Abstract
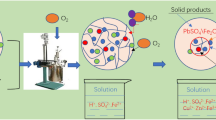
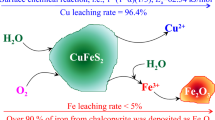
The leaching of copper from chalcopyrite in H2SO4 solution under pressure-oxidative conditions and its kinetics were investigated in this study. Leaching variables that affect the rate of copper dissolution from chalcopyrite are agitation speed (300–900 rpm), total pressure (0.8–2.0 MPa), temperature (160–180 °C), and sulfuric acid concentration (0.1–2.0 M). Results showed that dissolution of chalcopyrite increases with increasing agitation speed, total pressure, and temperature, whereas it decreases with the increasing sulfuric acid concentration. Under the optimal conditions, copper extraction of 94.5% was achieved after 90-min leaching, while a dissolution of iron at 4.2% was obtained. The kinetic study showed that the dissolution of chalcopyrite is represented by a shrinking core model with chemical reaction controlling mechanism given as (1 − (1 − α)1/3). The activation energy (E a) for the leaching reaction was calculated to be 42.4 kJ/mol. The reaction order with respect to total pressure was about 8.0, which indicates that total pressure, i.e., oxygen partial pressure, in an autoclave is the most important factor in controlling the dissolution of chalcopyrite in H2SO4 solution under pressure-oxidative leaching conditions. The effect of Fe/Cu mole ratio (1–20 mol/mol, adjusted by addition of pyrite) on chalcopyrite leaching from a copper ore was investigated. The results show that the sulfuric acid produced during pyrite oxidation promotes the chalcopyrite dissolution.
















Similar content being viewed by others
References
Padilla R, Vega D, Ruiz MC (2007) Pressure leaching of sulfidized chalcopyrite in sulfuric acid-oxygen media. Hydrometallurgy 86:80–88. doi:10.1016/j.hydromet.2006.10.006
Baba AA, Ghosh MK, Pradhan SR, Rao DS, Baral A, Adekola FA (2014) Characterization and kinetic study on ammonia leaching of complex copper ore. Trans Nonferrous Met Soc China 24:1587–1595. doi:10.1016/S1003-6326(14)63229-5
Antonijević MM, Janković ZD, Dimitrijević MD (2004) Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid. Hydrometallurgy 71:329–334. doi:10.1016/S0304-386X(03)00082-3
Harmer SL, Thomas JE, Fornasiero D, Gerson AR (2006) The evolution of surface layers formed during chalcopyrite leaching. Geochim Cosmochim Acta 70:4392–4402. doi:10.1016/j.gca.2006.06.1555
Sokić MD, Marković B, Živković D (2009) Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 95:273–279. doi:10.1016/j.hydromet.2008.06.012
Li Y, Kawashima N, Li J, Chandra AP, Gerson AR (2013) A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv Coll Interface Sci 197:1–32. doi:10.1016/j.cis.2013.03.004
Hua Y, Cai C, Cui Y (2006) Microwave-enhanced roasting of copper sulfide concentrate in the presence of CaCO3. Sep Purif Technol 50:22–29. doi:10.1016/j.seppur.2005.11.003
Dutrizac JE (1978) The kinetics of dissolution of chalcopyrite in ferric ion media. Metall Trans B 9:431–439. doi:10.1007/BF02654418
Dutrizac JE (1989) Elemental sulphur formation during the ferric sulphate leaching of chalcopyrite. Can Metall Q 28(4):337–344. doi:10.1179/cmq.1989.28.4.337
Lu ZY, Jeffrey MI, Lawson F (2000) The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 56:189–202. doi:10.1016/S0304-386X(00)00075-X
Marsden JO, Wilmot JC (2007) Medium-temperature pressure leaching of copper concentrates-Part II: Development of direct electrowinning and an acid-autogenous process. Miner Metall Process 24:205–217
Al-Harahsheh M, Kingman S, Al-Harahsheh A (2008) Ferric chloride leaching of chalcopyrite: synergetic effect of CuCl2. Hydrometallurgy 91:89–97. doi:10.1016/j.hydromet.2007.11.011
Olubambi PA, Potgieter JH (2009) Investigations on the mechanisms of sulfuric acid leaching of chalcopyrite in the presence of hydrogen peroxide. Miner Process Extr Metall Rev 30:327–345. doi:10.1080/08827500902958191
Dreisinger D (2006) Copper leaching from primary sulfides: Options for biological and chemical extraction of copper. Hydrometallurgy 83(1):10–20. doi:10.1016/j.hydromet.2006.03.032
Puvvada GVK, Murthy DSR (2000) Selective precious metals leaching from a chalcopyrite concentrate using chloride/hypochlorite media. Hydrometallurgy 58(3):185–191. doi:10.1016/S0304-386X(00)00083-9
Padilla R, Pavez P, Ruiz MC (2008) Kinetics of copper dissolution from sulfidized chalcopyrite at high pressures in H2SO4-O2. Hydrometallurgy 91:113–120. doi:10.1016/j.hydromet.2007.12.003
Misra M, Fuerstenau MC (2005) Chalcopyrite leaching at moderate temperature and ambient pressure in the presence of nanosize silica. Miner Eng 18:293–297. doi:10.1016/j.mineng.2004.06.014
Turan MD, Arslanoğlu H, Altundoğan HS (2015) Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system. J Taiwan Inst Chem Eng 50:49–55. doi:10.1016/j.jtice.2014.12.009
Akcil A, Ciftci H (2003) Metals recovery from multimetal sulphide concentrates (CuFeS2-PbS-ZnS): combination of thermal process and pressure leaching. Int J Miner Process 71:233–246. doi:10.1016/S0301-7516(03)00061-9
Al-Harahsheh M, Kingman S, Hankins N, Somerfield C, Bradshaw S, Louw W (2005) The influence of microwaves on the leaching kinetics of chalcopyrite. Miner Eng 18:1259–1268. doi:10.1016/j.mineng.2005.06.006
Antonijević MM, Janković Z, Dimitrijević M (1994) Investigation of the kinetics of chalcopyrite oxidation by potassium dichromate. Hydrometallurgy 35:187–201. doi:10.1016/0304-386X(94)90051-5
Arslan F, Bulut G, Kangal MO, Perek KT, Gül A, Gürmen S (2004) Studies on leaching of massive rich copper ore in acidic ferric sulfate solutions. Scand J Metall 33:6–14. doi:10.1111/j.1600-0692.2004.00662.x
Aydogan S, Ucar G, Canbazoglu M (2006) Dissolution kinetics of chalcopyrite in acidic potassium dichromate solution. Hydrometallurgy 81:45–51. doi:10.1016/j.hydromet.2005.10.003
Dreisinger D (2006) Copper leaching from primary sulfides: options for biological and chemical extraction of copper. Hydrometallurgy 83:10–20. doi:10.1016/j.hydromet.2006.03.032
Dutrizac JE (1981) The dissolution of chalcopyrite in ferric sulfate and ferric chloride media. Metall Trans B 12:371–378. doi:10.1007/BF02654471
Dutrizac JE (1990) Elemental sulphur formation during the ferric chloride leaching of chalcopyrite. Hydrometallurgy 23:153–176. doi:10.1016/0304-386X(90)90002-J
Dutrizac JE (1991) Ferric ion leaching of chalcopyrites from different localities. J Electron Mater 20:303–309. doi:10.1007/BF02816001
Hackl RP, Dreisinger DB, Peters E, King JA (1995) Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy 39:25–48. doi:10.1016/0304-386X(95)00023-A
Havlík T, Škrobian M, Baláž P, Kammel R (1995) Leaching of chalcopyrite concentrate with ferric chloride. Int J Miner Process 43:61–72. doi:10.1016/0301-7516(94)00040-7
Mahajan V, Misra M, Zhong K, Fuerstenau MC (2007) Enhanced leaching of copper from chalcopyrite in hydrogen peroxide-glycol system. Miner Eng 20:670–674. doi:10.1016/j.mineng.2006.12.016
Shinichi HEGURI, Satoshi ASANO, Atsushi IDEGAMI (2015) Behavior of Iron and Sulfur in the Pressure Leaching of Chalcopyrite. J MMIJ 131:470–475. doi:10.2473/journalofmmij.131.470
McDonald RG, Muir DM (2007) Pressure oxidation leaching of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 86:191–205. doi:10.1016/j.hydromet.2006.11.015
Prasad S, Pandey BD (1998) Alternative processes for treatment of chalcopyrite-a review. Miner Eng 11:763–781. doi:10.1016/S0892-6875(98)00061-2
Saxena NN, Mandre NR (1992) Mixed control kinetics of copper dissolution for copper ore using ferric chloride. Hydrometallurgy 28:111–117. doi:10.1016/0304-386X(92)90068-B
Tchoumou M, Roynette M (2007) Leaching of complex sulphide concentrate in acidic cupric chloride solutions. Trans Nonferrous Met Soc China 17:423–428. doi:10.1016/S1003-6326(07)60109-5
Rao KS, Ray HS (1998) A new look at characterisation and oxidative ammonia leaching behaviour of multimetal sulphides. Miner Eng 11:1011–1024. doi:10.1016/S0892-6875(98)00089-2
Brewer RE (2004) Copper concentrate pressure leaching-plant scale-up from continuous laboratory testing. Miner Metall Process 21:202–208
Wodka J, Chmielewski T, Ziołkowski B (2007) Pressure leaching of shale ore in oxygenated sulphuric acid. Physicochem Prob Miner Process 41:349–364
Tshilombo KG, Mulaba-Bafubiandi AF (2013) Ammonia/nitric acid leaching of copper-cobalt oxidized ore. In: International conference on mining, mineral processing and metallurgical engineering (ICMMME 2013) 15–16 April 2013, Johannesburg, South Africa
Huang K, Li QW, Chen J (2007) Recovery of copper, nickel and cobalt from acidic pressure leaching solutions of low-grade sulfide flotation concentrates. Miner Eng 20:722–728. doi:10.1016/j.mineng.2007.01.011
Tamagawa T, Tabaian SH, Fu NX, Kobayashi M, Iwasaki I (2000) Extraction of copper from chalcopyrite concentrates without sulfuric acid generation via chlorination-Part 1: gaseous chlorination of sulfide concentrates. Miner Metall Process 17:259–263
Park KH, Mohapatra D, Reddy BR, Nam CW (2007) A study on the oxidative ammonia/ammonium sulphate leaching of a complex (Cu–Ni–Co–Fe) matte. Hydrometallurgy 86:164–171. doi:10.1016/j.hydromet.2006.11.012
Qiu TS, Nie GH, Wang JF, Cui LF (2007) Kinetic process of oxidative leaching of chalcopyrite under low oxygen pressure and low temperature. Trans Nonferrous Met Soc China 17:418–422. doi:10.1016/S1003-6326(07)60108-3
Gok O, Anderson CG, Cicekli G, Cocen EL (2014) Leaching kinetics of copper from chalcopyrite concentrate in nitrous-sulfuric acid. Physicochem Probl Miner Process 50:399–413. doi:10.5277/ppmp140133
Yue G (2015) Speciation of the sulfuric acid-ferric sulfate-ferrous sulfate-water system and its application to chalcopyrite leaching kinetics up to 150°C. Ph.D. thesis, University of British Columbia
Guan YC, Han KN (1997) The leaching kinetics of chalcopyrite (CuFeS2) in ammonium lodide solutions with iodine. Metall Mater Trans B 28(6):979–985. doi:10.1007/s11663-997-0051-1
Rath PC, Paramguru RK, Jena PK (1988) Kinetics of dissolution of sulphide minerals in ferric chloride solution: 1. Dissolution of galena, sphalerite and chalcopyrite. Trans Inst Min Metall C 97:150–158
Murr LE, Hiskey JB (1981) Kinetic effect of particle size and crystal dislocation density on the dichromate leaching of chalcopyrite. Metall Trans B 12:255–267. doi:10.1007/BF02654458
Aydoğan S, Erdemoğlu M, Uçar G, Aras A (2007) Kinetics of galena dissolution in nitric acid solutions with hydrogen peroxide. Hydrometallurgy 88(1):52–57. doi:10.1016/j.hydromet.2007.03.005
Berry VK, Murr LE, Hiskey JB (1978) Galvanic interaction between chalcopyrite and pyrite during bacterial leaching of low-grade waste. Hydrometallurgy 3:309–326. doi:10.1016/0304-386X(78)90036-1
Kaskiala T (2002) Determination of oxygen solubility in aqueous sulphuric acid media. Miner Eng 15:853–857. doi:10.1016/S0892-6875(02)00089-4
Yu PH, Hansen CK, Wadsworth ME (1973) A kinetic study of the leaching of chalcopyrite at elevated temperatures. Metall Trans 4:2137–2144. doi:10.1007/BF02643279
Jackson E (1986) Hydrometallurgical extraction and reclamation. Ellis Horwood Ltd, Chichester
Acknowledgements
This research was supported by the Leading Graduates Schools Program, the “New Frontier Leader Program for Rare Metals and Resources” of Japan Society for the Promotion of Science (JSPS). We gratefully acknowledge their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Hongmin Zhu.
Rights and permissions
About this article
Cite this article
Han, B., Altansukh, B., Haga, K. et al. Leaching and Kinetic Study on Pressure Oxidation of Chalcopyrite in H2SO4 Solution and the Effect of Pyrite on Chalcopyrite Leaching. J. Sustain. Metall. 3, 528–542 (2017). https://doi.org/10.1007/s40831-017-0135-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-017-0135-3