Abstract
Pressure-sensitive adhesives, especially acrylate-based pressure-sensitive adhesives, are the main group of polymers used to produce a wide range of pressure-sensitive adhesives. Self-adhesive adhesives based on well-known natural resins and polymers have been known for a long time. The development of synthetic adhesives, especially those based on acrylate, began after World War II. BASF has made great contributions in this field. Therefore, because polyacrylates are relatively cheap, they are resistant to aging and the damaging effects of oxygen and UV radiation. Polyacrylate-based adhesives are known as solvent-based, water-dispersion, and solvent-free adhesives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since their introduction half a century ago, pressure-sensitive acrylic adhesives have been successfully applied in many fields. They are used in self-adhesive tapes, labels, and protective films as well as in dermal dosage systems for pharmaceutical applications; in biomedical electrodes; and the assembly of automotive parts, toys, electronic circuits, and keyboards. In the last 50 years or so, pressure-sensitive adhesive acrylics have made tremendous strides, from what was virtually a black art to what is now a sophisticated science; so much so that both the few large manufacturers of pressure-sensitive adhesive articles and their even larger suppliers now use very expensive equipment to study pressure-sensitive adhesive behavior: tack, adhesion, and cohesion.
Three properties that are useful in characterizing the nature of pressure-sensitive adhesives are tack, peel adhesion (adhesion), and shear strength (cohesion). The first measures the ability of the adhesive to adhere quickly, the second its ability to resist removal by peeling, and the third its ability to hold in position when shearing forces are exerted. Generally speaking, the first two are directly related to each other but are inversely related to the third.
The performance of pressure-sensitive adhesives, such as tack, peel, and shear, based on polyacrylates synthesized through copolymerization of acrylate monomers and formulated in organic solvent mixtures are, to a large degree, determined by the molecular weight of acrylic copolymer, polymerization method, and especially by the type and quantity of the crosslinking agents added to the polymerizate.
Although the production of solvent-borne acrylic pressure-sensitive adhesives in Europe is characterized by constantly increasing productivity, and in the year 2020 it had indeed grown to about 240,000 tons, up to now, a complex publication concerning synthesis, crosslinking, and technology of acrylic PSAs has not been published [1]. The review of scientific publications on this topic has shown that only negligible publications concern the modification possibilities of acrylic PSA properties. Similar information regarding the dependence of the main properties of acrylic PSAs on the type and amount of crosslinking agents used or crosslinking methods, are also fragmentary and dissipated and only negligible publications undertake an optimization of the problem of PSA application properties and the crosslinking agents and methods used.
A target of this work was the presentation of acrylic pressure-sensitive adhesives, especially solvent-borne acrylics with high application performances, through a selection among the most efficient crosslinking agents and the best crosslinking methods. This work brought to development a technology of a wide palette manufacturing of acrylic PSAs and self-adhesive products on an industrial scale.
The actual background of the knowledge and technique refers to available references and patents concerning solvent-borne acrylic pressure-sensitive adhesives and their crosslinking, using diverse crosslinkers and crosslinking methods presented in the introduction. The second part of this monograph describes the investigation conducted by the author and it applies to the influence of crosslinking agents on the tack, peel, and shear of crosslinked pressure-sensitive acrylic adhesive investigated by using multifunctional monomers during the polymerization process; by the use of room temperature reactive crosslinkers such as alkyl titanates, metal chelates, multifunctional isocyanates, polycarbodiimides, and propylene imines; and by the insertion of high-temperature reactive crosslinkers such as amino resins and monomers with crosslinking properties.
My further research concerns the influence of the UV-crosslinking of pressure-sensitive adhesive acrylics on tack, peel, and shear by using UV-radiation from UV-lamps or UV-lasers for a wide range of conventional and unsaturated copolymerizable photoinitiators. These unsaturated photointiators include also new products, synthesized and patented by the author.
The majority of the development investigations in this scientific publication were conducted during 20 years research and development at Lohmann Company (Germany) [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29], Technical University of Szczecin (Poland), and West Pomeranian University of Technology Szczecin (Poland).
The history of pressure-sensitive adhesives and adhesive tapes
Pressure-sensitive adhesives (PSAs) are nonmetallic materials used to bond other materials, mainly on their surfaces through adhesion and cohesion. Adhesion and cohesion are phenomena that may be described thermodynamically, chemically, and mechanically. It was shown that the most important bonding processes are bonding by adhesion and bonding with pressure-sensitive adhesives (PSAs).
In the long history of this technology, pressure-sensitive adhesives and tapes as we know them are a fairly recent concept. However, to trace their origins, one needs to study the history of adhesives as a whole, including the many failures and near misses along the way, as well as the fusion of various technologies, which eventually led to their development.
Since the dawn of history, people learned of the healing powers of certain leaves and plants. There is archaeological evidence indicating that adhesives have indeed been found on primitive tools. More than 6000 years ago, with the arrival of the Egyptian civilization, the art of healing was already a profession. A primitive tape concept utilized by Egyptians was the use of a paste of starch in water applied to cloth strips. This indicates that surgical bandages, made of a mixture of fat and honey, were in use. There is very little known of the other raw materials used in Egyptian/Greek times for surgical dressings. It is known, though, that resins, pitches, and so on, were in common use in other trades and professions, for instance, in the ship-building industry, and such resins would no doubt work well as tackifying resins in pressure-sensitive adhesive systems.
Pressure-sensitive adhesives were in wide use since the late nineteenth century, starting with medical tapes and dressings. The earliest being produced in 1845. This was for a surgical pressure-sensitive adhesive that used natural rubber as the base, pine gum as the tackifier, and with balsam of Peru, turpentine, and spirits of turpentine also being added. Ninety years later, the American inventor and entrepreneur Richard Stanton Avery (1907–1997) developed and introduced the self-adhesive label. Two major industries resulted from these innovations: pressure-sensitive tapes and labels (Fig. 1).
In the late 1800s and early 1900s, the development first of the bicycle and then the automobile and their need for tires, allowed the rubber industry to flourish. Greater demands were placed on the industry to develop improved rubber-based products, and this improved technology naturally filtered into the existing adhesive tape industry. Industrial tapes were introduced in the 1920s and 1930s followed by self-adhesive labels in 1935. While various materials in roll form were available early in the twentieth century that could have been used as adhesive tape backings, cotton cloth remained the backing of choice, with manufacturing geared to producing surgical tape.
Minnesota Mining and Manufacturing Company, popularly known as 3M, was the supplier of sandpaper to the automobile industry in the 1920s, their brand being known as “Wetordry.” Richard Gurley Drew (1899–1980), then a laboratory technician for 3M would occasionally call at the visited automobile plants and body repair shops to take developmental samples of sandpaper for testing. There followed a whole series of patents by 3M on pressure-sensitive adhesive tapes, which laid the cornerstone of the industrial adhesive tape industry. The patents were granted in 1933 for the transfer of his masking tape know how to cellophane film, making the first pressure-sensitive film tape, giving the world the generic name of “Scotch” tape. Take this tape back to those Scotch bosses of yours and tell them to put more adhesive on it.
The major raw materials for pressure-sensitive adhesives in the mid-thirties were natural rubber, either as pale crepe, smoked sheet rubber, or wild rubber, with reclaimed rubber for primer formulations; coumarone gum resin, burgundy pitch, pine oil, wood resin, and gum olibanum as tackifiers; liquid paraffin or mineral oil, lanolin, and beeswax as softeners; zinc oxide as filler, with whiting as filler for prime coats; and benzene or low-boiling-point aliphatic petroleum hydrocarbons as solvent. There was little else available.
The elastomers in common use were polyisobutylene, Oppanol B, polyvinyl isobutyl ether Oppanol C, and some styrene butadiene, or Buna S. It is significant to note that as early as in 1941, a poly(acrylic ester), known as Acronal 4, from I.G. Farben, was being used as a one component pressure-sensitive adhesive, the first example of an acrylic pressure-sensitive adhesive system. Oppanol B and C grades consist primarily of polyisobutene, therefore please amend accordingly.
In the 1940s, hot-melt adhesives were introduced in USA. The post-war times brought with them exploration; a lot of initial investigations began with balloons being sent into the stratosphere. It was soon learned that adhesives would be needed that were capable of functioning at extremely low temperatures. A research contract was to develop such an adhesive, and from it came Dow Corning’s silicone pressure-sensitive adhesive, which could perform in the range from –62 to +260 °C, the forerunner of other low/high temperature silicone pressure-sensitive adhesive systems.
However, the 1950s brought with them an acceleration of research to convert the practice from art to science, and the mystery of tack and adhesion was explored in depth. Also, industrial adhesive tape companies began to communicate with one another for the common good of the industry. Acrylic pressure-sensitive systems, although still more than twice the cost of rubber-based systems, were not viable. For most companies, this meant buying a commercial pressure-sensitive adhesive produced by someone other than themselves, so they lost the ability to manipulate by formula adjustment [30,31,32].
With the arrival of the 1970s, a very large proportion of the raw materials used by the adhesive tape industry were petroleum derived. The 1980s continued to bring raw material upgrades and new products, particularly in the area of alternate hot-melt elastomers, but little in the way of changes in pressure-sensitive adhesive technology. Product and process development in the industry continued to improve, as demonstrated by the number of related patents being granted worldwide each week. Ongoing environmental concerns now forced the industry to look for coating techniques other than solvent-based systems, with calendaring the original technique still holding its own as a 100% solids system capable of applying a thick layer of adhesive at reasonably high speeds. Work has continued to develop effective cross-linked hot melt adhesive systems to replace those based on natural rubber, and water-based adhesive systems are now becoming more practical, with a greater choice of raw materials and improving economics. However, the number and uses of pressure-sensitive adhesive and tape products continued to increase as the performance of the pressure-sensitive adhesive system has improved and the user continued to be educated about their potential.
At the end of the 1980s and during the early 1990s, 3M, Beiersdorf, BASF, and Lohmann presented the first solvent-free pressure-sensitive adhesive acrylics cross-linked with UV-radiation. Six years later, 3M presented a new adhesive tape with pressure-sensitive thermosetting adhesives, the semi-structural adhesive tape.
The term PSA has a very precise technical definition and was dealt with extensively in the chemical literature. The function of PSAs is to ensure instantaneous adhesion upon application of a light pressure. Most applications further require that they can be easily removed from the surface to which they were applied, through a light pulling force. Thus, PSAs are characterized by a built-in capacity to achieve this instantaneous adhesion to a surface without activation, such as a treatment with solvents or heat, and also by having sufficient internal strength so that the adhesive material will not break up before the bond between the adhesive material and the surface ruptures. The bonding and the debonding of PSAs are energy-driven phenomena. Pressure-sensitive adhesives must possess viscous properties to flow and to be able to dissipate energy during the adhesive bonding process.
Polymers employed as PSAs have to fulfill partially contradictory requirements; they need to adhere to substrates, to display high shear strength and peel adhesion, and not leave any residue on the substrate upon debonding. To meet all these requirements, a compromise is needed. When using PSAs, there appears another difference with wet adhesives, namely the adhesive does not change its physical state because film forming is inherent to PSAs.
Thus, PSAs used in self-adhesive tapes are adhesives, which, through their viscoelastic fluid state, can build up the joint without the need to change this flow state during or after application. On the other hand, their fluid state allows controlled debonding, giving a temporary character to the bond. Because of the fluid character of the bonded adhesive, the amount of adhesive (i.e., the dimensions of the adhesive layer) is limited; the joint works as a thin-layer tape, laminate, or composite. The solid-state components of the tape exert a strong influence on the properties of the adhesive in the composite. Therefore, there exists a difference between the measured properties of the pristine adhesive and of the adhesive enclosed within the laminate.
The properties, which are essential in characterizing the nature of PSAs, comprise tack, peel adhesion, and shear. The first measures the ability of the adhesive to adhere quickly, the second its ability to resist removal through peeling, and the third its ability to hold in position when shear forces are applied.
Solvent-borne pressure-sensitive acrylic adhesives
The difference between pressure-sensitive adhesives and other adhesives, such as contact adhesives, is in the permanent surface stickiness of the pressure-sensitive adhesives before, or after, the application.
In the giant field of adhesives, the pressure-sensitive adhesives make up but a low percentage, and the solvent-borne pressure-sensitive acrylic adhesives with their 150,000–210,000 tons per annum [33] in Europe are almost a quantity negligible within this group. Shortly after World War II, copolymers of the higher alkyl acrylates, i.e., the acrylics, were introduced into the PSA market. Other types of PSA in use nowadays include silicones, vinyl acetate copolymers, poly(vinyl alkyl ether)s, and ethylene–vinyl acetate copolymers, but volumes of these are in many cases relatively small compared with the acrylic polymers.
Pressure-sensitive adhesive acrylics can be applied as a solvent-borne, water-borne (dispersions), or a solvent-free system. Although the solvent-borne pressure-sensitive acrylic adhesives may be dwarfs in terms of quantity, they are giants when considered from the sales point of view. Only by means of the performance of these acrylic specialties was it possible to succeed in drafting the present surprisingly efficient generation of double-sided pressure-sensitive adhesive tapes for prominent assembly projects at justifiable cost. Other important applications are for medical products, protective masking films, films for graphics market, and various speciality products.
Pressure-sensitive adhesive acrylic solutions are nowadays predominantly manufactured by polymerization from a wide selection of acrylic and methacrylic groups, often with low levels of monomers having pendant functional groups in a refluxing organic solvent in the present of an initiator, such as organic peroxides or azo compounds.
The most important requirements for a pressure-sensitive adhesive, such as high tackiness (adhesion by the touch); high cohesion (inner stability); high stickiness (adhesion); and UV, solvent, and temperature stability are fulfilled by polyacrylates in an outstanding way.
Solvent-borne PSA acrylics offer several advantages such as excellent aging characteristics and resistance to elevated temperatures and plasticizers, exceptional optical clarity due to the polymer compatibility, and nonyellowing. They also have the highest balance of adhesion and cohesion and excellent water resistance. Acrylics are harder than rubbers. This can be seen in a less aggressive tack and slower build-up of peel strength. Lower adhesion to nonpolar polyolefins is caused by the polar chemistry of acrylics.
The numerous advantages of solvent-based PSA polyacrylics have led to their wide use in the manufacture of self-adhesive products (Fig. 2) [34]. Solvent-based acrylic pressure-sensitive adhesives represent more than 45% of the total PSAs produced.
Adhesives chemists can attain the required properties of acrylic adhesive by means of a wide range of possibilities; be it by exerting a direct influence on the composition of the acrylic polymer or, later on, by the kind of crosslinking or by varying additives. A crosslinker is usually added for improved cohesion.
Acrylate polymer chemistry is expanding through the introduction and utilization of new raw materials, polymerization techniques, novel crosslinking agents, and new crosslinking methods.
The concept of solvent-borne acrylic PSAs design
The versatility of acrylate chemistry is inherently useful in the design of high-performance pressure-sensitive adhesives. A broad raw material base and a versatility of polymerization processes lend themselves to the design of base polymers with unique properties.
The glass transition temperature (Tg) is the main issue for adhesion properties of various polymers, allowing the selection of raw materials for PSA applications. It is specific for homopolymers, but also reveals important information about the suitability of the homopolymers as pressure-sensitive adhesive, which is synthesized from various components. Its value defines the tack of PSAs; a low Tg is a prerequisite for tacky materials. On the other hand, the Tg alone does not permit to obtain a real image of the adhesive performance. For permanent adherent pressure-sensitive adhesives, Tg ranges from about –70 to –25 °C.
The design parameters utilized to produce high-performance solvent-borne adhesives include [35]:
-
Monomer selection
-
Selection of solvents
-
Initiators
-
Molecular weight
-
Polymerization methods
-
Crosslinking
Specific design parameters are used to achieve the desired surface properties of tack and peel in combination with the bulk property of cohesive strength. The balance of these properties is needed for high-performance products [36].
Monomer selection
For the manufacture of acrylic pressure-sensitive adhesives, primarily tackifying common acrylic acid esters are preferred with C4—C12 carbon atoms in the alkyl moiety together with other comonomers. The composition of acrylate polymers that are inherently pressure sensitive is a combination of soft (low Tg), hard (high Tg), and functional monomers. The most important monomers of this class are compiled in Table 1:
Table 1 contains typical hard and soft monomers, as well as the types of functionalities that can be incorporated into the polymer. The tack and the peel properties are imparted by the soft or low glass transition temperature monomers such as 2-ethylhexyl acrylate, isooctyl acrylate, or n-butyl acrylate. The harder monomers, such as methyl acrylate or isobornyl acrylate are included to provide internal strength. The functional groups containing monomers such as acrylic acid or 2-hydroxyethyl acrylate are incorporated into the balanced monomers for specific adhesion to desired substrates and to provide sites in form of active crosslinking centers for crosslinking.
Selection of solvents
The media applied for radical polymerization of acrylates are organic solvents or mixtures whose molecules buffer the heavily exothermic polymerization reaction. The selection of suitable solvents is distinctly limited due to following requirements.
The solvent has to be [37]:
-
Inexpensive
-
Reclaimable
-
Absolutely inert
-
Proper boiling point between 50 and 120 °C
-
A relatively low transfer constant
-
A good solvency for acrylics
The last-mentioned points on the check list for solvents influence the molecular weight, being crucial for the entire properties of pressure-sensitive adhesives, which should be as high as possible. The higher the boiling point of the solvent the more the properties decrease. Per se, very low molecular weights bring about solvents with a high transfer constant, such as petrol with special boiling point. This deficiency can be compensated to a great extent by a versed conduction of the polymerization process. Such endeavors make sense since aliphatic mixtures particularly meet the first three items of the above catalogue of requirements best.
In the pressure-sensitive acrylic adhesives available on the market, only a small group of solvents may be found, which is not surprising:
-
Ethyl acetate
-
Special boiling point petrol (BP = 60–95 °C)
-
N-hexane
-
Toluene
-
acetone
Ethyl acetate or solvent mixtures on ethyl acetate basis, only permit the formulation of pressure-sensitive acrylic adhesives with an outstanding performance level.
Initiators
The acrylic monomers are commonly polymerized utilizing free radical polymerization processes involving the unsaturated double bond contained in the acrylic monomers. The free radical polymerization process starts with the initiation step. This involves the introduction of free radicals into the reaction medium and subsequent reaction with the monomer unit. This is accomplished with initiator compounds that break down to yield reactive free radical species that will add to the acrylic double bond. The breakdown of the initiator can be accomplished via several routes. These include thermal breakdown, redox reactions, and with radiation such as UV radiation and Electron Beam (EB). Beyond starting the process, the initiators can influence the polymer structure. For instance, peroxides tend to cause hydrogen abstraction resulting in a higher degree of branching in the polymer, while azo-containing initiators tend to yield more linear polymers.
The propagation step involves the addition of monomer units to the reactive species from the initiator step. During this step, the available monomers are consumed in the polymerization. These growing polymer chains continue to add monomer units and build molecular weight until termination occurs. The termination can occur through a variety of mechanisms, such as coupling, disproportionation, radical transfer to the solvent, monomer, polymer, or chain transfer agents.
The various acrylic monomers copolymerize with each other to a high molecular weight quite readily. This allows for versatility in monomer selection and ease of incorporation into the polymer of the monomers that are at lower concentrations, such as the functional monomers. This ensures that it is likely to have functionality incorporated into all of the individual polymer chains. This regular distribution of functional groups is important for both adhesive properties and crosslinking.
By polymerization a polymer evolves, which is saturated and has a backbone of carbon-to-carbon bonds. The polymer is chemical and oxidative resistant, making it suitable for harsh environments including direct sunlight. The polymer is also resistant to chemical attack because of the stability of the carbon-to-carbon bonds in the polymer backbone.
Molecular weight
The molecular weight of a polyacrylate pressure-sensitive adhesive is of eminent importance for its mechanical and physical properties and has been shown to be key to the final adhesive performance.
The tack and peel performance increases with molecular weight to a maximum. with a decrease and leveling off as the failure mode changes from cohesive to adhesive failure. The cohesive or shear properties build to a maximum with increasing molecular weight. Even low shearing loads cause cohesion tearing, which would disqualify the polymer as pressure-sensitive adhesive. The molecular weight and branching is controlled in the polymerization process with initiator level and selection, process temperature, and chain transfer agents.
The influence of the molecular weight MW on the properties of the pressure-sensitive adhesive, such as tack, peel adhesion, and shear strength of an acrylate copolymer is shown in Fig. 3.
Highly efficient solvent-borne polyacrylate pressure-sensitive adhesives with a molecular weight between 450,000 Da and 1.5 MDa. UV curable hot-melt PSA (HMPSA) acrylics show a molecular weight at 100,000–300,000 Da. An increase of molecular weight in the case of hot-melt acrylics above 300,000 Da negatively affects the process viscosity and makes the hot melt unsuitable for the coating process.
Polymerization methods
The solution polymerization of acrylic and other suitable monomers to form copolymers being soluble in organic solvents is an important commercial process for the preparation of polymers for the use as pressure-sensitive adhesives. Typically, the polymerization is done batch wise by adding monomers to an organic solvent in the presence of a soluble peroxide or azo initiator. Since polymerization is a chemical reaction, its course is determined by the concentrations of the initiator and the monomers, as well as by the heat input and the heat loss. These factors are controllable on a large scale and are reproducible, but they are very difficult to predict on the basis of a small-scale laboratory work. It is for these reasons that the scale up of a polymer is not a fast process. It must be done on the basis of successive steps of increasing size, ultimately to full size and often calls for more laboratory work between each step to ensure that the desired product is produced in sufficient quality at commercial quantities.
Generally, the processability of solvent-borne PSAs depends on their viscosity and solids content. Coating devices are designed for a given viscosity, whereas the coating weight and the drying speed depend on the solids content. In general, the solids content and viscosity should be measured for formulated compositions. This method of synthesis is suitable for the preparation of high-performance solvent-borne pressure-sensitive adhesives with high molecular weights of about 600,000–1.8 MDa. Pressure-sensitive adhesives based on polyacrylates with higher molecular weight are not only difficult to prepare by this method, but also have viscosities too high for easy handling. The really high-performance adhesives are exclusively pressure-sensitive adhesive solvent-borne acrylics, or probably, the really high-performance adhesives are exclusively solvent-based pressure-sensitive adhesives.
Crosslinking
The properties of pressure-sensitive adhesives synthesized by copolymerization of acrylic monomers and formulated in organic solvent mixture are determined, to a great extent, by the kind and quantity of the crosslinking agent added to the polymerizate. As with molecular weight, crosslinking influences the bulk properties of the film and builds shear, heat, and chemical resistance, while it has a negative effect on tack and peelability. It is necessary to achieve interchain crosslinking for heat resistance, because the pressure-sensitive adhesive polymers are operating in the region above their glass transition temperature. Therefore, without crosslinks, the polymer would readily flow under heat, losing all cohesive strength. The crosslinking also builds water and chemical resistance, as well as final adhesive construction properties to enhance die cutting, slitting, and roll stability [38].
Acrylic copolymers whose chains are not only crosslinked via hydrogen bonds or interpenetrating systems (IPN) can hardly be cohesively loaded. But a few tenths of a percent of crosslinking agent make it a pressure-sensitive adhesive having good mechanical and thermal properties.
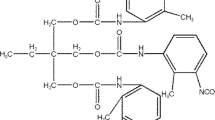
There are several different chemical agents that are employed to achieve the polymer crosslinking, which contain a list of the more common chemistries employed as multifunctional crosslinking agents, such as:
-
Multifunctional monomers
-
Metal salts
-
Metal chelates
-
Amino resins
-
Isocyanate crosslinking agents
-
Propylene imines
-
Monomers with crosslinking properties
-
UV-radiation causing crosslinking process
Several of these crosslinking agents allow stabilization and the preparation of single-package products. In this case, the crosslinker is activated in the oven during the film-drying operation. Other chemistries, such as isocyanates, require employment of two package systems where the crosslinking agent is compounded into the final product just prior to coating.
Performance of acrylic pressure-sensitive adhesives
The term pressure-sensitive describes adhesives, which in the dry form are aggressively and permanently tacky at room temperature and firmly adhere to a variety of dissimilar surfaces upon mere contact, without the need of more than finger or hand pressure.
Adhesion–cohesion balance
Pressure-sensitive adhesives possess adhesion, required for bonding and debonding, and cohesion necessary against debonding. Adhesion is characterized by tack and peel, whereas cohesion is described by shear resistance and partially by peel. The special balance of these properties, the adhesion/cohesion balance, embodies the pressure-sensitive character of the adhesive. The efficiency of the bonding process is related to the adhesive’s ability to exhibit viscous flow. To achieve peel adhesion, the bonding stage involves some dwell time. During this time, the adhesive must flow in the absence of any externally applied forces. The more liquid-like the behavior of the polymer under these conditions, the more pronounced the degree of bond formation. The debonding process involves a more rapid deformation of the adhesive mass. The polymer’s resistance to deformation at higher strain rates becomes very important; the higher this resistance, the higher the force that must be applied to separate the adhesive from the adherent (i.e., the peel resistance). Therefore, high tack, high peel strength adhesives should exhibit good flow at low strain rates, but good resistance to flow at higher strain rates [39].
A proper balance between high tack, peel adhesion, and high cohesion is necessary in most cases. The behavior of any pressure-sensitive adhesive can be reduced to three fundamental and interconnected physical properties: tack, adhesion (peel adhesion), and shear resistance (cohesion). A clear understanding of each property and term is essential.
Tack
Tack has been one of the favorite subjects of theorists over many years, often resulting in the derivation of complex formulae in an attempt to explain the property. Nevertheless, tack is still considered and rated by many as how well a pressure-sensitive adhesive sticks to the finger following only slight pressure and short dwell time. While in many cases this can be an approximate measure, this method is badly flawed in that it is highly subjective [40].
When a pressure-sensitive adhesive is applied to a surface, it takes time for that adhesive to wet out the surface until optimum contact area and adhesion is achieved. This time may be a small fraction of a second or may take days or even weeks. The wetting process can be aided by the degree of pressure applied and the length of time given to that pressure. The rate at which wetting takes place varies inversely with the amount of surface still available for wetting. Because the rate of wettability can be considerably accelerated by applied pressure, in a number of tests this application pressure is reduced to a minimum to increase the sensitivity of the test.
Tack tests are many and varied. However, if they are all carefully examined, they will be seen to be unique adhesion tests, each with its own method of application to a test surface, applied pressure, unique geometry, and rate of removal. A point in time is chosen during the wettability phenomenon when the adhesive is stripped from the test surface: the force required to do so is considered a measure of tack. Were the test to be repeated, but to allow optimum wetting, a comparison of the two results would be a better indication of how quickly the adhesive was capable of wetting out the surface. Note that an adhesive system can wet out the surface quite quickly, but if the final adhesion is low, then a low tack value will still be apparent.
Under the bottom line, the tack of a pressure-sensitive adhesive can be considered to be primarily a measure of the wettability of that adhesive under controlled application conditions, with due regard for its optimum adhesion value. Tack increases continuously upon adding soft, viscous components to the formulation (Fig. 4).
Peel adhesion (adhesion)
High peel adhesion requires a certain tack level for bonding and certain cohesion for debonding. The dependence of the peel on the ratio of elastic/viscous components is more complex, going through a maximum as a function of the level of the soft component.
There are two meanings of the term “adhesion.” On the one hand, adhesion is understood as the process through which two bodies are attached to each other when brought together. In this sense, adhesion characterizes the sum of all intermolecular and electrostatic forces acting across the interface. On the other hand, we may examine the process of breaking the already adhesive in contact. In this case adhesion is the force, or the energy, required to separate the two bodies, often called “practical adhesion” or “adherence” (Fig. 5).
One would believe that in describing the adhesion of a pressure-sensitive adhesive in the form of tapes, it would be a measure of the force that holds that pressure-sensitive adhesive tape to an applied surface. In fact, it is actually a measure of the force required to remove it from that surface. Removal involves work done in extension of the adhesive, the work done in distorting the backing during the stripping action, and the work done in separating the adhesive/surface interface; the last being the smallest of the three.
According to the American Society for Testing Materials (ASTM), adhesion is “a state in which two surfaces are held together by interfacial forces which may consist of valence forces or interlocking action, or both.”
A theoretical treatment of adhesion in terms of intermolecular interaction is not just confined to bond energies; other important factors are the number of contact points of the interacting atoms or molecules, intermolecular distances, the mobility of atomic groups, and the structure of neighboring matter.
Many theoretical models of adhesion have been proposed, which together are both complementary and contradictory. The most important will now be described:
-
Mechanical interlocking
-
Adsorption (or thermodynamic) theory
-
Electrostatic theory
-
Chemical bonding theory
-
Diffusion theory of adhesion
-
Adhesive effect of thin liquid films
-
Theory of weak boundary layers
Each of these theories of adhesion is supported by experimental analysis but for each there are also convincing counter arguments.
Shear strength (cohesion)
According to the ASTM, definitions of cohesion include “The propensity of a single substance to adhere to itself, the internal attraction of molecules towards each other; the ability to resist partition from the mass; internal adhesion; the force holding a single substance together.”
The most important means to influence the cohesion of pressure-sensitive adhesives are tackification and crosslinking. Pressure-sensitive adhesives possess typical viscoelastic properties, which allow them to respond to both a bonding and a debonding step. For permanent adhesives, the most important step is the debonding one; the adhesive should not break under debonding (mainly shear and peel) forces (i.e., permanent adhesives must provide a higher level of cohesive or shear strength than removable adhesives).
When an increasing shear force is applied to a pressure-sensitive adhesive tape, eventually a point will be reached when tape failure results. The nature of that failure is dependent on how quickly the liquid component of the adhesive can respond to the applied force. At one end of the spectrum, with a high stress or a rapidly increasing stress, the behavior will be largely elastic and the adhesive will separate at the interface leaving a trace of adhesive residue, or the tape backing will break. At the other end of the spectrum, the liquid component of the adhesion can respond fully, allowing molecular disentanglement within the adhesive resulting in cohesive failure (Fig. 6):
Typical shear resistance testing is performed with a controlled area of adhesive tape (pressure-sensitive adhesive layer) applied to a standard test surface. Because shear failure is the inability of the pressure-sensitive adhesive to resist a continuous stress, any task that is a measure of stress relaxation within the adhesive gives meaningful data. A high shear resistant adhesive will maintain the stress, while a poor shear resistant adhesive will relieve the stress quite rapidly (Fig. 7).
Shrinkage
If the dimensions of the test bar or plaque do not match precisely the mold from which was made, shrinkage takes place, either through differential thermal expansion between the mold material and the polymer or through microstructural changes in the polymer after molding. Although acrylic polymers have been used successfully as pressure-sensitive adhesives in a variety of industries, a property inherent to all acrylic PSAs that negatively impacts adhesion performance is shrinkage on PVC surfaces upon crosslinking. This phenomenon is attributed to the formation of a three-dimensional, covalently crosslinked network during crosslinking, which reduces intermolecular distances between the monomers used to form the crosslinked network.
For example, before crosslinking, the molecules, which comprise the PSA acrylics are separated by their characteristic van der Waals radii. Upon crosslinking, these intermolecular distances are reduced due to the formation of covalent bonds between monomers, which produces the desired highly crosslinked PSA material. This reduction of intermolecular distances creates internal stress throughout the crosslinked network, which is manifested by reduced adhesion of the PSA adhesive to both the substrate and the object attached thereto.
Shrinkage presents the percentage or millimeter change of dimensions of the PVC foil covered with PSA after PSA crosslinking and attached to the glass after keeping it 1 week at a temperature of 70 °C. With shrinkage greater than 0.5% or greater than 0.5 mm, other properties were neglected.
Summary
Acrylics play perhaps the most key role in technology for obtaining all types of self-adhesive adhesives used to produce a wide range of products, such as classic single-sided, double-sided, and carrier-less self-adhesive tapes in the form of assembly adhesive films. Acrylics are used to produce self-adhesive labels, protective foils, and marking and sign films, as well as a wide range of medical products in the form of plasters, self-adhesive bandages, OP tapes (surgical tapes), and bioelectrodes.
Professional concepts presented in the article, such as tack, peel adhesion, shear strength, and shrinkage, are described very precisely in the content of the study.
A decisive role in the application of polyacrylate self-adhesive adhesives is played by the selection of appropriate monomers for the synthesis process and the use of various crosslinking compounds, and in crosslinking technologies, the use of UV radiation for polymerization, crosslinking and application, emitted UV by both lamps and LEDs. The use of LED technology is a relatively new method of crosslinking self-adhesive adhesives and will be presented in a separate publication. Initial research has clearly indicated lower energy consumption when using LEDs than when using classic UV lamps.
Data availability
No datasets were generated or analysed during the current study.
References
Czech Z (1999) Vernetzung von haftklebstoffen auf polyacrylatbasis, wydawnictwo politechniki szczecińskiej. Szczecin (ISBN 83-87423-18-1)
Milker R, Czech Z (1984) Münchener klebstoff-und veredelungsseminar, München, Germany
Czech Z (2005) Synthesis of mew solvent-borne acrylic pressure-sensitive adhesives with low shrinkage, Polimery 50, Szczecin University of Technology
Czech Z (2005) Synthesis of removable and repositionable water-borne pressure-sensitive adhesive acrylics. J Appl Polym Sci:886–892
Milker R, Czech Z (1998) Münchener Klebstoff-und Veredelungsseminar, München, Germany
Czech Z (1991) WO 92/10553
Czech Z, Milker R, Nissing P, Wehmann J (1992) WO 93/03106
Czech Z, Herrmann F (1993) WO 93/20112
Czech Z, Blum W, Herrmann F (1993) WO 94/14853
Czech Z, Seeger K (1995) WO 95/31224
Czech Z, Wehmann J (1990) US Patent, 5.084.348
Czech Z, Seeger K (1990) US Patent 5.135.755
Czech Z, Seeger K (1992) US Patent 5:336–501
Czech Z (1991) US Patent 5:433–892
Czech Z, Seeger K (1990) US Patent 5.785.985
Müller W, Czech Z, Simon G (1986) EP 0:272–562
Czech Z (1989) EP 0:352–442
Czech Z, Wehmann J (1990) EP 0:379–932
Czech Z, Seeger K (1990) EP 0:382–128
Czech Z, Lindner E (1990) EP 0:413–301
Czech Z (1991) EP 0:561–854
Czech Z, Milker R, Nissing P, Wehmann J (1992) EP 0 597–999
Czech Z (1993) EP 0 604–709
Czech Z, Blum W (1994) EP 0 628–616
Czech Z, Herrmann F (1994) EP 0 658–610
Czech Z (1995) EP 0 670–338
Czech Z, Blum W (1995) EP 0 670–356
Czech Z, Sander D (1995) EP 0 701–822
Czech Z (1995) EP 0 710–708
Köhler R (1970) Adhäsion 3:90
Kaelble DH (1959) Trans Soc Rheol 3:161
Satas D (1982) Handbook of pressure sensitive technology. Van Nostrand-Rheinhold Co, New York
Aydin O, Dragon A (2001) Pressure-sensitive adhesives. In: Coating Handbook, BASF, Germany
Post LK (1994) Münchener Klebstoff- und Veredelungsseminar, München
Odian G (1991) Principles of Polymerization, 3rd edn. Whiley, New York
Milker R, Czech Z (1985) Adhäsion 29:3
Satas D (1989) Handbook of pressure-sensitive technology, Second Edition
Igarashi T (1975) J Polym Sci 13:2199
Kinloch AJ (1990) Adhesion and adhesives. Chapman and Cambridge University Press, Cambridge
Lee LH (1991) Fundamentals of adhesion. Plenum Press, New York
Author information
Authors and Affiliations
Contributions
Z.C. wrote the whole manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Robert Czech, Z. Pressure-sensitive acrylic adhesives (PSAs): how it began and the present state of art. ChemTexts 10, 6 (2024). https://doi.org/10.1007/s40828-024-00189-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40828-024-00189-w