Highlights
-
Through materials analysis and theoretical calculations, the defects of CsSnI3 nanowires (NWs) were effectively passivated via incorporating 1-butyl-2,3-dimethylimidazolium chloride into perovskites.
-
The high-performance CsSnI3 NW photodetectors (PDs) were achieved with a responsivity of up to 0.237 A W−1, a high detectivity of 1.18 × 1012 Jones and a linear dynamic range of 180 dB. These values are comparable to the reported high-performance Pb-based perovskite PDs and higher than those of the Pb-free perovskite PDs.
-
Our unpackaged devices exhibit ultra-high stability with no degradation after 60 days of storage in air (25 °C, 50% humidity).
Abstract
In recent years, Pb-free CsSnI3 perovskite materials with excellent photoelectric properties as well as low toxicity are attracting much attention in photoelectric devices. However, deep level defects in CsSnI3, such as high density of tin vacancies, structural deformation of SnI6− octahedra and oxidation of Sn2+ states, are the major challenge to achieve high-performance CsSnI3-based photoelectric devices with good stability. In this work, defect passivation method is adopted to solve the above issues, and the ultra-stable and high-performance CsSnI3 nanowires (NWs) photodetectors (PDs) are fabricated via incorporating 1-butyl-2,3-dimethylimidazolium chloride salt (BMIMCl) into perovskites. Through materials analysis and theoretical calculations, BMIM+ ions can effectively passivate the Sn-related defects and reduce the dark current of CsSnI3 NW PDs. To further reduce the dark current of the devices, the polymethyl methacrylate is introduced, and finally, the dual passivated CsSnI3 NWPDs show ultra-high performance with an ultra-low dark current of 2 × 10–11 A, a responsivity of up to 0.237 A W−1, a high detectivity of 1.18 × 1012 Jones and a linear dynamic range of 180 dB. Furthermore, the unpackaged devices exhibit ultra-high stability in device performance after 60 days of storage in air (25 °C, 50% humidity), with the device performance remaining above 90%.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Because of excellent photoelectronic properties and low fabrication cost, organic–inorganic hybrid perovskites have become a hot topic in recent years and are widely used in solar cells [1,2,3,4], photodetectors (PDs) [5,6,7], light-emitting diodes (LEDs) [8, 9], etc. Until now, the highest certified power conversion efficiency of perovskite solar cells has exceeded 25% [10, 12], and the LEDs based on lead-containing perovskite materials also show high performance; for example, the highest external quantum efficiency of green LEDs has exceeded 20% [13,14,15]. However, the presence of heavy metal lead severely limits the commercialization of lead halide materials, which will face security risks of heavy metal lead leakage during mass production, transportation, installation and operation.
In this context, lead-free perovskite materials based on tin (Sn) [14], bismuth [15], germanium [16], antimony [17] or copper [18] have attracted much attention. Among these lead-free perovskite materials, CsSnI3 is more popular in photovoltaic applications due to its similar crystal and electronic structure to its Pb-based counterpart. Besides, inorganic perovskite CsSnI3 has a narrow optical band gap close to the Shockley–Queisser limit, with long lifetime and high charge carrier mobility [19, 20]. Moreover, the melting point of the CsSnI3 is up to 451 °C, which means that it has excellent inherent thermal stability. Therefore, the development of the inorganic perovskite CsSnI3 shows great prospect, and many scientists have achieved meaningful results [21,22,23]. Jin et al. [24] reported the growth of CsSnX3 (X = Br, I) perovskite semiconductors with controlled orientation and size by high-temperature vapor-phase epitaxy on mica sheets. Yang et al. [25] reported the preparation of CsSnX3 (X = Cl, Br, and I) perovskite nanowire (NW) arrays by chemical vapor deposition with a responsivity of 54 mA W−1, a detectivity of 3.85 × 10–5 Jones, and fast rise and decay time constants of 83.8 and 243.4 ms, respectively. However, the efficiency of the solution-processed CsSnI3 devices was much lower than that of the Pb-based analogs, mainly due to the weak Sn-I bond of inorganic CsSnI3, which causes lower tin vacancy formation energy and the easy oxidation of Sn2+ to Sn4+, leading to a high level of self-P doping in inorganic CsSnI3 perovskites, and the reduction in the device performance and the decrease in the output stability [26]. Therefore, strategies to passivate the Sn defects in inorganic CsSnI3 perovskites while maintaining their environmental stability are urgently needed.
In this work, 1-butyl-2,3-dimethylimidazolium chloride (BMIMCl) salt is introduced to passivate the defects of perovskite CsSnI3 NWs. Through materials analysis and theoretical calculations, the BMIMCl has a strong passivating effect on Sn-related defects via large π-bonds in N–C = N, and the lone pair of electrons in large π-bonds enhances the electron density around Sn2+ in CsSnI3 and protects it from oxidation to Sn4+; thus, the fabricated CsSnI3 NWs with BMIMCl show high light absorption, low defect density and air stability. To further reduce the dark current of the devices, the polymethyl methacrylate (PMMA) was applied, and finally, the dual passivated CsSnI3 NW PDs show ultra-high performance with an ultra-low dark current of 2 × 10–11 A, a high responsivity of 0.237 A W−1, a high detectivity of 1.18 × 1012 Jones and a linear dynamic range (LDR) of 180 dB. Besides, our unpackaged devices exhibit good stability with less than 10% degradation in device performance after 60 days of storage in air (25 °C, 50% humidity), demonstrating good application potential.
2 Experimental and Calculation
2.1 Device Fabrication
A pre-etched indium-tin oxide (ITO) glass substrate was ultrasonically cleaned with detergent, deionized water, ethanol and iso-propyl alcohol for 15 min, respectively. To prepare the SnO2 precursor solution, the SnO2 stock solution (1 mL) was diluted in deionized water (4 mL). The as-cleaned ITO substrate was treated with UV ozone at 100 °C for 10 min. A compact layer of SnO2 was spin-coated on top of the ITO at 4000 rpm for 30 s. Then, it was heated at 150 °C for 30 min in air. After that, the samples were treated with UV ozone for 10 min. Subsequently, the samples were transferred into a N2 filled glovebox with H2O and O2 concentrations of < 0.1 ppm. A layer of PbI2 film was fabricated by spin-coating PbI2/BMIMCl (1 mol mL−1/0, 5, 8, 10 and 15 mg mL−1) in DMF at 3000 rpm for 30 s, followed by annealing at 70 °C for 10 min. Then, the substrate was soaked in the prepared CsI/SnI2/SnF2 (5 /4 /0.4 mg mL−1) solution in anhydrous methanol for 2 h. After that, the substrates were placed in an isopropyl alcohol solution for 20 s and then annealed at 180 °C for 10 min. Finally, a layer of PMMA was coated on the samples by spin-coating PMMA in CB (20 mg mL−1) at 2000/3000/4000/5000/6000/7000 rpm for 30 s, followed by annealing at 100 °C for 10 min. Then, a layer of carbon electrode was scraped on the samples and annealed at 120 °C for 15 min in air.
2.2 Calculations
All the calculations are performed in the framework of the density functional theory (DFT) with the projector augmented plane-wave method, as implemented in the Vienna ab initio simulation package. The generalized gradient approximation proposed by Perdew, Burke and Ernzerhof is selected for the exchange–correlation potential. The long-range van der Waals interaction is described by the DFT-D3 approach. The cutoff energy for plane wave is set to 400 eV. The energy criterion is set to 10–5 eV in iterative solution of the Kohn–Sham equation. The Brillouin zone integration is performed at the Gamma point for structural optimization, and a 3 × 3 × 1 k-mesh grid is used for electronic structure calculations. All the structures are relaxed until the residual forces on the atoms have declined to less than 0.05 eV Å−1.
3 Results and Discussion
A schematic diagram of the preparation of CsSnI3 NWs by a two-step solution method is shown in Fig. 1a. The aqueous SnO2 solution was first spin-coated onto a clean ITO substrate and then annealed on a hot table at 150 °C for 30 min to obtain the SnO2 films. The PbI2/BMIMCl (1 mol mL−1/0, 5, 8, 10 and 15 mg mL−1) DMF solution was spin-coated on the SnO2/ITO substrate and then annealed at 70 °C for 10 min to obtain the PbI2-BMIMCl films. The PbI2-BMIMCl film-covered substrates were immersed in a methanol solution of CsI/SnI2/SnF2 (5/4/0.4 mg mL−1) for 2 h. The yellow phase CsSnI3 (γ-CsSnI3) was obtained by the B-position exchange reaction. During the reaction, the perovskite also grows in a specific direction because of the strong anisotropy of γ-CsSnI3 and eventually forms one-dimensional γ-CsSnI3 NWs [27, 28]. After that, the CsSnI3 NWs-covered substrates were placed in an isopropyl alcohol solution for 20 s to wash away the impurities on it and then annealed at 180 °C for 10 min to obtain the black phase CsSnI3 NWs (B-CsSnI3 NWs). The top-view scanning electron microscopy (SEM) images of CsSnI3 NW films with different soaking times (2, 4, 8, 16, 24, and 48 h) are shown in Figs. 1b and S1, which show little changes in surface morphology. Besides, the energy-dispersive spectroscopy (EDS) results (Table S1) show that when the soaking time is above 2 h, the perovskite NWs indicate very little Pb content (~ 0.21%) and we think our perovskite nanowires are basically all converted to CsSnI3 NWs and 2 h of soaking time are enough.
When different BMIMCl concentrations (5, 8, 10 and 15 mg mL−1) are incorporated (Fig. S2), the surface coverage of the nanowires is more obvious with the increase in the concentration. In order to further study the effect of the incorporation of BMIMCl, the high-resolution transmission electron microscopy (HRTEM) is performed, and the results are shown in Figs. 1c and S4. From these figures, all perovskite NW samples show high crystallinity. The d-spacing value of the CsSnI3 without BMIMCl is estimated to be 0.31 nm, corresponding to the (202) plane of CsSnI3. For the BMIMCl + CsSnI3 sample, the spacing between the lattice fringe is 0.35 nm, which corresponds to the (220) plane of CsSnI3, and shows clearer lattice fringes of the perovskite grains, indicating higher crystallinity of the perovskite grains [29, 30]. Besides, the NW is covered by an amorphous BMIMCl molecular layer with a thickness of about 1.2 nm. This thin layer of BMIMCl will protects the underlying CsSnI3 from degradation when exposed to ambient air.
Figures 1d and S3a show the X-ray diffraction (XRD) patterns of CsSnI3 NW films with different concentrations of BMIMCl, which match well with the orthorhombic perovskite crystal structure. After the addition of BMIMCl, the peak intensity of the (220) plane of the CsSnI3 NW film is slightly enhanced compared with that of the unincorporated CsSnI3 NW film, the better crystal quality of the perovskite may improve the carrier transmission of the CsSnI3 [31]. Figures 1e and S3b show the optical properties of the perovskite with different concentrations of BMIMCl, which show the CsSnI3 NWs have capacity of absorbing from visible to infrared light. Steady-state photoluminescence (PL) spectroscopy is then performed to examine the emission characteristics of the CsSnI3 NW thin films (Figs. 1f and S3c). Compared with the CsSnI3 NW film without BMIMCl, the peak intensity of the CsSnI3 NW film gradually increases with increasing the concentration of BMIMCl and reaches a maximum at the concentration of 8 mg mL−1. Then, the peak intensity gradually decreases after further increasing the concentration of BMIMCl. This can be explained that the moderate BMIMCl concentration can effectively passivate the defects of the CsSnI3 NWs and suppress the non-radiative recombination of the perovskites [23, 32]. Besides, the time-resolved PL indicates that the CsSnI3 NW film with 8 mg mL−1 BMIMCl shows longest carrier lifetime (Fig. S4d), which further confirms moderate BMIMCl concentration is beneficial for passivating the defects of the CsSnI3 NWs.
Figure 2a shows the CsSnI3 NWPD with an ITO/SnO2/perovskite NWs/carbon structure, in which SnO2 is used as the electron transport layer, perovskite NWs are the photo-active layer, and carbon is as the top electrode also protects perovskites from corrosion by water and oxygen in air. As a high-performance PD, low dark current is essential, which has a positive impact on the linear dynamic range, signal-to-noise ratio and detectivity of the device. The relationship between the light and dark current of the PDs is shown in Fig. 2b, and 8 devices are used for the light/dark current statistic. The dark current gradually decreases as the BMIMCl concentration increases, and the dark current reaches a minimum until the concentration is at 8 mg mL−1. The drop in dark current is attributed to the passivation of the surface defects of perovskite, especially the Sn vacancies, which are the main culprit for the material to appear metallic [21]. However, too much ionic liquid will cause additional impurities, which will increase the dark current of the device. For the photocurrent, it shows a slightly downward trend. Considering the light and dark currents comprehensively, we believe that the NWPDs prepared at 8 mg mL−1 are best.
To investigate the passivation interaction of the BMIMCl on Sn-related defects and verify our conjecture, Fourier transform infrared spectroscopy (FTIR) measurements are performed on BMIMCl and BMIMCl + CsSnI3. The peak of the pure BMIMCl sample is located at 1589 cm−1 (Fig. 3a), which corresponds to the N–C = N stretching vibration of the imidazole ring. For the BMIMCl + CsSnI3 sample, this peak is red-shifted to 1584 cm−1, indicating a strong interaction between N–C = N and CsSnI3. It is inferred that the N–C = N of BMIM+ has a strong passivating interaction with Sn-related defects such as Sn vacancies. The trap density of the Sn-based perovskites is strongly associated with the Sn4+ content, which is considered as the Sn vacancies and can be distinguished by X-ray photoelectron spectroscopy (XPS) measurement. As shown in Fig. 3b, compared with ordinary CsSnI3 samples, the proportion of Sn4+ in the BMIMCl + CsSnI3 sample is greatly reduced, and the proportion of Sn2+ rises, which indicates that the oxidation of Sn2+ to Sn4+ in the BMIMCl + CsSnI3 sample is significantly suppressed. In order to further verify our conjecture, the DFT calculations are used to study the effect of the introduction of BMIMCl on CsSnI3. Since the BMIMCl is coated on the surface of CsSnI3 perovskite, a model of BMIMCl on CsSnI3 is established, and the calculated differential charge density of BMIMCl + CsSnI3 (220) indicates the effective charge extraction of BMIMCl, as shown in Fig. 3c. Besides, the density of state (DOS) of CsSnI3 perovskites with or without BMIMCl is calculated, as shown in Fig. 3d. It can be clearly observed that compared with CsSnI3, the DOS of CsSnI3 after BMIMCl passivation is significantly reduced in electron defects near the Fermi level, indicating the reduction in charge carrier recombination in CsSnI3 after BMIMCl passivation and the promotion of electron transport in the perovskite.
Previous reports showed that the PMMA can fill the voids of the nanowires, passivate the surface defects of the perovskites [33] and can also generate Fowler–Nordheim tunneling to enhance device performance [34]. Based on these, we further adopt the PMMA to reduce the dark current and enhance the device performance. Through optimize the rotational speed, the appropriate thickness of the PMMA is obtained at 5000 rpm (Fig. S5a), and at this rotational speed, the device filled by the PMMA shows the lowest dark current and the biggest light current.
The detailed device performances are shown in Fig. 4. As shown in Fig. 4a, under the irradiation of 405 nm laser with different illumination intensities, our device exhibits good photoresponse characteristics to both strong and weak light. From this figure, there is a three-order-of-magnitude difference in dark current under different light intensities. We think the main reason of the three-order-of-magnitude increase in dark current is attributed to the perovskite CsSnI3, in which a few Sn vacancies still exist. When the device is illuminated by light, a large number of photo-generated carriers will be generated, and some of them may be trapped by these vacancies and remain. After the light is removed, these remaining photo-generated carriers will be slowly released and cause the rise of the dark current. When stronger light is applied, more photo-generated carriers are generated and more left, resulting in a three-order of magnitude difference in dark current under different light intensities.
The LDR of the PD is an important parameter for evaluating the performance of the PD, which can be calculated by the formula:
where Psat and Plow are the strongest and weakest light intensities, respectively, when the incident light begins to deviate from the linear photocurrent. The calculated LDR is as high as 180 dB, indicating that our device can work stably in a wide range of the light intensity (Fig. 4b), and the ultra-low dark current of 2 × 10–11 A of the device is obtained. Besides, the noise of the device affects the sensitivity of the PD to light, and shot noise is an important noise source which affects the dark current. The device noise power spectrum (Fig. 4c) shows that the noise (in) of the CsSnI3 NW PD is very low with the value of about 4 × 10–13 A Hz−1. Through the dark current, the shot noise of the device is further calculated, and it is close to the total noise, indicating that the main device noise is the shot noise. To further study the performance of the device, the responsivity R and the detectivity D* are measured to evaluate the sensitivity of the photoelectric response of the device to incident light, which are calculated by the formulas:
where Iph is the photocurrent, A is the effective light irradiation area of the PD, Popt is the light intensity, and B is the detection line width. As shown in Fig. 4d, both the R and the D* show a linear drop with increasing the light intensity. Under weak light, our PD shows high device performance with a R of 0.237 A W−1, and a D* of up to 1.18 × 1012 Jones, which are comparable to the reported high-performance Pb-based perovskite PDs and higher than those of the Pb-free perovskite PDs [35,36,37,38,39,40,41,42,43,44]; the detailed comparison is shown in Table S2. The achievement of the high performance is due to the dual passivation of perovskite defects through the BMIMCl and the PMMA, which greatly reduce the dark current, thereby reduce the device noise level and improve the detection capability of the device. Moreover, the broad light absorption range of the CsSnI3 enables the wide work region of our PD ranging from ultraviolet to infrared radiation, as shown in Fig. S5b. Furthermore, our device shows the f-3 dB bandwidth of 1196 Hz (Fig. 4e) and the rise/fall time (τrise/τfall) of 0.23/0.19 ms (Fig. 4f).
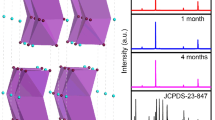
Device stability is a key parameter of the PD, which is an important reference for commercialization. To confirm the improvement in device stability by the dual passivation strategies, we perform the stability of the unpackaged PDs under different conditions in air (25 °C, 50% humidity), as shown in Fig. 5. We find the performance of the passivated device shows no degradation when it is exposed to continuous light in air for more than 1200 s, but the light current of the original device indicates a significantly decline (Fig. 5a). We further place the devices in air for more than 60 days, and the performance of the original CsSnI3 PDs decreases to about 30% of the initial value. However, the device performance of the passivated devices remains 90% of the initial value after the same storage time. These results demonstrate that the introduction of the BMIMCl and PMMA not only enhances the optoelectronic performance of the device, but also improves the stability of the device.
4 Conclusions
In conclusion, the BMIMCl was introduced in CsSnI3 NWs to passivate the Sn vacancies and reduce the dark current of the CsSnI3 NWPDs. Experimental analysis and theoretical calculation demonstrate that BMIM+ ions can effectively suppress the oxidation of Sn2+ to Sn4+ in the BMIMCl + CsSnI3 sample. For further reduce the dark current of the devices, the PMMA was applied, and finally, the dual passivated CsSnI3 NW PDs show ultra-high performance with an ultra-low dark current of 2 × 10–11 A, a responsivity of up to 0.237 A W−1, a high detectivity of 1.18 × 1012 Jones and a linear dynamic range of 180 dB. Furthermore, our unpackaged devices exhibit ultra-high stability in device performance after 60 days of storage in air (25 °C, 50% humidity), with the device performance remaining above 90%. This work provides a simple and effective method for the preparation of high-performance, highly stable Pb-free perovskite photoelectric devices.
References
P. Guo, H. Zhu, W. Zhao, C. Liu, L. Zhu et al., Interfacial embedding of laser-manufactured fluorinated gold clusters enabling stable perovskite solar cells with efficiency over 24%. Adv. Mater. 33(36), 2101590 (2021). https://doi.org/10.1002/adma.202101590
Y. Zhang, Y. Ma, Y. Wang, X. Zhang, C. Zuo et al., Lead-free perovskite photodetectors: progress, challenges, and opportunities. Adv. Mater. 33(26), 2006691 (2021). https://doi.org/10.1002/adma.202006691
B.B. Yu, Z. Chen, Y. Zhu, Y. Wang, B. Han et al., Heterogeneous 2D/3D tin-halides perovskite solar cells with certified conversion efficiency breaking 14%. Adv. Mater. 33(36), 2102055 (2021). https://doi.org/10.1002/adma.202102055
C. Li, Y. Ma, Y. Xiao, L. Shen, L. Ding, Advances in perovskite photodetectors. InfoMat 2(6), 1247–1256 (2020). https://doi.org/10.1002/inf2.12141
N. Ma, J. Jiang, Y. Zhao, L. He, Y. Ma et al., Stable and sensitive tin-lead perovskite photodetectors enabled by azobenzene derivative for near-infrared acousto-optic conversion communications. Nano Energy 86, 21106113 (2021). https://doi.org/10.1016/j.nanoen.2021.106113
Z. Shuang, H. Zhou, D. Wu, X. Zhang, B. Xiao et al., Low-temperature process for self-powered lead-free Cs2AgBiBr 6 perovskite photodetector with high detectivity. Chem. Eng. J. 433, 22134544 (2022). https://doi.org/10.1016/j.cej.2022.134544
L. Zhang, X. Pan, L. Liu, L. Ding, Star perovskite materials. J. Semiconduct. 43(3), 030203 (2022). https://doi.org/10.1088/1674-4926/43/3/030203
X. Tang, H. Zhou, X. Pan, R. Liu, D. Wu et al., All-inorganic halide perovskite alloy nanowire network photodetectors with high performance. ACS Appl. Mater. Interfaces 12(4), 4843–4848 (2020). https://doi.org/10.1021/acsami.9b21666
Z. Chu, Q. Ye, Y. Zhao, F. Ma, Z. Yin et al., Perovskite light-emitting diodes with external quantum efficiency exceeding 22% via small-molecule passivation. Adv. Mater. 33(18), 2007169 (2020). https://doi.org/10.1002/adma.202007169
H. Min, D.Y. Lee, J. Kim, G. Kim, K.S. Lee et al., Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 598(7881), 444–450 (2021). https://doi.org/10.1038/s41586-021-03964-8
X. Pan, J. Zhang, H. Zhou, R. Liu, D. Wu et al., Single-layer ZnO hollow hemispheres enable high-performance self-powered perovskite photodetector for optical communication. Nano-Micro Lett. 13, 70 (2021). https://doi.org/10.1007/s40820-021-00596-5
J.J. Yoo, G. Seo, M.R. Chua, T.G. Park, Y. Lu et al., Efficient perovskite solar cells via improved carrier management. Nature 590(7847), 587–593 (2021). https://doi.org/10.1038/s41586-021-03285-w
Z. Liu, W. Qiu, X. Peng, G. Sun, X. Liu et al., Perovskite light-emitting diodes with EQE exceeding 28% through a synergetic dual-additive strategy for defect passivation and nanostructure regulation. Adv. Mater. 33(43), 2103268 (2021). https://doi.org/10.1002/adma.202103268
T. Ye, K. Wang, Y. Hou, D. Yang, N. Smith et al., Ambient-air-stable lead-free CsSnI3 solar cells with greater than 7 5% efficiency. J. Am. Chem. Soc. 143(11), 4319–4328 (2021). https://doi.org/10.1021/jacs.0c13069
N.K. Tailor, P. Maity, S. Satapathi, Observation of negative photoconductivity in lead-free Cs3Bi2Br9 perovskite single crystal. ACS Photon. 8(8), 2473–2480 (2021). https://doi.org/10.1021/acsphotonics.1c00702
L.J. Chen, Synthesis and optical properties of lead-free cesium germanium halide perovskite quantum rods. RSC Adv. 8(33), 18396–18399 (2018). https://doi.org/10.1039/c8ra01150h
A. Singh, P.T. Lai, A. Mohapatra, C.Y. Chen, H.W. Lin et al., Panchromatic heterojunction solar cells for Pb-free all-inorganic antimony based perovskite. Chem. Eng. J. 419, 21129424 (2021). https://doi.org/10.1016/j.cej.2021.129424
C. Pareja-Rivera, D. Solis-Ibarra, Reversible and irreversible thermochromism in copper-based halide perovskites. Adv. Opt. Mater. 9(15), 2100633 (2021). https://doi.org/10.1002/adom.202100633
H.H. Fang, S. Adjokatse, S. Shao, J. Even, M.A. Loi, Long-lived hot-carrier light emission and large blue shift in formamidinium tin triiodide perovskites. Nat. Commun. 9, 243 (2018). https://doi.org/10.1038/s41467-017-02684-w
M.A. Kamarudin, D. Hirotani, Z. Wang, K. Hamada, K. Nishimura et al., Suppression of charge carrier recombination in lead-free tin halide perovskite via lewis base post-treatment. J. Phys. Chem. Lett. 10(17), 5277–5283 (2019). https://doi.org/10.1021/acs.jpclett.9b02024
I. Chung, B. Lee, J. He, R.P. Chang, M.G. Kanatzidis, All-solid-state dye-sensitized solar cells with high efficiency. Nature 485(7399), 486–489 (2012). https://doi.org/10.1038/nature11067
K. Marshall, M. Walker, R. Walton, R. Hatton, Enhanced stability and efficiency in hole-transport-layer-free CsSnI3 perovskite photovoltaics. Nat. Energy 1, 16178 (2016). https://doi.org/10.1038/nenergy.2016.178
T. Ye, L. Pan, Y. Yang, Q. Liang, Y. Lu et al., Synthesis of highly-oriented black CsPbI3 microstructures for high-performance solar cells. Chem. Mater. 32(7), 3235–3244 (2020). https://doi.org/10.1021/acs.chemmater.0c00427
J. Chen, Z. Luo, Y. Fu, X. Wang, K.J. Czech et al., Tin(IV)-tolerant vapor-phase growth and photophysical properties of aligned cesium tin halide perovskite (CsSnX3, X = Br, I) nanowires. ACS Energy Lett. 4(5), 1045–1052 (2019). https://doi.org/10.1021/acsenergylett.9b00543
M. Han, J. Sun, M. Peng, N. Han, Z. Chen et al., Controllable growth of lead-free all-inorganic perovskite nanowire array with fast and stable near-infrared photodetection. J. Phys. Chem. C 123(28), 17566–17573 (2019). https://doi.org/10.1021/acs.jpcc.9b03289
M. Konstantakou, T. Stergiopoulos, A critical review on tin halide perovskite solar cells. J. Mater. Chem. A 5(23), 11518–11549 (2017). https://doi.org/10.1039/c7ta00929a
M. Lai, Q. Kong, C.G. Bischak, Y. Yu, L. Dou et al., Structural, optical, and electrical properties of phase-controlled cesium lead iodide nanowires. Nano Res. 10(4), 1107–1114 (2017). https://doi.org/10.1007/s12274-016-1415-0
Y. Zhou, J. Luo, Y. Zhao, C. Ge, C. Wang et al., Flexible linearly polarized photodetectors based on all-inorganic perovskite CsPbI3 nanowires. Adv. Opt. Mater. 6(22), 1800679 (2018). https://doi.org/10.1002/adom.201800679
M. Li, W.W. Zuo, Y.G. Yang, M.H. Aldamasy, Q. Wang et al., Tin halide perovskite films made of highly oriented 2D crystals enable more efficient and stable lead-free perovskite solar cells. ACS Energy Lett. 5(6), 1923–1929 (2020). https://doi.org/10.1021/acsenergylett.0c00782
T. Ye, B. Zhou, F. Zhan, F. Yuan, S. Ramakrishna et al., Below 200 °C fabrication strategy of black-phase CsPbI3 film for ambient-air-stable solar cells. Solar RRL 4(5), 2000014 (2020). https://doi.org/10.1002/solr.202000014
T. Ye, A. Bruno, G. Han, T.M. Koh, J. Li et al., Efficient and ambient-air-stable solar cell with highly oriented 2D@ 3D perovskites. Adv. Funct. Mater. 28(30), 1801654 (2018). https://doi.org/10.1002/adfm.201801654
X. Zheng, Y. Hou, C. Bao, J. Yin, F. Yuan et al., Managing grains and interfaces via ligand anchoring enables 22 3%-efficiency inverted perovskite solar cells. Nat. Energy 5(2), 131–140 (2020). https://doi.org/10.1038/s41560-019-0538-4
H. Zhou, Z. Song, C.R. Grice, C. Chen, J. Zhang et al., Self-powered CsPbBr3 nanowire photodetector with a vertical structure. Nano Energy 53, 880–886 (2018). https://doi.org/10.1016/j.nanoen.2018.09.040
J. Zeng, C. Meng, X. Li, Y. Wu, S. Liu, H. Zhou, H. Wang, H. Zeng, Interfacial-tunneling-effect-enhanced CsPbBr 3 photodetectors featuring high detectivity and stability. Adv. Funct. Mater. 29(51), 1904461 (2019). https://doi.org/10.1002/adfm.201904461
C. Bao, J. Yang, S. Bai, W. Xu, Z. Yan et al., High performance and stable all-inorganic metal halide perovskite-based photodetectors for optical communication applications. Adv. Mater. 30(38), 1803422 (2018). https://doi.org/10.1002/adma.201803422
J. Li, G. Zhang, Z. Zhang, J. Li, Z. Uddin et al., Defect passivation via additive engineering to improve photodetection performance in CsPbI2Br perovskite photodetectors. ACS Appl. Mater. Interfaces 13(47), 56358–56365 (2021). https://doi.org/10.1021/acsami.1c19323
X. Li, D. Yu, F. Cao, Y. Gu, Y. Wei et al., Healing all-inorganic perovskite films via recyclable dissolution–recyrstallization for compact and smooth carrier channels of optoelectronic devices with high stability. Adv. Funct Mater. 26(32), 5903–5912 (2016). https://doi.org/10.1002/adfm.201601571
Z. Li, X. Liu, C. Zuo, W. Yang, X. Fang, Supersaturation-controlled growth of monolithically integrated lead-free halide perovskite single-crystalline thin film for high-sensitivity photodetectors. Adv. Mater. 33(41), 2103010 (2021). https://doi.org/10.1002/adma.202103010
S.K. Shil, F. Wang, Z. Lai, Y. Meng, Y. Wang et al., Crystalline all-inorganic lead-free Cs3Sb2I9 perovskite microplates with ultra-fast photoconductive response and robust thermal stability. Nano Res. 14(11), 4116–4124 (2021). https://doi.org/10.1007/s12274-021-3351-x
A. Waleed, M.M. Tavakoli, L. Gu, Z. Wang, D. Zhang et al., Lead-free perovskite nanowire array photodetectors with drastically improved stability in nanoengineering templates. Nano Lett. 17(1), 523–530 (2017). https://doi.org/10.1021/acs.nanolett.6b04587
J. Wang, S. Xiao, W. Qian, K. Zhang, J. Yu et al., Self-driven perovskite narrowband photodetectors with tunable spectral responses. Adv. Mater. 33(3), 2005557 (2021). https://doi.org/10.1002/adma.202005557
X. Wu, J. Sun, H. Shao, Y. Zhai, L. Li et al., Self-powered UV photodetectors based on CsPbCl3 nanowires enabled by the synergistic effect of acetate and lanthanide ion passivation. Chem. Eng. J. 426, 131310 (2021). https://doi.org/10.1016/j.cej.2021.131310
X. Xu, C. Fan, Z. Qi, S. Jiang, Q. Xiao et al., CsCu2I3 nanoribbons on various substrates for UV photodetectors. ACS Appl. Nano Mater. 4(9), 9625–9634 (2021). https://doi.org/10.1021/acsanm.1c02041
F. Zhu, G. Lian, B. Yu, T. Zhang, L. Zhang et al., Pressure-enhanced vertical orientation and compositional control of ruddlesden–popper perovskites for efficient and stable solar cells and self-powered photodetectors. ACS Appl. Mater. Interfaces 14(1), 1526–1536 (2021). https://doi.org/10.1021/acsami.1c18522
Acknowledgements
We acknowledge grants from the National Natural Science Foundation of China (Nos. 51972101, 62074117, 12134010) and the Shenzhen Fundamental Research Program (No. JCYJ20190808152609307).
Funding
Open access funding provided by Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, Z., Zhou, H., Dong, K. et al. Defect Passivation on Lead-Free CsSnI3 Perovskite Nanowires Enables High-Performance Photodetectors with Ultra-High Stability. Nano-Micro Lett. 14, 215 (2022). https://doi.org/10.1007/s40820-022-00964-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-022-00964-9