (2018) Structural and electrochemical properties of TiFe alloys synthesized by ball milling for hydrogen storage. J Solid State Electrohem 22(1):17–29. https://doi.org/10.1007/s10008-017-3718-9
Xiao W, Lu XG, Zou XL et al (2013) Phase transitions, micro-morphology and its oxidation mechanism in oxidation of ilmenite (FeTiO3) powder. Trans Nonferr Met Soc China 23:2439–2445. https://doi.org/10.1016/S1003-6326(13)62752-1
Qi C, Hua Y, Chen K et al (2016) Preparation of ferrotitanium alloy from ilmenite by electrochemical reduction in chloride molten salts. JOM 68:668–674. https://doi.org/10.1007/s11837-015-1710-3
Yong ZHU, Meng MA, Dihua W et al (2006) Electrolytic reduction of mixed solid oxides in molten salts for energy efficient production of the TiNi alloy. Chin Sci Bull 51:2535–2540. https://doi.org/10.1007/s11434-006-2105-1
Zhou Z, Hua Y, Xu C et al (2016) Preparation of ferrotitanium from ilmenite by electrolysis-assisted calciothermic reduction in CaCl2-NaCl molten salt. JOM 68:532–539. https://doi.org/10.1007/s11837-015-1723-y
Zadorozhnyy V, Klyamkin S, Zadorozhnyy M et al (2012) Hydrogen storage nanocrystalline TiFe intermetallic compound: synthesis by mechanical alloying and compacting. Int J Hydrogen Energy 37:17131–17136. https://doi.org/10.1016/j.ijhydene.2012.08.078
Kim C, Lee J (1985) The effect of surface conditions on the activation of FeTi. J Less Common Met 105:247–253
Schober T (1983) On the activation of FeTi for hydrogen storage. J Less-Common Met 89:63–70. https://doi.org/10.1016/0022-5088(83)90249-7
Sarac B, Zadorozhnyy V, Berdonosova E et al (2020) Hydrogen storage performance of the multi-principal-component CoFeMnTiVZr alloy in electrochemical and gas-solid reactions. RSC Adv 10:24613–24623. https://doi.org/10.1039/d0ra04089d
Subbaraman R, Tripkovic D, Chang KC et al (2012) Trends in activity for the water electrolyser reactions on 3d M(Ni Co, Fe, Mn) hydr(oxy)oxide catalysts. Nat Mater 11:550–557. https://doi.org/10.1038/nmat3313
Abrashev B, Spassov T, Pandev M et al (2017) Hydrogen sorption and electrochemical properties of Ti-Fe based alloys synthesized by mechanical alloying. Bulg Chem Commun 49:247–253
Bernardini M, Comisso N, Davolio G, Mengoli G (2000) Electrolytic hydriding of TiFe 50/50 alloy. J Electroanal Chem 487:1–15. https://doi.org/10.1016/S0022-0728(00)00144-3
Haraki T, Oishi K, Uchida H et al (2008) Properties of hydrogen absorption by nano-structured FeTi alloys. Int J Mater Res 99:507–512. https://doi.org/10.3139/146.101669
Morris S, Dodd SB, Hall PJ, Mackinnon AJ (1999) The effect of novel processing on hydrogen uptake in FeTi- and magnesium-based alloys. J Alloys Compd 293–295:458–462
Vega LER, Leiva DR, Leal Neto RM et al (2019) Improved ball milling method for the synthesis of nanocrystalline TiFe compound ready to absorb hydrogen. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.11.035
Emami H, Edalati K, Matsuda J et al (2015) Hydrogen storage performance of TiFe after processing by ball milling. Acta Mater 88:190–195. https://doi.org/10.1016/j.actamat.2014.12.052
Vega LER, Leiva DR, Neto RML et al (2017) Mechanical activation of TiFe for hydrogen storage by cold rolling under inert atmosphere. Int J Hydrogen Energy 43:2913–2918. https://doi.org/10.1016/j.ijhydene.2017.12.054
Comisso N, Davolio G, Soragni E, Mengoli G (2001) The cycle life of 50 / 50 TiFe alloy electrodes for charge storage. J Electroanal Chem 512:92–100
Dematteis EM, Berti N, Cuevas F et al (2021) Substitutional effects in TiFe for hydrogen storage: a comprehensive review. Mater Adv. https://doi.org/10.1039/d1ma00101a
Bowman RC, Tadlock WE (1979) Hydrogen diffusion in β-phase titanium iron hydride. Solid State Commun 32:313–318. https://doi.org/10.1016/0038-1098(79)90954-2
Bowman RC, Attalla A, Tadlock WE (1977) NMR studies of structure and diffusion in metal hydrides. Int J Hydrogen Energy 1:421–426. https://doi.org/10.1016/0360-3199(77)90095-7
Acknowledgements
SPP and UKC are thankful to the Indian Institute of Technology (IIT) Bhubaneswar and Ministry of Human Resource Development (MHRD), Government of India (GOI) for providing financial support. SP is thankful to the Department of Science and Technology (DST), Govt. of India for funding under the Multi-Institutional Centers on Materials for Energy Conservation and storage Platform (MECSP)-2017 program and Indian Rare Earth Limited, India (IREL) for supplying raw materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Adam Clayton Powell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Padhee, S.P., Chanda, U.K., Singh, R. et al. Electro-deoxidation Process for Producing FeTi from Low-Grade Ilmenite: Tailoring Precursor Composition for Hydrogen Storage. J. Sustain. Metall. 7, 1178–1189 (2021). https://doi.org/10.1007/s40831-021-00412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00412-9