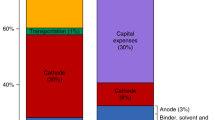
Abstract
The global growth of clean energy technology deployment will be followed by parallel growth in end-of-life (EOL) products, bringing both challenges and opportunities. Cumulatively, by 2050, estimates project 78 million tonnes of raw materials embodied in the mass of EOL photovoltaic (PV) modules, 12 billion tonnes of wind turbine blades, and by 2030, 11 million tonnes of lithium-ion batteries. Owing partly to concern that the projected growth of these technologies could become constrained by raw material availability, processes for recycling them at EOL continue to be developed. However, none of these technologies are typically designed with recycling in mind, and all of them present challenges to efficient recycling. This article synthesizes and extends design for recycling (DfR) principles based on a review of published industrial and academic best practices as well as consultation with experts in the field. Specific principles developed herein apply to crystalline-silicon PV modules, batteries like those used in electric vehicles, and wind turbine blades, while a set of broader principles applies to all three of these technologies and potentially others. These principles are meant to be useful for stakeholders—such as research and development managers, analysts, and policymakers—in informing and promoting decisions that facilitate DfR and, ultimately, increase recycling rates as a way to enhance the circularity of the clean energy economy. The article also discusses some commercial implications of DfR.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the traditional linear economy, products are typically landfilled at end of life (EOL), and new products from virgin materials are manufactured to replace them. In contrast, a circular economy optimizes energy and material use over product life cycles by remanufacturing, refurbishing, repairing, or reusing EOL products. When products can no longer be remanufactured, refurbished, repaired, or reused, recycling is the final circular option. Yet many products comprise a complex mix of materials that are difficult to recover during recycling. One approach to bringing such products into the circular economy is to design them from the beginning with recycling in mind. Design for recycling (DfR) holds potential to increase the quantity and value of materials recovered and reused from EOL products.
Researchers have proposed engineering practices that resemble DfR under a variety of other names and classifications [1,2,3,4,5]. For example, the classification by Fiksel [2] identifies common pathways and methods among different design for the environment (DfE) strategies, where DfE encompasses DfR under the category of design for revalorization; other DfE categories include design for dematerialization, design for detoxification, and design for capital protection and renewal.
DfR is optimally applied when looking beyond just the recycling process itself to the facets of the larger system in which it fits (Fig. 1). Knowledge of the kind of recycling process that will be applied to a product (e.g., hydrometallurgical, pyrometallurgical) can affect the design choices that enhance recyclability (DfR), elaborated further below. Recycling often consists of multiple processing stages. In ideal (but uncommon) situations, DfR can enable direct reuse of disassembled, intact, recovered components (denoted by the dashed blue line in Fig. 1), which minimizes intermediate losses and overall recycling energy use.

Figure adapted from [6] (Color figure online)
The role of DfR within a circular system. The large red-dotted boundary encompasses various activities which should be considered in DfR, whereas the small red-dotted circle encompasses stakeholders that directly implement DfR; service providers are included because their feedback can inform product manufacturers on DfR. The dashed blue line denotes direct reuse of intact recovered components.
Although multiple studies discuss DfR in some capacity, few studies have attempted to identify specific strategies for implementing it [4, 7,8,9]. Others provide broad DfR strategies and comment on DfR’s relationship within broader sustainability practices [2, 5]. This article synthesizes and extends identified DfR principles based on a review of published industrial and academic best practices as well as consultation with experts in the field; these experts are listed in the Acknowledgements. The resulting high-level DfR principles are meant to be useful for stakeholders—such as research and development (R&D) managers, analysts, and policymakers—in informing and promoting decisions that facilitate DfR.
We focus on the application of DfR to several clean energy technologies because of their recent and projected growth (e.g., to fulfill renewable portfolio standards,Footnote 1 100% renewable energy targets,Footnote 2 and other initiativesFootnote 3) and to help avoid future waste-management challenges. Cumulatively, by 2050, estimates project 78 million tonnes of raw materials embodied in the mass of EOL photovoltaic (PV) modules (which are ~ 95% crystalline-silicon modules), 12 billion tonnes of wind turbine blades, and by 2030, 11 million tonnes of lithium-ion batteries [10]. Thus, we provide DfR principles specific to crystalline-silicon PV modules, batteries like those used in electric vehicles (EVs), and wind turbine blades, along with a set of broader principles applicable to all three of these technologies and potentially others. These principles are based on the current state of knowledge and technologies, and revisions will be needed as the field continues to evolve. The article concludes with a discussion of commercial DfR applications in clean energy and a summary of the principles.
Principles Applicable to All Three Selected Clean Energy Technologies
Based on our literature review—see the Supplementary Information (SI)—and consultation with experts, we identify nine DfR principles that broadly apply to crystalline-silicon PV modules, EV batteries, and wind turbine blades, as described below. Readers could consider whether these broad principles might also apply to other clean energy technologies. Principles specific to the selected clean energy technologies are provided in “PV DfR Principles” section (crystalline-silicon PV), “EV Battery DfR Principles” section (EV batteries), and “Wind Turbine Blade DfR Principles” section (wind turbine blades).
Product Requirements Such As Functionality, Longevity, Reliability, and Cost Are Critical for Market Acceptance; DfR Should Support or Enhance These Aspects but May Result in Trade-Offs Between Recyclability and Product Performance and Cost
DfR products must be accepted in the market for the recycling benefits to be achieved, which requires that the function, reliability, and cost be acceptable to consumers. In some cases, improving a product’s recyclability may also improve its performance and/or cost, but in some case these different objectives conflict. If a high degree of recyclability impairs a product’s commercial viability, designing for less recyclability may be necessary [4]. “Crystalline-Silicon PV DfR Principles” section (Principle 4) provides an example of such a trade-off, in relation to the laminate-free NICE-PV module that sacrifices some efficiency to facilitate disassembly and recycling. Conversely, if a product is subject to recycling targets (e.g., due to government policies), lower performance or higher cost may be trade-offs for greater recyclability.
Material Choice and the Ability to Liberate Separate Materials Are Critical to DfR Outcomes
DfR outcome metrics like material recovery rate and purity of recovered materials depend on the choice and distribution of materials within the product as well as use of adhesives (see Principle 6) [4, 7, 8]. Materials entering secondary material markets must meet purity specifications. If incompatible materials are used in a product, but are not adhered together, they can sometimes be adequately separated to avoid commingling of the recycling output streams—for instance, when the incompatible materials are isolated in easily separable components. However, even if incompatible materials are in the same component, they might be separable during a recycling process. For example, shredding might be able to liberate and separate copper (Cu) and iron sufficiently from a product to provide a steel mill with a stream of recycled iron that is acceptably uncontaminated with Cu [7].
Recycling Outcomes Can Be Enhanced by Minimizing Hazardous Materials in Products or Making These Materials Completely Recoverable via DfR
Hazardous materials should be avoided when possible (e.g., when not contrary to Principle 1) so the product is not classified as hazardous waste at EOL, to reduce the cost of recycling (for handling and treating hazardous materials), and to reduce environmental impacts. When hazardous materials must be used, DfR that enables full and separately controlled recovery of these materials can avoid contamination of recycling outputs and characterization of the outputs as hazardous waste. For example, recycling rates for lead-acid car batteries exceed 90%. Most of the lead (Pb) is recovered and used in new battery applications, which keeps this hazardous material out of landfills and avoids contaminating other waste streams [11]. Similarly, cadmium telluride (CdTe) PV modules are subject to a specialized process that recovers Cd and Te, which prevents environmental and human health hazards related to Cd [12, 13].
Minimizing and Managing Hard-to-Recycle Materials Can Improve Overall Recycling Yield
Hard-to-recycle materials vary by recycling situation and may include materials degraded by recycling or those not recyclable more than a few times, such as treated wood [4, 5]. When such materials must be used, DfR that makes them easy to isolate early in the recycling process can facilitate recycling of the product’s other materials.
Minimizing Non-Reversible Adhesives or Similar Bonds, Especially Over Whole Surfaces and for Dissimilar Materials, Can Facilitate Disassembly and Material Liberation
Non-reversible adhesives or similar difficult-to-break bonds may impede product disassembly and material liberation [4]. This is especially true when two dissimilar and incompatible materials are bonded together; however, in some cases, such bonds can facilitate recycling, such as when strategically placed welds (non-reversible) provide a better path of preferential breakage and liberation during shredding, compared with using bolts (reversible).
Design for Disassembly (DfD) Can Improve Recyclability
DfD promotes modular product construction, which can facilitate separation and then recycling of individual component groups at EOL (as well as repair of components individually, as opposed to needing to disassemble a whole device to repair the faulty component). In addition, product disassembly trials by manufacturers can reveal implications of various aspects of the product’s design with regard to DfR, such as choice and location of fasteners and joining methods [9, 14], and these insights can be applied in designing a recycling process. Design for remanufacturing exhibits similar synergies with DfR [4, 5].
Being Able to Estimate Improvements in Recyclability and Economic and Environmental Impacts Due to DfR Is Important for Continuous Improvement, Identification, and Weighing of Trade-Offs and Communicating Value
In recent years, methods designed to quantitatively [7,8,9, 14] or qualitatively [4] analyze product recyclability have emerged. These methods, which vary in complexity and ease of use, are most useful when validated with experimental observations, and they can continue to be improved. DfR can be beneficial even if product designers can only assess its impacts at an informal level [4, 7,8,9, 14]. Benefits from analyzing effects of recycling include the ability to learn and continuously improve, to identify trade-offs and weigh their effects for future redesign, and to enable communication about the value of DfR to internal and external stakeholders.
Using Labels to Identify Recyclable and Non-Recyclable Materials Helps Recyclers Classify Feedstocks; Labeling Standardization Is Important for Uptake and Utilization
Product labeling options include stickers with identifying information, symbols, embossed or engraved information, bar codes, radio-frequency identification (RFID), blockchain,Footnote 4 material passports,Footnote 5 or other methods. The label composition, placement, and application method can have positive or negative recycling implications, but the label’s information is most important [4, 7, 8]. Clear labels help recyclers classify feedstocks and thus facilitate their entire recycling operations [2, 11]. Standardization is key to efficiency of use, degree and ease of uptake, and broad utilization. Confidential or proprietary information can be maintained in some systems, with potential trade-offs in ease and speed of identification by multiple parties. Labels that provide information beyond material composition—such as appropriate handling, repair, remanufacturing, and recycling strategies—can also be helpful.
Designing Products to Use Recycled Materials Promotes Circular Manufacturing
Although using recycled materials in a product does not inherently improve that product’s recyclability, doing so bolsters the circularity of the manufacturing system and encourages DfR; building markets for recycled materials gives impetus to companies and R&D managers to enhance design of products for recycling, because the materials within the products will have greater value at EOL [2, 4, 5]. In turn, increasing the prevalence of DfR may lead to higher-quality recycled feedstocks and greater utilization of these resources.
PV DfR Principles
This section starts with principles focused on the world’s dominant PV technology, crystalline-silicon PV modules (“Crystalline-Silicon PV DfR Principles” section), followed by a short discussion of DfR considerations for thin-film PV modules, which hold the remaining market share (“Discussion of Thin-Film PV” section). Various crystalline-silicon PV module recycling concepts exist [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30], including three that have achieved pilot scale or larger: the hot-knife (thermal) glass separation process offered by NPC [18, 19], a mechanical-only process run by Veolia in France [20,21,22,23], and a mechanical-chemical process designed in the Full Recovery End Life Photovoltaic (FRELP) research project—see the SI [15, 24, 25]. The basic design of crystalline-silicon modules has not changed for decades, although manufacturers have created thousands of variations. Such variability presents a challenge to recycling systems and PV DfR. In addition, PV systems are expected to perform with high reliability for warranty periods of 2–3 decades under extreme outdoor environmental conditions. Such stringent performance requirements have incentivized a design philosophy leading to a sealed, durable, sandwich-like module structure that hinders separation and liberation of constituent materials. DfR for PV modules must consider current market (price, performance) and safety expectations as the starting point for any proposed changes.
Crystalline-Silicon PV DfR Principles
Durable Identification of Module Construction and Composition Could Enable Safer and More Efficient Recycling Processes
Identifying module composition and construction may permit higher tolerance for variable module designs that are otherwise suboptimal from a recycling perspective, because the recycling process can be designed to accommodate known variability. Known composition could also facilitate batch processing of categorized groups, enable isolation of problematic or incompatible chemical compositions, and avoid contamination of recycling products. Because modules may outlive their manufacturers, it would be helpful for labeling to be durable (on the scale of decades) and for any linked databases of construction or composition to remain accessible after a manufacturer goes out of business. Steps in this direction are being taken. Although currently limited, a new voluntary sustainability certification standard, NSF-457, includes provisions for identifying PV module compounds [30]. Emerging regulations in France surrounding minimization of PV’s carbon footprint will require manufacturers to identify module composition details [31, 32].
Backsheet Composition Has Particularly Important Implications for Recyclability
The design of PV module backsheets has implications for cost, performance, and recyclability [25]. One important issue relates to backsheets containing fluorinated polymers, which produce hazardous fluorine (F) gases under thermal processing and thus increase thermal recycling costs or restrict treatment options. For example, use of pyrolysis is challenged because the pyrolysis oil becomes contaminated with F [25, 33,34,35,36,37]. Some segments of the PV industry have reduced module F content to reduce production costs (see the SI) [38]. Recently, recyclability has become a driver of F-free backsheet manufacturing [37], particularly for higher-end modules. Transparent backsheets exist in both fluorinated [39] and F-free [40] varieties. If thermal processing is not used, backsheet composition has less effect on recyclability.
If fluoropolymers must be used in backsheets for a particular module design, lower F content is preferable for three reasons. First, air emissions from thermal processing can be controlled at lower cost owing to lower use of reagent to neutralize F. Second, the resulting gases will be less corrosive. Finally, Cu smelters will pay more for recovered materials with lower levels of F contamination.
The cost implications of recycling fluorinated backsheets have been debated. Some stakeholders say the incremental cost impact is limited, particularly where regionally available thermal treatment infrastructure is already equipped for fluoropolymers. However, the incremental cost could reach 1 U.S. dollar (USD) per module when a primary Cu smelter treats an F-bearing backsheet [41], which could be roughly equivalent to the recovered Cu value per module [30], thus reducing the economic feasibility of recycling. When a module’s polymer content represents a potentially salable recycling output (as with the Veolia process), backsheet content considerations depend on the local or regional recycled plastics market [20].
Future recycling processes may be able to remove fluoropolymers from backsheets mechanically [19, 42, 43]. In such a process, designs that minimize fluoropolymer coatings on the inner layers of the module would likely be easier to treat. If a future recycling option can remove and isolate a thin (~ 50 μm) layer from the exterior of a backsheet (of fluoropolymer and/or non-fluorinated base layer materials), this could improve the tolerance of recycling systems to the use of exterior fluoropolymer layers in future module designs [44]. The SI contains descriptions of R&D aimed at mitigating recyclability problems due to fluorinated backsheets. Glass/glass or double-glass PV modules that eliminate the backsheet in favor of a second layer of glass would likely bypass the F issue.
Beyond F content, other backsheet attributes can affect module recyclability. For example, polymers containing nitrogen can result in nitrogen oxides during thermal treatment. Nano-titanium dioxide (TiO2) pigment may become subject to additional regulatory hurdles, particularly in thermal treatment applications [44]. The recycling implications of using carbon black pigments or conductive backsheets are unclear [45].
Metal Choices Can Have Significant Impacts on Recycling Processes and Costs
Substitution of silver (Ag) metallization with Cu/nickel (Ni) presents recycling trade-offs, which depend in part on the recycling process used. Ag, one of the largest sources of module recycling revenues, is also one of the most resource-constrained PV feedstocks. Reducing Ag use in modules may be necessary to sustain rapid global PV growth [46,47,48,49,50]. Partial substitution with Cu/Ni is less favorable than total substitution in recycling processes that use leaching, such as FRELP, owing to the additional complexity of handling both Ag and Ni in the leach solution. The presence of Ag makes the mixture unsuitable to alternative and less expensive leaching chemistries [51]. Costs for recycling processes without leaching, such as the hot-knife/Cu smelter process, likely would be less affected by partial or total substitution of Ag with Cu/Ni. In some cases, Ni can be recovered when present in a Cu smelter feed, and penalties likely would not be incurred if Ni concentrations are below 1% mass in the dry smelter feed [52].
Various candidates could replace Pb in module solder alloys, with a range of impacts on recyclability. Cu or Ag could increase recycling revenues, although using Ag may strain feedstock availability and increases cost. Tin (Sn) is effectively interchangeable with Pb in terms of recycling impacts; Sn is scarcer and more expensive than Pb, but less so than Ag. Bismuth (Bi) presents the most recycling problems among Pb-replacement candidates. Bi can result in higher penalties when selling Cu-bearing recycling outputs to a Cu smelter, reaching 3–10 USD per tonne of smelter feed per 0.1% of excess Bi for a typical feed consisting of 22%–30% Cu [52]. In contrast, Sn and Pb are tolerated at much higher concentrations (3%–5% Pb + Sn + zinc) and incur lower penalties (1 USD per tonne per 1% mass) [52]. Bi interferes with downstream electro-refining of smelter Cu outputs, increasing complexity and cost [52]. Failure to remove Bi results in lower electro-refining efficiencies, higher energy intensity for PV recycling, and potential contamination of the Cu cathode; the mechanical properties of Cu products suffer with even 0.2% mass Bi contamination [53]. Cu refiners rely on a sulfuric acid process, but the same issues may manifest in more sophisticated PV recycling flowsheets, such as FRELP’s nitric acid-based process, and could require further recycling stages. In addition, not all Bi-isolation methods yield salable Bi outputs, so circularity of Bi is not assured. Pb and Sn interfere much less with such circuits and can be readily managed in FRELP’s nitric acid-based process [15]. Should Bi be required in crystalline-silicon PV applications [54], some portion of the recycling impacts could eventually become more tolerable [55]. See the SI for additional discussion of this issue.
Minimizing Laminate Use or Using Reversible Laminates Can Facilitate Disassembly of PV Modules
Delamination poses a challenge to many PV recycling processes. The FRELP and hot-knife processes use high temperatures to volatilize the laminate from the cells [15, 18, 24, 25]. The Veolia process separates materials laminated together through a complex mechanical process. This process may have more tolerance for existing laminates but would likely perform better without them owing to reduced cross-contamination of recycling products, improved liberation, and more homogenous plastic outputs [20,21,22,23].
The manufacturers of the NICE-PV module offer a laminate-free design that facilitates disassembly and recycling, but module efficiency declines because of the air gap and electrical connections held together by internal module vacuum pressure; changes in temperature and altitude would change the electrical efficiency [56,57,58,59,60]. One study demonstrated that the NICE design also enabled relatively easy recovery of intact wafers and glass panes from a simulated EOL module, which is currently infeasible for modules with laminates [60]. TPedge is another laminate-free module design. It uses adhesive pins, which cover only 0.02% of the cell surface, and soldered electrical connections that require no specific under-pressure but might exhibit some sensitivity to temperature or altitude. Both NICE and TPedge eliminate laminate from the face of the cells with the help of edge-sealing methods like silicone. Still, some glue-like material is required on portions of the front glass and rear materials. Although recyclability has not been specifically demonstrated on a TPedge module, the design indicates it could be more successful than most typical modules [61,62,63].
Ideally, future laminate designs would offer the necessary durability but also be more easily reversed during recycling than the currently typical module laminate, ethylene vinyl acetate (EVA) [64]. Other materials, such as silicone, may offer benefits accompanied by trade-offs in the form of increased unit cost or different life cycle impacts [65]. A study found, for instance, that silicone laminates could increase short-circuit current density and cell efficiency under certain conditions [65, 66]. This result may, however, not apply to dusty conditions [66]. Moreover, silicone encapsulants have been found to lose adhesive properties in extreme weathering conditions [67].
Decreasing the Number and Complexity of Module Materials Presents Trade-Offs Related to Recyclability and Economics
Two trends in PV module designs exemplify trade-offs with regard to reducing the number and complexity of materials. Frameless modules are one trend. PV modules are typically designed with frames, but they can be designed without frames. Framing helps protect the module during transportation, installation, and EOL removal while easing the installation process and providing torsional rigidity throughout the life cycle. Frameless modules are more prone to breakage, although certain transportation strategies and, for instance, reusable corner protectors can reduce breakage. On the other hand, frameless modules simplify recycling. De-framing a module adds a recycling step and increases the potential for glass and cell breakage [68]; however, the frames are relatively easy to recover, and the aluminum (Al) can add more than 2 USD/module in recycling revenue [16, 30]. Thus, the frame’s impact on recycling economics is comparable to module Ag content (~ 2.70 USD/module [30]), particularly for recycling processes such as FRELP and hot-knife [15, 18, 24, 25]. As yet another trade-off, frameless modules are lighter and more compact and can cost less to transport at EOL. Removing the frame (whether in design as frameless or through frame removal at EOL) can increase up to sevenfold the number of modules that fit in the same container [20].
Glass/backsheet versus glass/glass module designs present other trade-offs. Glass/glass designs increase the potential glass cullet revenue per module and eliminate use of a backsheet, which is often fluorinated [16, 30]. If the rear and front glass are of different grades and are not isolated from each other throughout the recycling process, introducing the multiple grades into outputs may degrade the glass recycling economics. Experimental recycling data are sparser for glass/glass modules than for the typical glass/backsheet varieties. Because the mechanical properties of glass differ from those of polymer backsheets, recycling processes that intentionally or coincidentally exploit such attributes could be impacted in unpredictable ways. The mechanical Veolia process has undergone preliminary testing with glass/glass modules, and future glass/glass recycling is possible with some retrofitting, according to a La Mia Energia representative (personal communication, 2019).
Discussion of Thin-Film PV
The crystalline-silicon PV DfR principles pertaining to structural aspects of a module generally remain valid for a range of thin-film module chemistries; however, the metal-recovery stages of the recycling process may vary as a function of underlying module chemistry. A copper-indium-gallium-selenide (CIGS) module may require different treatment [69, 70] than CdTe [12, 13] or perovskite [71,72,73] modules. First Solar manufactures and also recycles their CdTe modules [12]. Integrating these functions in the same facility and supply chain enabled the establishment of a best practice for DfR implementation: First Solar’s recycling division is part of the approval chain for new module designs, ensuring recyclability is considered in developing new module designs. Additionally, raw material values are higher for CdTe than for crystalline silicon, inducing interest in enhancing the security of their supply chain by participating directly through EOL recovery of precious metals like Te. Handling of Cd, a metal with high toxicity, must be carefully controlled to ensure no environmental release, something that the company had to prove early on to ensure their social license to operate. Laboratory-scale recycling of several CIGS and perovskite module designs has been demonstrated [69,70,71,72,73]. Despite their infancy, circularity appears possible for perovskite PV, despite concerns about Pb content [71, 72].
EV Battery DfR Principles
Many battery DfR principles depend on the battery chemistry and recycling flowsheet [11, 36, 74,75,76,77]. This section lists several principles applicable to current and next-generation EV batteries. These principles focus on the battery exterior housing, because they can be applied well to the range of different battery types. Overall, standardization of product size and design can help streamline and optimize operations for battery recyclers, but it is not the current reality. Internal configuration and material choices are also important recyclability considerations, but the variability of these characteristics hinders identification of broadly applicable principles. The principles listed here are generally applicable to batteries sized for EVs and smaller batteries. Where possible, commentary on lithium-ion batteries is provided, but these batteries consist of a highly diverse set of chemistries and recycling options [75,76,77]. The principles are generally less applicable to large-scale vanadium redox flow batteries (VRFBs); performance, durability, and recyclability are complementary, rather than competing, criteria in this application—at least with regard to the vanadium (V) electrolytes. In addition, the larger the scale, the better the capacity for built-in equipment redundancy, isolation, and repairability during operation, all of which further improve recyclability [78, 79].
Recyclability Is Usually Improved When Removal of the Battery Is Made Easier
Easy battery removal improves recyclability of the battery (and the host product), which increases the probability that batteries will end up at dedicated recycling outlets [4, 9, 11, 14, 80,81,82]. However, in some situations (e.g., with EVs), a hard-to-remove battery may enhance recyclability by making it more likely that only trained personnel attempt removal, which may improve safety and control over the recycling supply chain.
Clear Identification of Battery Chemistries Can Mitigate Recycling Safety Hazards
Improper mixing of battery chemistries during recycling can be a safety hazard. Conversely, avoiding such mixing can improve the probability of any given battery material being reintroduced into a manufacturer’s supply chain. Clear identification of chemistries can mitigate this issue, using approaches such as color coding, RFID, barcodes, and ultraviolet or infrared scanning. Recent advances in sensor-based sorting technology may help battery recyclers use such identifiers to reject incompatible batteries early and cost-effectively [11]. The battery industry has historically resisted color coding owing to concerns about loss of brand recognition, but even partial adoption of such an approach (e.g., on battery areas that are never seen by consumers) could offer recycling benefits.
Matching the Battery Exterior’s Chemical Compatibility to the Intended Recycling Process Can Avoid Unwanted Chemical Interactions and Facilitate Recycling
It is important to ensure chemical compatibility between the exterior battery materials and the recycling process, because some exterior materials may not react well to some chemical or high-temperature processes. In some cases, using a chemically ideal exterior may enable skipping of early physical separation. Alternatively, if the material can be physically isolated upstream of the chemistry-intensive recycling stages, chemical incompatibility may not be relevant; for example, removing an Al exterior casing will limit the amount of Al exposed to chemical processing.
When durable, rigid, non-polymer, non-detachable exteriors are required, some materials are more amenable to existing recycling systems. It can be advantageous to isolate exterior or internal materials prior to downstream recycling in an attempt to separate at the earliest steps and provide the best opportunity for homogeneous recovered materials. For example, when subjecting lithium (Li), Ni, or cobalt (Co) compounds to acid leaching, it is beneficial to separation and performance outcomes to remove any Al exterior material before the acid-leaching process [36], to limit the amount of Al exposed to leaching.
Rigid Polymer Exteriors Are Often Well-Suited to Recyclability
Rigid, durable metal exteriors, especially those that cannot be readily detached, can hinder access to internal components and damage recycling equipment, such as crushers. Rigid polymer exteriors, by contrast, pose little threat to recycling equipment and can offer high material recovery; they are typically used for lead-acid battery exteriors. Some of these polymers float, which can facilitate dedicated polymer separation and recovery [11]. Fiberglass, another exterior material choice, currently has poor recyclability [80, 81]. Flexible or foil-like exteriors likely have fewer negative implications for recyclability, but this cannot be assumed for all situations. Metal exteriors are typical for EVs owing to structural strength requirements. Battery designs that can facilitate removal of the exterior (e.g., bolts instead of welds) can facilitate the recycling process.
For exteriors subjected to crushing or shredding, magnetic materials can be rejected pre- or post-crushing, or eddy current separators can enable rejection of Al/magnesium (Mg) alloy exteriors [83]. Magnetic or eddy current separation could also be used to remove higher-cost Ni/Co alloys [83], which—with additional processing—likely would be chemically compatible with many lithium-ion and some nickel-metal-hydride recycling flowsheets. Carbon fiber exteriors may be acceptable when thermal treatment is considered [84], if adding carbon does not hinder the intended chemical reactions. If mechanical separation of the exterior is intended, then carbon fiber is less desirable owing to the current challenges of recycling broken carbon fiber into new applications. Finally, titanium and austenitic stainless steels do not respond to magnetic or eddy current separation [83] and thus lack suitable physical rejection methods, making them more likely to increase crushing equipment wear; methods would need to be identified to ensure these materials are not sent into the shredder.
Using F-free Binders or Otherwise Minimizing F Content Can Facilitate Recycling
The F in fluoropolymer binders may hinder recycling, but few F-free binder substitution candidates exist. However, if other sources of F are already present in lithium-ion batteries (e.g., lithium hexafluorophosphate [LiPF6] electrolyte), substitution with a lower F-content (rather than F-free) fluoropolymer may be easier to rationalize, because F removal will still need to be included in EOL material-recovery processes.
Reducing the challenges of separating F from valuable materials at EOL can improve the economics of material recovery. In nickel-manganese-cobalt (NMC) lithium-ion batteries, these metals and Li are present in the “black mass fraction,” which is attached to electrode foils (such as Al) via a binder. The black mass fraction represents most of the battery’s rare metals and much of the Li content, and thus much of its value [36]. For this case, recovery of the black mass fraction requires separation from the Al electrode foils as well as the F binder; however, the prospects for maximum recovery decrease with increases in thermal treatment temperatures. There are some recycling advantages to enabling lower-temperature and faster binder destruction via substituting a polyvinylidene difluoride (PVDF) binder with a more readily volatilized fluoropolymer. Additionally, it is important to consider what types of materials might be volatilized in these thermal reactions to minimize hazardous emissions.
When considering alternatives to PVDF-based binders, materials based on terpolymer of tetrafluoroethylene, hexafluoropropylene, and vinylidene fluoride (THV) should be avoided. THV has a higher F content than other binders and is not prone to significant thermal decomposition until approximately 700 °C [85]. This forces the use of unfavorably high temperatures for thermal treatments for NMC lithium-ion batteries outside of recycling options that include smelters.
Wind Turbine Blade DfR Principles
Current wind turbine recycling challenges mostly focus on the blades and, to a lesser extent, the magnets, because the remaining turbine components have simpler designs and are made of more homogenous materials, in particular easy-to-recycle metals. The following DfR principles focus on blades. The SI discusses the potential DfR implications of magnet innovations. Ongoing developments in materials science, manufacturing, and recycling processes may radically shift the particulars of any wind DfR principles, and thus the principles should be reviewed regularly to ensure applicability.
Some Blade Materials Are More Recyclable Than Others, but Blade Technologies Are Changing
Composite materials, such as fiberglass and carbon fiber, are currently less recyclable than homogenous materials, because they are not easily separated into homogenous materials and at best are ground up and used as either filler or in other down-cycled products (Phuon Anh Vo Dong, 2018). Research is underway to evaluate blade compositions, fabrication methods, and recycling processes.Footnote 6
Because recycling of wind turbine blades is currently challenging and not commonly done, reuse of blades is another circular economy option to consider. The current large and ever-increasing size of the blades, however, likely makes this option impractical. The large size of the blades also hinders recycling, but on-site EOL size reduction could make transport of blades to recycling facilities easier [86].
Minimize Use of Additional Blade Materials Other Than Resin and Fibers
Research is underway6 to evaluate the potential for recovering composite resins using thermoplastics in place of thermoset materials; further progress could provide a favorable recycling option for wind turbine blades. Short of existing material-recovery options, DfR would suggest prioritizing materials with the highest potential for energy recovery (while considering fire safety) though this priority is only in relation to disposal since energy recovery is the least favored CE pathway (see Fig. 1). Carbon fiber materials consist nearly entirely of reactive combustible material, whereas traditional fiberglass leaves behind up to 40% mass in an ash fraction. While combustible, fiberglass requires additional fuel to combust, and thus is not desirable as a fuel substitute except in cement operations where the ash fraction is a cement filler [87, 88]. However, carbon fiber production has a very high energy intensity, so the life cycle perspective should be considered to ensure an overall benefit.
Some Blade Designs Enable Retention of Material Value After Recycling
Designing wind turbine blades such that materials maintain value after recycling is a key research need. For example, recycling processes that can recover long, straight fibers may provide higher value and performance potential, compared with processes that can only recover odd geometries which have limited uses for other designs or applications.
Commercial Implications of DfR
The successful application of DfR principles offers potential for environmental benefits as well as economic benefits to manufacturers and end users. Emerging business models such as product-as-a-service (PaaS) may enable manufacturers to leverage recyclability to reduce prices beyond what competitors can offer with comparable but less-recyclable products.
Rentable V electrolytes for VRFBs are one prominent clean-energy example of a PaaS business model [89]. Because the vertically integrated V miner/electrolyte manufacturer knows that the V will remain recoverable and recyclable after 10–20 years of operation, they are willing to enter into rental contracts with an end user of the battery [90]. If the V miner/electrolyte manufacturer rents V to the end user, both the battery maker and end user are decoupled from full exposure to the volatile cost of V. This reduces the end user’s upfront VRFB purchase cost (by as much as 30% in one estimate [90]), making it competitive with the cost of lithium-ion batteries [90]. The result is a more circular economy with regard to material flow and a business model in which a finite mineral reserve translates into recurring annual payments for the V miner long after digging has ceased. Such alternative business models inherently rely on assurance that EOL collection and recycling are possible.
To maintain a high degree of recyclability, product developers must maintain a working awareness of potentially applicable recycling processes. Thus, DfR is easier to implement when a manufacturer recycles its own products, whether through PaaS models or through extended producer responsibility schemes like those for lead-acid batteries in the United States or PV under Europe’s Waste Electronic and Electrical Equipment (WEEE) Directive—or, even more directly, First Solar’s takeback and recycling of its modules.
Conclusion
Table 1 summarizes the DfR principles described in this article. Because DfR is product and situation specific, these principles must be adapted to particular cases. For example, DfR may need to consider the recycling capabilities of regions in which a product is likely to reach EOL [9]. Specific technical considerations are also important, such as whether the product will require only mechanical separation or whether it will require thermal processing.
More generally, realizing a circular economy requires attention to many factors and coordination among numerous stakeholders. Recycling and DfR are important aspects of this effort, but they should be considered after other circular approaches—such as design for reuse, product longevity, and remanufacturing—that provide better material value retention (shown as tighter circles in Fig. 1). DfR is inherently challenged by the time that elapses between manufacturing of a product and the product’s EOL. A conservative approach is to design products with some assurance of the recycling system’s ability to handle the materials at EOL. Manufacturers can take calculated risks based on knowledge of potential future recycling methods, and they can mitigate risk further by becoming directly involved in planning future recycling capabilities or even operating their own recycling operations for their products (such as with the First Solar example above). The market for materials and the supply and demand will drive the success of recycling. Economics, product performance, and environmental impacts must be considered; recycling at EOL may not always be the best solution for the existing manufacturing and recycling ecosystem. Ultimately, DfR is desirable to achieve system-wide goals, such as ensuring material supply, maximizing material value, or minimizing life cycle impacts. There is a need for analytical tools to better evaluate the impacts of recycling, DfR, and other circular-economy strategies.
Notes
For example, http://there100.org/companies.
For example, www.everledger.io.
References
Institute of Scrap Iron and Steel, Inc. (1986) Design for recycling. Phoenix Q 18(1):8–10
Fiksel J (2009) Design rules and guidelines. Design for environment: a guide to sustainable product development. McGraw-Hill Companies, New York, pp 117–162
Den Hollander MC, Bakker CA, Hultink EJ (2017) Product design in a circular economy: development of a typology of key concepts and terms. J Ind Ecol 21:517–525. https://doi.org/10.1111/jiec.12610
Hultgren N (2012) Guidelines and design strategies for improved product recyclability—how to increase the recyclability of consumer electronics and domestic appliances through product design. Thesis, Chalmers University of Technology. https://pdfs.semanticscholar.org/ddbd/c91c3fb5fedfa094cc32bf07c2554aab81c9.pdf
Gaedel T, Allenby B (1996) Design for recycling. In: Design for environment. Prentice Hall, Upper Saddle River, pp 89–103
Ellen MacArthur Foundation (2013) From linear to circular accelerating a proven concept. In: Towards the circular economy, p 24. https://www.ellenmacarthurfoundation.org/assets/downloads/publications/Ellen-MacArthur-Foundation-Towards-the-Circular-Economy-vol.1.pdf. Accessed 8 July 2019
Reuter MA, Van Schaik A (2015) Product-centric simulation-based design for recycling: case of LED lamp recycling. J Sustain Metall 1(2015):4–28. https://doi.org/10.1007/s40831-014-0006-0
Van Schaik A, Reuter MA (2014) Material-centric (aluminium and copper) and product-centric (cars, WEEE, TV, lamps, batteries, catalysts) recycling and DfR rules. In: Worrel E, Reuter MA (eds) Handbook of recycling. Elsevier, Amsterdam, pp 307–378
Dender L, Gates C, Jackson N, O’Malley G, Okrasinski T, Rifer W, Schaffer M, Wolenski K (2019) A practical means for assessing circular economic value of an ICT product, iNEMI. https://www.inemi.org/care-innovation-2018-papers?submissionGuid=732791e0-4191-4ce8-814f-5aee7aada2a9. Accessed June 2019
IRENA, IEA-PVPS (2016) End-of-life management solar photovoltaic panels. International Renewable Energy Agency and International Energy Agency Photovoltaic Power Systems. https://www.irena.org/publications/2016/Jun/End-of-life-management-Solar-Photovoltaic-Panels. Accessed June 2019
Gaines L (2014) The future of automotive lithium-ion battery recycling: charting a sustainable course. Sustain Mater Technol 1–2(December):2–7. https://doi.org/10.1016/j.susmat.2014.10.001
Wade A (2013) Evolution of first solar’s module recycling technology. First Solar, Inc. http://iea-pvps.org/fileadmin/dam/public/workshop/07_Andreas_WADE.pdf. Accessed 14 Nov 2019
Sinha P, Wade A (2018) Addressing the hotspots in the product environmental footprint of CdTe photovoltaics. IEEE J Photovoltaics 8(3):793–797. https://doi.org/10.1109/JPHOTOV.2018.2802786
Vanegas P, Peeters JR, Cattrysse D, Tecchio P, Ardente F, Mathieux F, Dewulf W, Duflou JR (2018) Ease of disassembly of products to support circular economy strategies. Resour Conserv Recycl 135(August):323–334. https://doi.org/10.1016/j.resconrec.2017.06.022
Ramon L, Ercole P, Favaro N et al (2014) Full recovery end of life photovoltaic. Presented at: IEA-PVPS Task 12 Open Workshop, Amsterdam, 23 September 2014. http://iea-pvps.org/index.php?id=292
Deng R, Chang NL, Ouyang Z, Mun Chong C (2019) A techno-economic review of silicon photovoltaic module recycling. Renew Sustain Energy Rev 109(July):532–550. https://doi.org/10.1016/j.rser.2019.04.020
Lunardi MM, Alvarez-Gaitan JP, Bilbao J, Corkish R (2018) A review of recycling processes for photovoltaic modules. In: Solar panels and photovoltaic materials. IntechOpen, London, pp 9–27. https://doi.org/10.5772/intechopen.74390
Ito M (2016) Development of recycling technology of glass and metals from photovoltaic panels by separation with a heated cutter. NPC Incorporated, Japan. https://www.nedo.go.jp/content/100806683.pdf
Komoto K (2016) Approaches to PV waste management in Japan. Presented at Workshop on PV end-of-life management: challenges and opportunities, EU-PVSEC, 21 June 2016. https://www.photovoltaic-conference.com/images/2016/2_Programme/parallel_events/PvProductionQualityInnovation/Keiichi_KOMOTO.pdf
La Mia Energia Scarl (2019) Circular economy, solar panel recycling. Presented at PV in the circular economy (PViCE) workshop, 1 March 2019, Golden, CO
Veolia Group (2018) World premiere in recycling photovoltaic panels. https://www.youtube.com/watch?v=PaUlSZ2biI8. Accessed 1 July 2019
La Mia Energia Scarl (2014) PV-Morede photovoltaic panels mobile recycling device, deliverable D 3.3, vehicle homologation. Agreement number: ECO/12/333078/SI2.658616. http://www.pvmorede.it/public/D%203.2%20VEHICLE%20HOMOLOGATION.PDF
La Mia Energia Scarl PV-Morede photovoltaic panels mobile recycling device, deliverable D 4.1, presentation slide. Agreement number: ECO/12/333078/SI2.658616. http://www.pvmorede.it/public/D%204.1%20PRESENTATION%20SLIDE.pdf
Latunussa C, Ardente F, Blengini GA, Mancini L (2016) Life cycle assessment of an innovative recycling process for crystalline silicon photovoltaic panels. Sol Energy Mater Sol Cells 156(November):101–111. https://doi.org/10.1016/j.solmat.2016.03.020
Ardente F, Latunussa C, Blengini GA (2019) Resource efficient recovery of critical and precious metals from waste silicon PV panel recycling. Waste Manage 91(May):156–167. https://doi.org/10.1016/j.wasman.2019.04.059
Li HY, Luo-Hoffman Y, Ballif C, Perret-Aebi LE (2011) Re-use of c-Si solar cells from failed PV modules. EUPVSEC, January. https://doi.org/10.4229/26thEUPVSEC2011-4AC3.52. https://www.eupvsec-proceedings.com/proceedings?paper=11151
Prado P, Tenorio J, Espinosa D (2017) Alternative method for materials separation from crystalline silicon photovoltaic modules. Energy Technol. https://doi.org/10.1007/978-3-319-52192-3_27
D’Adamo I, Miliacca M, Rosa P (2017) Economic feasibility for recycling of waste crystalline silicon photovoltaic modules. Int J Photoenergy. https://doi.org/10.1155/2017/4184676
Bellmann MP, Roligheten R, Park GS et al (2016) Eco-solar factory: 40% plus eco-efficiency gains in the photovoltaic value chain with minimised resource and energy consumption by closed loop systems. EUPVSEC, pp 1758–1763. https://www.eupvsec-proceedings.com/proceedings?paper=38127
Heath G (2019) PV modules end of life management—towards a recycling R&D roadmap. Presented at PViCE workshop, 1 March 2019, National Renewable Energy Laboratory, Golden, CO
Bellini E (2019) The weekend read: playing by the carbon footprint rules. PV Magazine International. https://www.pv-magazine.com/2019/04/27/the-weekend-read-playing-by-the-carbon-footprint-rules/. Accessed 19 April 2019.
Beetz B (2019) Interview: JinkoSolar on why it has chosen a cradle to cradle path. PV Magazine International. https://www.pv-magazine.com/2019/05/16/interview-jinkosolar-on-why-it-has-chosen-a-cradle-to-cradle-path/?fbclid=IwAR1NB66Zx-GXCUFkTTz-gFm%E2%80%A6. Accessed 24 June 2019
Fraunhofer (2017) Final report: end-of-life pathways for photovoltaic backsheets. Fraunhofer Institute UMSICHT, Sulzbach-Rosenberg, Germany. https://www.coveme.com/files/documenti/news/fraunhofer_final_report_eol_pathways_abstract.pdf
Stapler J, Barnes W, Yelland W (1968) Thermal degradation of polyvinylidene fluoride and polvinyl fluoride by oven pyrolysis. Technical report 69-7-CM. Clothing and Organic Materials Laboratory, U.S. Army Natick Laboratories, Natick, MA. https://apps.dtic.mil/dtic/tr/fulltext/u2/672509.pdf
Madorsky SL, Hart VE, Straus S, Sedlak VA (1953) Thermal degradation of tetrafluoroethylene and hydrofluoroethylene polymers in a vacuum. J Res Natl Bur Stand 51(6):327–333
Lombardo G (2019) Effects of pyrolysis and incineration on the chemical composition of Li-ion batteries and analysis of the by-products. Thesis, Chalmers University of Technology. https://research.chalmers.se/en/publication/508111
Beetz B (2019) Interview: DSM’s circular solar ambitions. PV Magazine International. https://www.pv-magazine.com/2019/05/17/interview-dsms-circular-solar-ambitions/?fbclid=IwAR0752MTtzl02x4_qks261mD1q6E6Cw-cUUTVYIHMG6%E2%80%A6. Accessed 6 June 2019
DSM (2018) Endurance backsheets, an innovative view on backsheet performance. https://www.dsm.com/solutions/dsm-in-solar/en_us/technologies/pv-backsheets.html. Accessed 3 April 2018
DuPont (2014) DuPont teldar polyvinyl fluoride (PVF) films: general properties. https://www.dupont.com/products-and-services/solar-photovoltaic-materials/technical-resources/dupont-tedlar-polyvinyl-fluoride-pvf-films-general-properties.html. Accessed June 2019
Coveme (2015) Coveme photovoltaic backsheet for PV modules. https://www.coveme.com/photovoltaic/. Accessed June 2019
BolidenMineral AB (2013) Socio-economic analysis. https://echa.europa.eu/documents/10162/18584504/afa_sd-0042-01-sea_en.pdf. Accessed 11 Apr 2019
Duflou J, Peeters J, Altamirano D, Bracquene E, Dewulf W (2018) Demanufacturing photovoltaic panels: comparison of end-of-life treatment strategies for improved resource recovery. CIRP Ann 67(1):29–32. https://doi.org/10.1016/j.cirp.2018.04.053
Fiandra V, Sannino L, Andreozzi C, Corcelli F, Graditi G (2019) Silicon photovoltaic modules at end-of-life: removal of polymeric layers and separation of materials. Waste Manage 87(March):97–107. https://doi.org/10.1016/j.wasman.2019.02.004
Geretschlager K, Wallner G, Fischer J (2016) Structure and basic properties of photovoltaic module backsheet films. Sol Energy Mater Sol Cells 144(January):451–456. https://doi.org/10.1016/j.solmat.2015.09.060
Bennet I (2018) Conductive backsheet for manufacture of PV modules with back contact cells. Presented at EU Photovoltaic Solar Energy Conference, 26 September 2018. https://www.eupvsec-planner.com/presentations/c47361/development_of_conductive_back-sheet_for_manufacture_of_pv_modules_with_back-contact_cells.htm
Louwen A, Van Sark W, Schropp R, Faaij A (2016) A cost roadmap for silicon heterojunction solar cells. Sol Energy Mater Sol Cells 147(April):295–314. https://doi.org/10.1016/j.solmat.2015.12.026
Grandell L, Hook M (2015) Assessing rare metal availability challenges for solar energy technologies. Sustainability 7(9):11818–11837. https://doi.org/10.3390/su70911818
Wang X (2017) Cost-effective and reliable copper plated metallisation for silicon solar cells: a development path. Thesis, University of New South Wales. http://unsworks.unsw.edu.au/fapi/datastream/unsworks:46100/SOURCE02?view=true
Hernandez JL, Yoshikawa K, Feltrin A et al (2012) High efficiency silver-free heterojunction silicon solar cell. Jpn J Appl Phys 51(October):10NA04. https://doi.org/10.1143/JJAP.51.10NA04
Reinwarnd D, Pysch D, Bay N, Burschik J, Kuenlein HH, Madon F, Einhaus R, Brand A, Arya V, Smith B, Richter D, Kray D (2018) All copper NICE modules. In: 2018 IEEE 7th world conference on photovoltaic energy conversion (WCPEC) (A joint conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC). https://ieeexplore.ieee.org/document/8547749
Bilczuk D, OlveraO AE (2016) Kinetic study of the dissolution of metallic nickel in sulphuric acid solutions in the presence of different oxidants. Can J Chem Eng 94(June):1872–1879. https://doi.org/10.1002/cjce.22576
Ecometales (2017) The economic implications of impurities and contaminants in semi-finished mining products. http://www.ecometales.cl/index-1.htm?lang=en. Accessed 11 Apr 2019
Steinacker S, Antrekowitch J (2017) The role and influence of impurities on the quality of copper cathodes. In: MultiScience—XXXI. microCAD International Multidisciplinary Scientific Co. https://doi.org/10.26649/musci.2017.023. http://www.uni-miskolc.hu/~microcad/publikaciok/2017/b1/B1_5_Steinacker_Stephan.pdf
Hsiao P-C (2015) Eutectic Sn-Bi alloy for interconnection of silicon solar cells. Thesis, University of New South Wales. http://unsworks.unsw.edu.au/fapi/datastream/unsworks:36365/SOURCE02?view=true
Risopatron C (2018) Impurities in copper raw materials and regulatory advances in 2018: a global overview. Presented at International seminar on impurities in copper raw materials: regulation and social receptivity about impurities in copper raw materials, Tokyo, Japan, October 2018. http://www.jogmec.go.jp/content/300358430.pdf
Dupuis J, Saint-Sernin E, Nichiporuk O et al (2012) NICE module technology—from the concept to mass production: a 10 years review. In: 2012 38th IEEE photovoltaic specialists conference. https://ieeexplore.ieee.org/document/6318254. Accessed June 2019
Madon F, Colin H, Sicot L et al (2015) Results from extended degradation and outdoor tests of NICE modules. http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.711.6142&rank=7. Accessed June 2019
Nichiporuk O, Dupuis J, Saint-Sernin E et al (2012) Secured-intrinsic under-pressure in NICE modules—the oxygen gettering approach. https://doi.org/10.4229/27thEUPVSEC2012-4BV.3.10. http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.704.3311&rank=1. Accessed June 2019
Couderc R, Amara M, Degoulange J, Madon F, Einhaus R (2017) Encapsulant for glass-glass PV modules for minimum optical losses: gas or EVA? Energy Procedia 124(September):470–477. https://doi.org/10.1016/j.egypro.2017.09.283
Einhaus R, Madon F, Degoulange J, et al (2018) Recycling and resuse potential of NICE PV-modules. Presented at World conference on photovoltaic energy conversion, Waikoloa, Hawaii, June 2018. https://ecosolar.eu.com/2018/05/24/recycling-and-reuse-potential-of-nice-pv-modules-presentation-at-wpsec-on-the-11th-of-june-2018/
Fraunhofer ISE (2017) Reliability of TPedge PV modules successfully tested. Fraunhofer ISE, Freiburg, Germany. https://www.ise.fraunhofer.de/en/press-media/press-releases/2017/reliability-of-tpedge-pv-modules-successfully-tested.html
Mittag M, Haedrich I, Neff T et al (2015) TPedge: qualification of a gas-filled encapsulant-free glass-glass photovoltaic module. In: 31st European photovoltaic solar energy conference and exhibition, Hamburg. https://www.researchgate.net/publication/318226202_Tpedge_qualification_of_a_gas-filled_encapsulation-free_glass-glass_photovoltaic_module. Accessed June 2019
Mittag M, Eitner U, Neff T (2017) TPedge: progress on cost-efficient and durable edge-sealed PV modules. Presented at: 33rd EUPVSEC, 25–29 September 2017, Amsterdam. https://www.eupvsec-planner.com/presentations/c40935/tpedge_progress_on_cost-efficient_and_durable_edge-sealed_pv_modules.htm
Goris, MJAA (2014) Recycling friendly design, the Cu-PV project for sustainable photovoltaics. Presented at EUPVSEC, Amsterdam, 23 September 2014. http://iea-pvps.org/fileadmin/dam/public/workshop/11_Maurice_GORIS.pdf
McIntosh K, Cotsell J, Cumpston J, Norris A, Powell N, Ketola B (2009) An optical comparison of silicone and EVA encapsulants for conventional silicon PV modules: a ray-tracing study. In: 34th IEEE Photovoltaic Specialists Conference (PVSC), 2009, pp. 000544–000549. https://ieeexplore.ieee.org/document/5411624
Walwil HM et al (2017) Comparative studies of encapsulation and glass surface modification impacts on PV performance in a desert climate. Sol Energy 142:288–298
Cai C et al (2016) Degradation of thermally-cured silicone encapsulant under terrestrial UV. Sol Energy Mater Sol Cells 157:346–353
Goris M, Rosca V, Geerligs L, Gier BD (2015) Production of recyclable crystalline Si PV modules. EU Photovoltaic Solar Energy Conference. https://www.eupvsec-proceedings.com/proceedings?paper=33753. Accessed June 2019
Gustafsson A (2014) Recycling of CIGS solar cell waste materials. Thesis, Chalmers University of Technology, Gothenburg, Sweden
Amarakoon S, Vallet C, Curran MA, Haldar P, Metacarpa D, Fobare D, Bell J (2018) Life cycle assessment of photovoltaic manufacturing consortium (PVMC) copper indium gallium (di)selenide (CIGS) modules. Int J Life Cycle Assess 23(4):851–866. https://doi.org/10.1007/s11367-017-1345-4
Andreas B, Petrus M, Huber N, Bristow H, Hu Y, Bein T, Docampo P (2016) Recycling perovskite solar cells to avoid lead waste. ACS Appl Mater Interfaces 8:12881–12886. https://doi.org/10.1021/acsami.6b03767
Kadro J, Pellet N, Giodano F, Ulianov A, Muntener O, Maier J, Gratzel M, Hagfeldt A (2016) Proof-of-concept for facile perovskite solar cell recycling. Energy Environ Sci 9:3172–3179. https://doi.org/10.1039/c6ee02013e
Cheacharoen R, Boyd C, Burkhard G et al (2018) Encapsulating perovskite solar cells to withstand damp heat and thermal cycling. Sustain Energy Fuels 11:2398–2406. https://doi.org/10.1039/c8se00250a
Korkmaz K, Alemrajabi M, Rasmuson A, Forsber K (2018) Sustainable hydrometallurgical recovery of valuable elements from spent nickel-metal hydride HEV batteries. Metals 8(12):1062–1079. https://doi.org/10.3390/met8121062
Li L, Zhang X, Li M, Chen R, Wu F, Amine K, Lu J (2018) The recycling of spent lithium-ion batteries: a review of current processes and technologies. Electrochem Energy Rev 1(4):461–482. https://doi.org/10.1007/s41918-018-0012-1
Leisegang T, Treffer F (2016) Recycling of electrochemical storage devices. AIP Conf Proc 1765:02006. https://doi.org/10.1063/1.4961898
Dorella G, Borges Mansur M (2007) A study of the separation of cobalt from spent Li-ion battery residues. J Power Sources 170(1):210–215. https://doi.org/10.1016/j.jpowsour.2007.04.025
Park JH, Park JJ, Lee HJ, Min BS, Yang JH (2018) Influence of metal impurities or additives in the electrolyte of a vanadium redox flow battery. J Electrochem Soc 165:A1263-1268
Weber S, Peters J, Baumann M, Weil M (2018) Life cycle assessment of a vanadium redox flow battery. Environ Sci Technol. https://doi.org/10.1021/acs.est.8b02073
U.S. Advanced Battery Consortium (2013) 2014 recommended practice for recycling of xEV electrochemical energy storage systems. https://www.uscar.org. Accessed June 2019
Schutte R (2015) HEV and EV batteries—design for recycle. Retrieved Technologies
Peiro LT, Ardente F, Mathieux F (2017) Design for disassembly criteria in EU product policies for a more circular economy: a method for analyzing battery packs in PC-Tablets and subnotebooks. J Ind Ecol 21:731–741. https://doi.org/10.1111/jiec.12608
Settimo F, Bevilacqua P, Rem P (2004) Eddy current separation of fine non-ferrous particles from bulk streams. Phys Sep Sci Eng 13(1):15–23. https://doi.org/10.1080/00207390410001710726
Halliwell S (2006) National composites network best practice guide – repair of fiber reinforced polymer (FRP) structures. TWI Ltd. https://compositesuk.co.uk. Accessed June 2019
Teng H (2012) Overview of the development of the fluoropolymer industry. Appl Sci 2(2):496–512. https://doi.org/10.3390/app2020496
Psomopoulos C, Kalkanis K, Kaminaris S (2019) A review of the potential for the recovery of wind turbine blade waste materials. Recycling. https://doi.org/10.3390/recycling4010007
Chatziaras N, Psomopoulos C, Themelis N (2016) Use of waste derived fuels in cement industry: a review. Manage Environ Qual Int J 27:178–193. https://doi.org/10.1108/MEQ-01-2015-0012
Brandhorst H, Kaldunski B (2019) Environmental aspects of renewables wind turbine blade end-of-life disposal options, current & future research. Presented at Electric Power Research Institute, 4 February 2019
Mining Review Africa (2019) Innovative vanadium electrolyte rental product for industrial use. https://www.miningreview.com/energy/innovative-vanadium-electrolyte-rental-product-for-industrial-use/. Accessed 11 June 2019
Spector J (2019) A new path to market for flow batteries: rent an electrolyte. Green Tech Media. https://www.greentechmedia.com/articles/read/new-path-to-market-for-flow-batteries-rent-an-electrolyte#gs.hs5jkf. Accessed 11 June 2019
Acknowledgements
The authors wish to thank reviewers from the research, R&D management, and industrial communities, including K. Podkaminer, J. Cresko, J. Engel-Cox, M. Kempe, R. Murray, D. Berry, A. Eberle, A. Burrell, M. Mann, S. Santhanagopalan, A. Wade, P. Sinha, F. Dross, I. Goudswaard, L. Mansfield, J. Bilbao, M. Lunardi, R. Corkish, S. Jaako, M. O’Connor, S.M. Vettese, L. Gaines, S. Gillard, L. Tinker, J. Spangenberger, B. Polzin, K. McMahon, J. Cory, F. Della Rosa, K. Wambach, and M. Arbabzadeh for feedback on the earlier versions of the DfR principles presented herein, and A. Hicks and J. Zuboy for graphics and editorial support. This work was authored by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding was provided by U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Office of Strategic Programs, Advanced Manufacturing Office and Solar Energy Technologies Office. The work was conducted under the Clean Energy Manufacturing Analysis Center (CEMAC) operated by the Joint Institute for Sustainable Energy Analysis (JISEA). The views expressed in the article do not necessarily represent the views of the DOE or the U.S. Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Markus Reuter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Norgren, A., Carpenter, A. & Heath, G. Design for Recycling Principles Applicable to Selected Clean Energy Technologies: Crystalline-Silicon Photovoltaic Modules, Electric Vehicle Batteries, and Wind Turbine Blades. J. Sustain. Metall. 6, 761–774 (2020). https://doi.org/10.1007/s40831-020-00313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00313-3