Abstract
In this research, a recycling process for palladium using “dry aqua regia,” which consists of iron(III) chloride–potassium chloride, was proposed. Palladium was dissolved in “dry aqua regia,” and the dissolved palladium was recovered by leaching with potassium chloride solution with added ammonium chloride and nitric acid. Palladium was almost completely dissolved in 3 h at 600 K, and the recovery ratio of dissolved palladium was up to 80%. In addition, the dissolution of palladium in coexistence with platinum and the dissolution of platinum-palladium alloy by “dry aqua regia” were also tested. The dissolved palladium and platinum were separated and recovered by solid–liquid separation technique using the difference in solubility of their compounds in potassium chloride and sodium chloride solutions. As a result, pure compounds of each element were recovered. This result suggested the possibility of using “dry aqua regia” for the separation of platinum-group metals.
Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Platinum (Pt) and palladium (Pd) are platinum-group metals (PGMs), which are important metals for jewelry, coins, and other industrial applications, particularly owing to their high catalytic abilities and chemical stability. They are often used as automotive catalysts, approximately 40% of Pt and 75% of Pd and rhodium (Rh) were consumed [1, 2].
While the ore grade of PGMs is very low, their grade as automotive catalysts is relatively high; therefore, recycling efforts have been actively pursued. These recycling efforts are roughly divided into pyrometallurgical and hydrometallurgical processes and operated commercially and industrially. Panda et al. summarized these efforts in the previous review [3]. However, both pyrometallurgical and hydrometallurgical recycling impose large environmental loads due to the energy consumption and the generation of waste liquids, use of chlorine (Cl2), etc. [4] Therefore, various new methods have been proposed, including one that utilizes a molten salt [5, 6]. However, it lacks user-friendliness because of additional treatments required, such as electrolysis or Cl2 gas blowing.
In the US patent, a combined molten salt consists of ammonium nitrate (NH4NO3) and ammonium chloride (NH4Cl) was introduced as “dry aqua regia” [7]. This molten salt can dissolve Pt in ore by direct chlorination without electrolysis or Cl2 blowing and recover its compound. However, the treatment is difficult, owing to the use of NH4NO3 and NH4Cl, which are raw materials for gunpowder.
Based on the above, the authors have proposed a new recycling method that uses iron chloride(III) (FeCl3)–potassium chloride (KCl)-based molten salt [6, 8]. Using this method, Pt is chlorinated into potassium(IV) (K2[PtCl6]) by the reaction shown in Eq. (1)
In this reaction, Pt is directly chlorinated by the chlorine (Cl) contained in iron chloride(III) without Cl2 gas blowing or electrolysis. Moreover, Pt can be dissolved at approximately 600–650 K by adding KCl to FeCl3. This is a much lower temperature than that used in conventional processes that require approximately 800–1600 K [6].
Dissolved Pt was purified and recovered by leaching and precipitation as depicted in Eqs. (2) and (3), respectively.
K2[PtCl6] is insoluble in KCl (aq) and soluble in NaCl (aq). Thus, the sodium hexachloroplatinate(IV) (Na2[PtCl6]) solution can be recovered by leaching using KCl (aq) and NaCl (aq), eliminating the impurities, especially iron oxide (FeOx). Pure ammonium hexachloroplatinate(IV) ((NH4)2[PtCl6]) was obtained by the addition of NH4Cl. Moreover, high-purity Pt was recovered by heat treatment of this compound. This result suggests that using “dry aqua regia” is an eco-friendly method to recover Pt. However, previous studies have targeted only Pt, while Pd is also used as an auto-catalyst. Pd in spent auto-catalyst could be dissolved using a solution containing FeCl3, hydrochloric acid (HCl), and NaCl. The dissolved Pd could be recovered by the addition of NH4Cl and ammonia (NH3) [9]. This is an eco-friendly recovery process because of its small environmental impact compared with the conventional process using aqua regia. However, this process only targeted pure Pd. The auto-catalyst contains other PGMs such as Pt or Rh [1, 2], and these PGMs in spent auto-catalysts become alloy by the high temperature of exhaust gases [10]. The availability of the process for other PGMs and their alloys and the recovery of PGMs from spent auto-catalysts are not yet confirmed.
In the case of recycling several PGMs from auto-catalysts, the contained PGMs are dissolved simultaneously and their separation is required [4, 5, 11,12,13]. The conventional processes mainly use solvent extraction and ion-exchange resins to separate and recover each element of PGMs. However, the environmental impact was caused by the chemical sludge production from used solvents and the regeneration of used ion-exchange resins.
Considering the above situation, the authors, in this research, evaluated the usability of the recycling process for Pd in spent auto-catalyst using “dry aqua regia.” The authors also carried out the treatment of Pd in coexistence with Pt and the treatment of Pt–Pd alloy. Moreover, Pd and Pt were recovered using selective dissolution and precipitation.
Methods and Materials
Treatment of Pure Pd
Dissolution of Pd
Figure 1 is the Ellingham diagram of Pd–Fe–Cl system calculated by FactSage [14]. By this thermodynamic calculation, Pd can be chlorinated by the following reaction.
Ellingham diagram of Pd–Fe–Cl system [14]
In addition, the authors added equimolar KCl to FeCl3 to depress the melting point in reference to the previous studies [6, 8].
In this research, Pd wire (ϕ = 0.2 mm, L = approx. 100 mm) was used as the sample. An equimolar mixture of FeCl3 and KCl was used as the dry aqua regia and adjusted to achieve a weight of 3 g [8]. This adjusted mixture, along with a Pd wire sample of approximately 40 mg, was deposited in a porcelain crucible (≥ 58% SiO2, ≥ 33% Al2O3). The crucible was set in a glass reaction vessel in which air was substituted with atmospheric argon (Ar). Thereafter, it was heated in a heating mantle (TAIKA, GBR-5). After the thermocouple near the crucible showed a reading of 523 K, the processing time was adjusted to 1.0, 2.0, and 3.0 h. The processing temperature was varied from 560 to 600 K.
After the completion of the reaction, the reaction vessel was removed from the heater and air-cooled so that the dry aqua regia could solidify. The recovered dry aqua regia was leached with 20 mL of water, and the sample residue was recovered and weighed. The dissolution amount and rate were evaluated from the change in the sample weight and its processing time. Because the weight of the Pd samples varied in the range of 39–42 mg, the initial weight was converted to 40 mg using Eq. (5). In this equation, wdissolution is the actual dissolved amount, and wsample is the weight of the input sample.
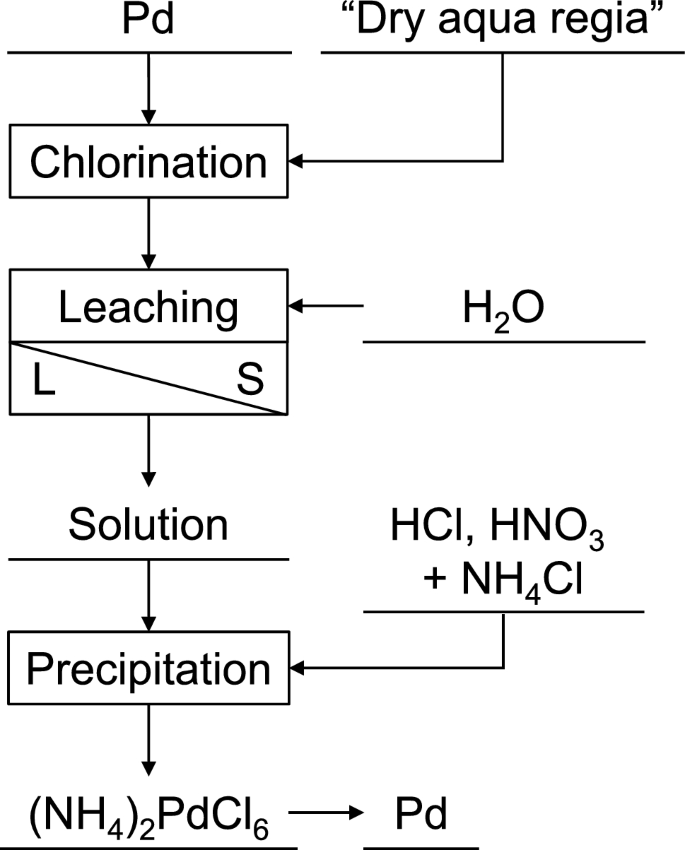
Precipitation and Recovery of Pd
Pd treated by the procedure mentioned in “Dissolution of Pd” section is predicted to be chlorinated as potassium tetrachloropalladate (K2[PdCl4]), based on the stability of Pd(II) ions in water [15] and a previous study that treated Pt [6, 8]. Pd chloride(II) (PdCl2) could be obtained by the dissolution of HCl (aq) and ammonium tetrachloropalladate(IV) ((NH4)2[PdCl6]) by the addition of NH4Cl and nitric acid (HNO3) [16, 17]. In this study, the authors precipitated (NH4)2[PdCl6] by the addition of HCl, HNO3 and NH4Cl to the solvent obtained by the leaching of frozen dry aqua regia after Pd dissolution by 20 mL of water. The recovered precipitate was decomposed into pure Pd by thermo-decomposition at 650 K under an Ar atmosphere. The recovery ratio was evaluated by comparing the dissolved and recovered weight of Pd.
Figure 2 shows the overall flow of the Pd recovery process, including dissolution and precipitation, described in “Dissolution of Pd” and “Precipitation and Recovery of Pd” sections.
Treatment of Pd in Coexistence with Pt
The FeCl3-KCl dry aqua regia can dissolve Pt, which can be recovered as pure Pt by solid–liquid separation using KCl (aq) and NaCl (aq) [8]. This separation uses the difference in solubility of K2[PtCl6], that is, insoluble in KCl (aq) and soluble in NaCl (aq). In contrast, K2[PdCl4] is extremely soluble in aqueous KCl [17]. Thus, by the leaching of frozen dry aqua regia containing both Pd and Pt using KCl (aq), Pd was dissolved in the solution and separated from Pt remaining in the residue. In the conventional refining or recycling process, coexisting PGMs are difficult to be separated into individual elements by solvent extraction using organic solvents [10]. Considering some auto-catalysts use Pt–Pd alloy or contain several PGMs [18, 19], a simple process that can separate each element is important for the treatment of PGMs from spent auto-catalyst.
Figure 3 shows the overall flow of Pd dissolution in coexistence with Pt and their separation and precipitation using dry aqua regia. Both Pd and Pt were dissolved by FeCl3-KCl dry aqua regia. The Pd sample mentioned in “Treatment of Pure Pd” section in this study was the same as that used in a previous study (ϕ = 0.2 mm, L = approx. 50 mm) [6, 8].
After dissolution, the frozen dry aqua regia containing Pd and Pt was leached using KCl (aq) and separated into the supernatant and the residue. The dissolution rate was evaluated by the change in the sample weight. The change in the sample weight was converted by Eqs. (5) and (6) as the initial weight of 35 mg.
Pd was precipitated using the procedure shown in “Precipitation and Recovery of Pd” section and Pt, by the procedure described in a previous study [8]. In this study, the separation of Pd and Pt and their recovery ratio were evaluated. The dissolved Pd and Pt were precipitated by the same condition of their maximum recovery ratio as that in previous studies treating isolated samples. The recovery ratio was calculated by multiplying the compound weight by its metal content.
Treatment of Pt–Pd Alloy
Pt–Pd alloy (Pt80%-Pd20%, ϕ = 0.2 mm, L = approx. 100 mm, w = approx. 60 mg) was treated with dry aqua regia under the same conditions of “Treatment of Pd in Coexistence with Pt” section. The difference in the weight between the samples was corrected by the following Eq. (7).
After dissolution, dissolved Pd and Pt were recovered by the same procedure described in “Treatment of Pd in Coexistence with Pt” section.
Results and Discussion
Treatment of Pd
Dissolution
Figure 4 shows the dissolution results for Pd. The dotted line in the figure shows the converted initial weight of the sample (40 mg). The effect of the temperature on the dissolution ratio was small, ranging between 560 and 600 K. All of the treated Pd was dissolved within 2 h at 600 K. In the cases under 580 K, approximately 70% of the sample was dissolved within 3 h. The initial dissolution rate was 1.72 mol m−2 h−1 at 560 K, which was relatively larger than that of Pt (0.45 mol m−2 h−1 at 655 K) [6]. From this result, it is suggested that Pd can be more easily dissolved than Pt by treatment with dry aqua regia.
Precipitation and Recovery of Pd
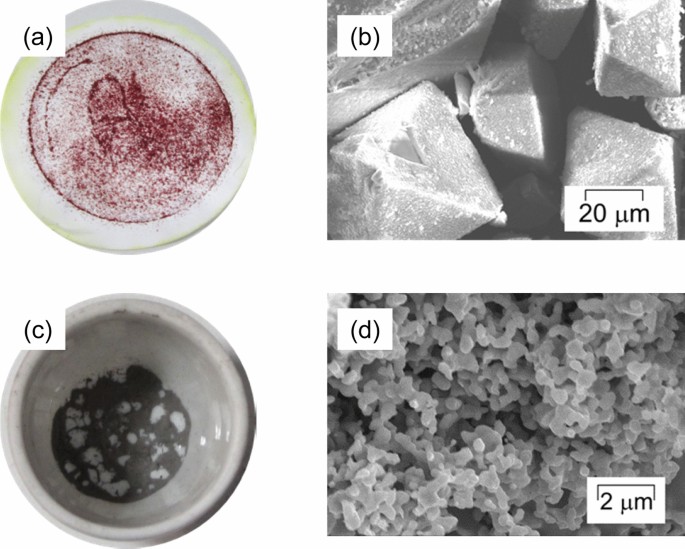
Figure 5a, b shows the recovered material by the addition of HCl, HNO3, and NH4Cl to the obtained solvent after leaching and its SEM image. Figure 5c, ds show the material recovered by thermal treatment and its SEM image. Table 1 shows their content by SEM–EDX analysis. Figure 6 shows the XRD analysis of recovered material (shown in Fig. 5a).
XRD result of recovered material [20]
The recovered material was dark-red in color, and its structure was octahedron. As shown in Table 1, the atomic ratio of Pd and Cl was determined by SEM–EDS as 15.8:81.8 which is near to 1:6 and little impurities like K and Fe. This recovered material was determined as (NH4)2[PdCl4] from the content analysis, its color and crystal shape, XRD result [19], and the reactions shown in Eqs. (8) and (9):
The recovered material after the thermal treatment was dark-gray in color, and its structure was porous. The suppression of contamination by impurities, especially iron (Fe), was confirmed from the SEM–EDX analysis. This result suggests that the solid–liquid separation and the addition of HCl and HNO3 prevented the contamination.
The porous structure of the final recovered material was similar to that obtained in a previous study [21], even though the recovery processes were completely different from each other. In this study, the recovery process was thermal decomposition, while precipitation was based on redox potential control in previous research. This result suggests that the similarity of recovered materials was caused by the characteristics of Pd, which agrees with a previous study [22]. In addition, we obtained nearly pure Pd in this study, while the contamination of copper (Cu) was confirmed in a previous study [21]. This result suggests the effectiveness of the solid–liquid separation shown in Fig. 2.
Table 2 shows the change in the recovery ratio in the recovery conditions. The recovery ratio was brought up to approximately 80% by the addition of a sufficient amount of HCl and HNO3 for the leaching and NH4Cl for precipitation. However, the recovery ratio decreased approximately 15–30% if some of the conditions were not optimized. These results suggest that the recovery process requires to be optimized for the effective recovery of Pd.
Dissolution of Pd in Coexistence with Pt
Figure 7a, b shows the dissolution rates of Pd and Pt at 650 and 600 K, respectively. In these experiments, the initial weight of sample was converted as 40 mg (Pd) and 35 mg (Pt). These results were compared with the Pt dissolution rate at 650 K [6] and the Pd dissolution rate at 600 K.
In the experiment at 650 K, most of the Pd was dissolved within 3 h, while Pt dissolution was suppressed. The dissolution of Pt started after the dissolution of Pd, and the dissolution rate was approximately the same as in previous research [6]. These results suggest that the dissolution of Pt was suppressed in the presence of Pd.
In the experiment at 600 K, approximately 70% of the Pd was dissolved by a 6-h treatment, while under the same conditions, most of the Pd was dissolved within 2 h in the experiment of solo-dissolution. In addition, most of the Pt was not dissolved. These tendencies suggest the requirement of high-temperature treatment for auto-catalysts containing several PGMs and the preferential dissolution of Pd in coexistent Pd and Pt.
Dissolution of Pt–Pd Alloy
Figure 8 shows the dissolution rate of the Pt–Pd alloy. Approximately 20 mg of alloy was dissolved in 8 h for an initial sample weight of 60 mg, which contained 48 mg of Pt and 12 mg of Pd. In the case of solo-dissolution of 48 mg of Pt and 12 mg of Pd, 35.5 mg of Pt in 8 h and 12 mg of Pd in 3 h were dissolved. From these calculations, the dissolution of Pd and Pt from the alloy was drastically less compared with that of the pure metal samples. In previous research, the electro-dissolution of Pd, Pt, and Pt–Pd alloy was performed. The research pointed out that alloying of Pd with a highly noble metal such as Pt decreases the alloy ability to be oxidized [23]. As mentioned in the introduction, Pd and Pt often form their alloy in the spent auto-catalyst, and this highly anticorrosive alloying must be considered during the treatment of spent auto-catalysts.
As mentioned in the introduction, a solvent containing FeCl3, HCl, and NaCl can dissolve Pd without high temperatures [9]; however, there is no evidence that this solvent can dissolve the Pt–Pd alloy. In this study, the authors attempted the treatment of Pt–Pd alloy using this solvent, and no change in weight was detected after 24 h of treatment at approximately 300 K. This result suggested that Pt–Pd alloy cannot be dissolved by this hydrometallurgical procedure and dry aqua regia can be effective for the treatment of Pt–Pd alloy present in spent auto-catalyst [10].
Separation and Precipitation of Pd and Pt Using Solid–Liquid Separation
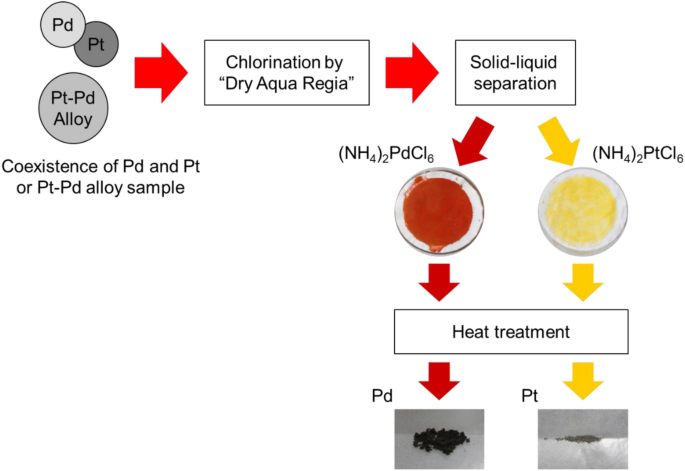
Figure 9 shows the recovered materials. (a) Is material recovered from the liquid phase and (b) is its SEM image, (c) is material recovered from the solid phase, and (d) is its SEM image. Table 3 shows the content of the recovered materials analyzed using SEM–EDX. Figure 10a–d shows the recovered materials and their SEM images from the Pt–Pd alloy treatment and Table 4 shows the content of recovered materials by SEM–EDX analysis. Tables 3 and 4 also show the content in the final recovered material.
In both cases of the treatment of Pd in coexistence with Pt and treatment of Pt–Pd alloy, red material (a) was recovered from the liquid phase and the yellow material (c) was recovered from the solid phase. As shown in Tables 3 and 4, the red material is a Pd compound, while the yellow material is a Pt compound. These results suggest the separation of Pd and Pt using solid–liquid separation, as shown in Fig. 11.
In conventional processes, the separation of PGMs requires, for example, solvent extraction, which is a complex procedure. In addition, these processes cause environmental impacts due to the chemicals and their chemical sludge [24, 25]. The process used in this research does not require a solvent for extraction and uses relatively mild chemicals. This result suggests that implementing this process could reduce the environmental impact of the separation and recovery of PGMs.
However, the Pd content in the final recovered materials was approximately 30% in both cases, while nearly pure Pt was recovered. Other contents in the Pd compound comprise mainly K and Cl, which were not detected in the recovery from solo-Pd treatment. For the effective recovery of Pd from the coexisting Pt, the suppression of the precipitation of these impurities is necessary.
Table 5 shows the recovery ratio of Pd and Pt from simultaneous dissolution. Approximately 60% Pd was recovered in all conditions, whereas only 20–40% of Pt was recovered. These recovery ratios were lower than those of solo-dissolution, and the decrease in the case of Pt was particularly large. To enhance the recovery of PGMs, effective separation and precipitation are required.
Conclusions
Through this research, the authors confirmed the dissolution of Pd using dry aqua regia and its recovery by the precipitation process. The authors also confirmed the Pd dissolution in coexistence with Pt and dissolution of the Pt–Pd alloy. The separation and recovery of Pd from Pt were also confirmed. These results suggest the availability of an effective recycling and simple separation process for PGMs using dry aqua regia.
However, the recovery ratio of Pd and Pt was still low and recovered Pd contained some impurities like potassium (K) or Cl. In future work, the authors will improve the recovery ratio and purity of recovered Pd in case of coexistence with Pt. In addition, the authors will also apply this process to other PGM elements, such as Rh, and will carry out the recovery of PGMs from spent auto-catalyst to construct the industrial recycling process.
References
Japan Oil, Gas and Metals National Corporation (2017) Kobutsu shigen material flow 2017 Hakkinzoku (PGM). http://mric.jogmec.go.jp/wp-content/uploads/2018/03/material_flow2017_PGM.pdf. Accessed 30 Sept 2020
Thomson Reuters (2017) “GFMS PT & PD survey 2017”. http://images.financial-risk-solutions.thomsonreuters.info/Web/ThomsonReutersFinancialRisk/%7B1680fcf5-2b58-4950-9651-1004a9b39aad%7D_GFMS_Pt_Group_Metals_Survey_2017.pdf. Accessed 30 Sept 2020
Panda R, Jha MK, Pathak DD (2018) Commercial processes for the extraction of platinum group metals (PGMs). In: Kim H et al (eds) Rare metal technology 2018. TMS 2018. Springer, Cham
Okabe TH, Nakata H, Morita K (2008) Recovery technology of platinum group metals. Hyomen Kagaku 29:592–600. https://doi.org/10.1380/jsssj.29.592
Yoshida T (2007) Kikinzoku rare metal no recycle Gijutsu Shusei. NTS Inc., Tokyo, pp 76–148
Yoshimura A, Matsuno Y (2019) A fundamental study of platinum recovery from spent auto-catalyst using “dry aqua regia.” J Jpn Inst Met Mater 83:23–29. https://doi.org/10.2320/jinstmet.J2018042
Barr WM (1976) Recovery of precious metal values from ores. US Patent 3(988):415
Yoshimura A, Matsuno Y (2019) The improvement of platinum recovery ratio in the recycling process using “dry aqua regia.” Mater Trans 60:2223–2228. https://doi.org/10.2320/matertrans.MT-M2019167
Ding Y, Zheng H, Li J, Zhang S, Liu B, Ekberg C (2019) An efficient leaching of palladium from spent catalysts through oxidation with Fe (III). Materials 12:1205
Sagawa Y (2015) Exhaust emission control catalyst, US. Patent 8,940,657 B2
Bernardis FL, Grant RA, Sherrington DC (2005) A review of methods of separation of the platinum-group metals through their chloro-complexes. React Funct Polym 65:205–217. https://doi.org/10.1016/j.reactfunctpolym.2005.05.011
Nikoloski AN, Ang KL (2014) Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner Process Extr Metall Rev 35:369–389. https://doi.org/10.1080/08827508.2013.764875
Homchuen P, Alorro RD, Hiroyoshi N, Sato R, Kijitani H, Ito M (2016) A study on the utilization of magnetite for the recovery of platinum group metals from chloride solution. Miner Process Extr Metall Rev 37:246–254. https://doi.org/10.1080/08827508.2016.1181629
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Hack K, Jung IH, Kang YB, Melançon J, Pelton AD, Robelin C, Petersen S (2009) FactSage thermochemical software and databases: recent developments. Calphad 33:295–311
Kılıç Y, Şireli GK, Timur S (2016) Production of Pure Platinum and Palladium from Dore Metals via Hydrometallurgical Methods, Proceedings of 18th International Metallurgy & Materials Congress 587–590
Arai Y (2011) Fundamentals of rare metal and precious metal recovery technology. Kogyo Kyoiku Shiryo 337:8–11
Garcia AG, Gervasio DF (2014) Pd–Pt nanostructures on carbon nanofibers as an oxygen reduction electrocatalyst. RSC Adv 79:42009–42013. https://doi.org/10.1039/C4RA06507G
Twigg MV (2011) Catalytic control of emissions from cars. Catal Today 163:33–41. https://doi.org/10.1016/j.cattod.2010.12.044
Miki T (2015) Reduction technique in amount of platinum used for diesel oxidation catalyst. AIST Today 55:33
Hooper TJN (2017) Solid state nuclear magnetic resonance on quadrupolar nuclei in disordered catalysis based materials, PhD thesis, University of Warwick, https://pugwash.lib.warwick.ac.uk/record=b3159493~S15. Accessed 30 Sept 2020
Komenami T, Sato M, Sato C, Matsuno Y (2018) Development of a production method for palladium micrometer-sized particles using DMSO solvent containing CuCl2. J Jpn Inst Met Mater 82:461–466. https://doi.org/10.2320/jinstmet.J2018030
Chen A, Ostrom C (2015) Palladium-based nanomaterials: synthesis and electrochemical applications. Chem Rev 115:11999–12044. https://doi.org/10.1021/acs.chemrev.5b00324
Łukaszewski M, Czerwinski A (2009) Anodic oxidation of Pd alloys with Pt and Rh. J Alloys Compd 473:220–226. https://doi.org/10.1016/j.jallcom.2008.05.037
Narita H, Suzuki T, Motokawa R (2017) Recent research in solvent extraction of platinum group metals. J Jpn Inst Met Mater 81:157–167. https://doi.org/10.2320/jinstmet.JE201604
Kato K, Ishiwatari Y, Oshiro Y, Osawa K, Igarashi S (2014) Development of high efficiency metal recovery system from precious metal plating waste fluid. Rep Ibaraki Prefect Ind Technol Center 43:6
Acknowledgements
This research was performed with the support of a JSPS research grant 19K 20483 and donations from the Hitachi Metals—Materials Science Foundation. We also received invaluable support from Professor T.H. Okabe and Associate Professor S. Yagi, from the Institute of Industrial Science, The University of Tokyo. In addition, Mr. Hidekazu Kato, a research associate at Chiba University, provided valuable research advice. We wish to express our gratitude to them all.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
The contributing editor for this article was U. Pal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshimura, A., Tochigi, S. & Matsuno, Y. Fundamental Study of Palladium Recycling Using “Dry Aqua Regia” Considering the Recovery from Spent Auto-catalyst. J. Sustain. Metall. 7, 266–276 (2021). https://doi.org/10.1007/s40831-020-00335-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00335-x