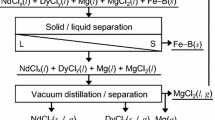
Abstract
To develop an efficient recycling process for the selective recovery of rare earth elements (REEs) from neodymium (Nd)-iron (Fe)-boron (B) magnets, a selective chlorination process using zinc chloride (ZnCl2) was investigated. A mixture of Nd-Fe-B magnet powder and ZnCl2 was set in a gas-tight quartz tube, and the tube was placed in a vertical furnace preheated to 1000 K. During the experiment, the magnet powder reacted with ZnCl2 for 1.5 – 5 h to selectively chlorinate the REEs in the magnet. The influence of the particle size of the magnet powder, reaction time, and premelting of ZnCl2 on the chlorination efficiency of REEs was investigated. Under certain conditions, the chlorination efficiencies of Nd, dysprosium (Dy), and praseodymium (Pr) were 96.5%, 57.2%, and 97.6%, respectively. In addition, Fe and neodymium chloride (NdCl3) were found to have been generated after the selective chlorination reactions. The results of this study demonstrate that the selective recovery of REEs, such as Nd, Dy, and Pr, from Nd-Fe-B magnets is feasible by utilizing the selective chlorination process using ZnCl2.
Graphical Abstract












Similar content being viewed by others
References
Haider SK, Lee J-Y, Kim D, Kang YS (2020) Eco-friendly facile three-step recycling method of (Nd-RE)2Fe14B magnet sludge and enhancement of (BH)max by ball milling in ethanol. ACS Sustainable Chem Eng 8:8156–8163
Future Market Insights (2015) Rare earth metals market: global industry analysis and opportunity assessment, 2016–2026. Future Market Insights, London
Takeda O, Okabe TH (2014) Current status on resource and recycling technology for rare earths. Metall Mater Trans E 1:160–173
Nakamura T (2016) NEDO report: review of rare metals. New Energy and Industrial Technology Development Organization, Japan (in Japanese)
Kang J (2020) Current status of rare earth industry in Japan. Ceramic Korea 3:72–79 (in Korean)
Akahori T, Miyamoto Y, Saeki T, Okamoto M, Okabe TH (2017) Optimum conditions for extracting rare earth metals from waste magnets by using molten magnesium. J Alloys Compd 703:337–343
Okabe TH, Takeda O, Fukuda K, Umetsu Y (2003) Direct extraction and recovery of neodymium metal from magnet scrap. Mater Trans 44:798–801
Takeda O, Okabe TH, Umetsu Y (2005) Phase equilibria of the system Fe-Mg-Nd at 1076 K. J Alloys Compd 392:206–213
Takeda O, Okabe TH, Umetsu Y (2006) Recovery of neodymium from a mixture of magnet scrap and other scrap. J Alloys Compd 408–412:387–390
Chae HJ, Kim YD, Kim BS, Kim JG, Kim T-S (2014) Experimental investigation of diffusion behavior between molten Mg and Nd-Fe-B magnets. J Alloys Compd 586:S143–S149
Nam S-W, Park S-M, Kim D-H, Kim T-S (2020) Thermodynamic calculations and parameter variations for improving the extraction efficiency of Dy in ternary alloy system. Met Mater Int 27(3):538–544
Takeda O, Okabe TH, Umetsu Y (2004) Phase equilibrium of the system Ag-Fe-Nd, and Nd extraction from magnet scraps using molten silver. J Alloys Compd 379:305–313
Nohira T, Kobayashi S, Kobayashi K, Hagiwara R, Oishi T, Konishi H (2010) Electrochemical formation of Nd-Ni alloys in molten LiF-CaF2-NdF3. ECS Trans 33:205–212
Kobayashi S, Kobayashi K, Nohira T, Hagiwara R, Oishi T, Konishi H (2011) Electrochemical formation of Nd-Ni alloys in molten LiF-CaF2-NdF3. J Electrochem Soc 158:E142–E146
Saito T, Sato H, Ozawa S, Yu J, Motegi T (2003) The extraction of Nd from waste Nd-Fe-B alloys by the glass slag method. J Alloys Compd 353:189–193
Saito T, Motegi T (2004) Extraction of Tb from Tb-Fe alloys by the glass slag method. Scripta Mater 51:1069–1073
Saito T, Sato H, Motegi T (2005) Extraction of Sm from Sm-Fe alloys by the glass slag method. J Alloys Compd 387:274–278
Saito T, Sato H, Motegi T, Kobayashi K (2005) Extraction of Sm from Sm-Fe-N magnets by the glass slag method. J Alloys Compd 403:341–344
Saito T, Sato H, Motegi T (2006) Recovery of rare earths from sludges containing rare-earth elements. J Alloys Compd 425:145–147
Shirayama S, Okabe TH (2018) Selective extraction and recovery of Nd and Dy from Nd-Fe-B magnet scrap by utilizing molten MgCl2. Metall Mater Trans B 49:1067–1077
Uda T (2002) Recovery of rare earths from magnet sludge by FeCl2. Mater Trans 43:55–62
Itoh M, Miura K, Machida K (2009) Novel rare earth recovery process on Nd-Fe-B magnet scrap by selective chlorination using NH4Cl. J Alloys Compd 477:484–487
Hua Z, Wang J, Wang L, Zhao Z, Li X, Xiao Y, Yang Y (2014) Selective extraction of rare earth elements from NdFeB scrap by molten chlorides. ACS Sustainable Chem Eng 2:2536–2543
Barin I (1995) Thermochemical data of pure substances. VCH Verlagsgesellschaft mbH, Weinheim
Hatada N (2014) Chesta: Software for Creating Chemical Potential Diagram, version 3.2.6.9, http://www.aqua.mtl.kyoto-u.ac.jp/wordpress/chestaEng.html
Jacob KT, Dixit A, Rajput A (2016) Stability field diagrams for Ln-O-Cl systems. Bull Mater Sci 39:603–611
Windholz M, Budavari S, Blumetti RF, Otterbein E.S (1983) The merch index – An encyclopedia of chemicals, drugs, and biologicals, 3rd ed. Rahway N.J., pp. 9932
Acknowledgements
The authors are grateful to Ms. Jieun Ahn and all the members of the Geoanalysis Department of KIGAM for their technical assistance. This research was supported by the Korea Evaluation Institute of Industrial Technology funded by the Korean Ministry of Industry in Korea (Project No.: 20000970, 20-9805).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
The contributing editor for this article was Hojong Kim.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, KH., Choi, C.U., Moon, G. et al. Selective Chlorination of Rare Earth Elements from a Nd-Fe-B Magnet Using Zinc Chloride. J. Sustain. Metall. 7, 794–805 (2021). https://doi.org/10.1007/s40831-021-00380-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00380-0