Abstract
This work reports the abstractive potential of Parkia Biglobosa seed waste (PSW) and Parkia Biglobosa cellulosic extract (PBCE) for the removal of Cr(VI), Pb(II), methylene blue (MB) and congo red (CR) from aqua system. Physicochemical analyses carried out for these biomaterials were proximate analysis, Fourier transform infra red (FTIR) Spectrophotometry and scanning electron microscopy (SEM). The FTIR data showed that –O–H, –C=C–, –C=O, –C≡C– and –S–H functional groups were responsible for the sequestration of Cr(VI), Pb(II), MB and CR from aqua system. The equilibrium data fitted best to Langmuir isotherm model with the highest adsorption obtained for MB, being 1498.42 mg/g at 298 K and 403.23 mg/g at 298 K for PSW and PBCE respectively. Pseudo-second order model gave the best fits for the kinetic data among two other kinetic models used. The PSW and PBCE biomaterials demonstrated good potentials for the removal of toxic Cr(VI), Pb(II), MB and CR from aqua systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quest for good quality living for flora, fauna and human beings has drawn researchers’ enthusiasm in recent times to toxic cations and ionic dyes generated from global industrial activities. Various water sources contaminated by these pollutants have become a serious environmental challenge. Heavy metals and dyestuffs pose serious threat to man, terrestrial and aquatic habitats, and to a very great extent hamper ecological sustainability (Omorogie et al. 2012).
Chromium is one of the most dangerous toxic metals, widely found in industrial wastewater comprising metal finishing, electroplating, tanning, textile, leather, mining, fertiliser and photography industries (Barnhart 1997) and effluents produced from the aerospace (Owlad et al. 2010). Cr(VI) exists as extremely soluble, highly toxic and recalcitrant chromate oxoanions (HCrO4− or Cr2O7 2−) that can transfer freely in aqueous environments (Mitra et al. 2011). Persistent exposure to Cr(VI) causes cancer in the digestive tract and lungs, skin dermatitis, bronchitis, perforation of the nasal septum, severe diarrhoea and haemorrhage (Deng and Alan 1996, Marjanovic et al. 2011). In natural waters, the range of Cr(VI) concentration is as high as 5.2–208,000 mg/L (Richard and Bourg 1991).
Lead is a known environmental pollutant that can be found in industrial wastewaters such as acid battery, ceramic and glass manufacturing, metal planting and finishing, printing, tanning, and production of lead additives for gasoline. Efforts in reducing lead concentration in industrial wastewaters are driven by the hazards it poses on the ecosystem. Current US Environmental Protection Agency (US EPA) drinking water standard for lead is 0.015 mg/L (Li and Wang 2009). Acute lead poisoning in humans causes severe damage to the kidneys, liver, brain, and nervous system while a long term exposure may induce sterility, abortion and neonatal death (Gercel and Gercel 2007; Sulaymon et al. 2009).
Synthetic dyes have been increasingly utilised in textile industries, dye manufacturing industries, paper and pulp mills, tanneries, electroplating factories, food companies, etc. It is reported that approximately 100 tonnes of dyes are discharged into waste streams by the textile industry/year (Wong et al. 2003). Dyes are considered recalcitrant pollutant on aquatic environment because they give undesirable colours to water (Al-Degs et al. 2004) and reduce light penetration and photosynthesis (Wang et al. 2005; Ponnusami et al. 2007; Mane et al. 2007). Most dyes are considered to be non-oxidisable substances by traditional biological and physical treatment (Weber and Morris 1962). The disposal of coloured wastes such as dyes into flowing waters consequent in damaging the environment as they constitutes toxins to aquatic life. The difficulties experienced in removing toxic metals and dyes from industrial wastewaters have led to the use of different adsorbents by researchers (Mahir et al. 2004).
The panacea to rescue polluted environment from these harmful substances led the utilisation of some conventional techniques such as ion exchange, chemical co-precipitation, electrochemical precipitation, reverse osmosis, solvent extraction, solid phase extraction, evaporation, membrane filtration and adsorption (for toxic metals) (Say et al. 2008) and sonochemical degradation (Minero et al. 2005), chemical oxidation, flocculation, photocatalytic decomposition (Wu and Chern 2006) and electro-catalytic degradation (for dyes) (Ma et al. 2009).
Over the years, researchers have used various biosorbents and materials in sequestering toxic metals and dyes from aqueous solutions. These include Nauclea diderrichii seed biomass waste (Omorogie et al. 2012), Zea mays cob, sugarcane bagasse (Sharma and Forster 1994), Japanese cedars (Aoyama et al. 2005), Thuja orientalis (Oguz 2005), reed (Rawajfih and Nsour 2008), rice bran (Singh et al. 2005), wheat bran (Dupont and Guillon 2003), soya cake (Daneshvar et al. 2002), coconut husk (Tan et al. 1993), rice husk (Krishnani et al. 2008), walnut hull (Wang et al. 2009), Fomes fomentarius, Phellinus igniarius (Maurya et al. 2006), Hydrilla verticillata (Low et al. 1993), algae Gelidium (Vilar et al. 2007), Ulva lactuca (El Sikaily et al. 2006), Posidonia oceania, Enteromorpha spp. (Ncibi et al. 2009), Aspergillus niger (Fu and Viraraghavan 2000), Caulerpa lentillifera (Marungrueng and Pavasant 2007), waste red mud (Namasivayam and Arasi 1997), waste orange peel (Namasivayam et al. 1996), cellulose/magnetite/activated carbon (Zhu et al. 2011), Barbula lambarenensis (Olu-Owolabi et al. 2012), Zea mays seed chaff (Babalola et al. 2016a), mesoporous SiO2/graphene oxide, TiO2 and MnO2 nanoparticles-modified Nauclea diderrichii biomass waste (Omorogie et al. 2014a, b, 2015, 2016a), Cedrela odorata (Babalola et al. 2016b), among others.
Parkia biglobosa paste is a local food seasoning agent known as ‘dawadawa’ in Ghana, ‘iru’ in Nigeria and Benin Republic, and ‘soumbala’ in Burkina Faso. It is obtained from fermented dried seeds of the African locust bean (Parkia biglobosa). This plant is a perennial leguminous tree which belongs to the family and sub family Mimosoideae and Leguminosae respectively. It grows in the savannah region of West Africa (Campbell-Platt 1980). The tree is not normally cultivated, but can be seen in population of two or more in the savannah region of West Africa (Gupta et al. 1992). The Parkia tree plays a vital ecological role in cycling of nutrients from deep soils, by holding the soil particles to prevent soil erosion with the aid of its roots. It also provides shade where it is found (Campbell-Platt 1980; Gupta et al. 1992; Irvine 1961; Hagos 1962).
As local food seasoning agent, it serves as a nutritive non-meat protein and flavouring agent for local soups (Poopal and Laxman 2009) in Nigeria, Northern Ghana and some other West African countries. Parkia biglobosa paste has a good nutritional and medicinal value that reduces high blood pressure. Parkia biglobosa has a wide distribution ranging across nineteen African countries including Nigeria, Sudan, Senegal, Gambia, Guinea Bissau, Guinea, Sierra Leone, Mali, Côte d’Ivoire, Burkina Faso, Ghana, Togo, Benin, Niger, Cameroon, Chad, Central African Republic, Zaire, Sudan and Uganda (Hall et al. 1991). Parkia biglobosa tree also plays a vital economic role in recycling nutrients from the soil. Parkia biglobosa seed that is grown into the tree plant has an outer covering that is thrown away, hence, constituting environmental pollution problem. This Parkia biglobosa seed waste (PSW) was collected from one of its growing sites in Ibadan (7°23′16″N, 3°53′47″E), Nigeria, West Africa. The cellulose extracted from Parkia biglobosa was used for this research.
Various biological materials comprise lignin and cellulose as their major components and possess polar functional groups such as alcohols, aldehydes, ketones, carboxylic acids, phenolics, sulphydryls aminos, amines, thiols and ethers. That have the ability to bind to heavy metals by donation of a lone pair of electrons to form complexes with the metal ions in solution (Pagnanelli et al. 2003). Celluloses have been known to be good adsorbents for the removal of toxic cations and ionic dyes (Pagnanelli et al. 2003). Therefore, this work evaluates the abstractive potential of PSW and PBCE for toxic cations and ionic dyes.
Experimental
Preparation of PSW and PBCE
Parkia biglobosa seeds were obtained from the Takie market in Ogo-Oluwa Local Government Area of Ogbomoso (8°08′N, 4°15′E), Southwest Nigeria. They were stored in a cool dry place till the time of usage. The leaves and fruits of the plant specimen were taken to the Herbarium section of the Department of Botany, University of Ibadan (7°23′16″N, 3°53′47″E), Nigeria for botanical identification. Fifty grammes of the peel waste was removed from the outer coverings of the seeds. It was dried under the sun for few days and pulverised.
Cellulose powder was extracted from the dried peel waste of Parka biglobosa by a process of extraction using toluene-ethanol (2:1 v/v) for 6 h to remove the waxy components. Thereafter, this peel waste was hydrolysed using 0.5% sulphuric acid at 100 °C for 2 h. The hydrolysed peel waste was washed with water and filtered. The resulting pulp was treated with solutions containing NaOH and Na2S (21:7 w/w) at a temperature of about 100 °C in 1 L and then washed with de-ionized water by filtration until it became colourless. The white cellulose extract obtained was washed with de-ionized water and filtered, until the pH of the filtrate was 7.0. The cellulose extract obtained from PSW was then air dried and ground to powder (particle size between 300 and 450 µm). It was then stored in a sealed glass container and kept for experimental use. The cellulose obtained was dried in an oven and weighed. The percentage yield of the PBCE was calculated using:
Chemical reagents
All chemical reagents were of analytical grade. Deionized water, Pb(NO3)2, CrO3, methylene blue (MB) and congo red (CR). Accurately weighed amounts of toxic metals and ionic dyes were used in preparing aqueous solutions of 1000 mg/L Cr6+, Pb2+, MB and CR. These solutions were diluted to different working concentrations when needed.
Physicochemical characterisations of PSW and PBCE
Fourier transform infra red (FTIR) was used in determining the functional groups present in loaded and unloaded biomass of PSW and PBCE. FTIR spectra of unloaded biomass and metal loaded biomass were recorded at 400–4000 cm−1 using FTIR Spectrophotometer JASCO-430 Model.
Proximate analysis was carried out on PSW and PBCE according to the published protocol of the Association of Official Analytical Chemist, (AOAC 1990). This constitutes the classes of food present in samples such as carbohydrate, crude fibre, crude protein, crude fat, ash content and moisture content. The particle size of the PSW and PBCE used was between 300 and 450 µm. The PSW and PBCE were further characterised by scanning electron microscopy (SEM) using the JEOL JSM-6390 LV Model, available at the Nigerian Liquified Natural Gas (NLNG) Multi-user Laboratories, Faculty of Engineering, Ahmadu Bello University, Zaria, Nigeria.
Optimization of experimental variables
Initial solution pH Study
For initial solution pH study, 10 mg of PSW and PBCE was contacted with 25 mL of 150 mg L−1 Cr6+, Pb2+, MB and CR solutions in 60 mL plastic bottles (high-density polyethylene) at agitation speed of 200 rpm using thermostatic water bath (Haake Wia Model) at temperature of 298 K. The pHs of the toxic metals and dye solutions were adjusted by pH meter Jenway 3520 Model from 3.0–7.0 and 2.0–12.0 using 0.1 M HCl or NaOH respectively. The clear supernatants of toxic metals and dyes were obtained by centrifugation using Hitachi high-speed refridgerated centrifuge. The residual concentrations of Cr6+ and Pb2+ in the aqueous solutions were analysed using atomic absorption spectrophotometer (AAS) Buck 205 Model. The residual concentrations of MB and CR were analysed by UV/Vis Spectrophotometer Jenway 6505 Model at maximum wavelength of absorption, λ max = 606 nm for MB and λ max = 518 nm for CR respectively. The optimum pH values obtained from pH study were used for further studies.
Equilibrium, kinetic and thermodynamics studies
Equilibrium, thermodynamic and temperature studies were carried out in batch mode, with initial concentrations of 150–1000 mg/L of 25 Ml Cr6+, Pb2+, MB and CR solutions in 60 mL high-density polyethylene bottles contacted with 10 mg of PSW and PBCE at optimum pHs obtained from pH study at 298–328 K respectively, agitation speed of 200 rpm using thermostatic water bath (Haake Wia Model).
Kinetic and contact time studies were also carried out by contacting 10 mg of PSW and PBCE with 25 mL Cr6+, Pb2+, MB and CR solutions of initial concentration of 150 mg/L in 60 mL high-density polyethylene bottles at various time intervals of 1–120 min respectively, at optimum pHs, agitation speed of 200 rpm using thermostatic water bath (Haake Wia Model) at 298 K. The amounts of Cr6+, Pb2+, MB and CR adsorbed by PSW and PBCE were calculated using:
where \(C_{\text{i}}\) is the initial concentration of metal ion (mg/L), \(C_{e}\) is the equilibrium concentration of residual metal ion in the solution (mg/L), \(V\) is the volume of the aqueous solution containing metal ions (\(L\)), \(W\) is the weight of adsorbent (g) and \(q_{e}\) is the amount of metal ion adsorbed by the adsorbent (mg/g).
Effects of biomass dose were carried out by contacting 10–200 mg of PSW and PBCE with 25 mL of 150 mg L−1 Cr6+, Pb2+, MB and CR solutions in 60 mL high-density polyethylene bottles at optimum pHs, agitation speed of 200 rpm using thermostatic water bath (Haake Wia Model) at 298 K.
Experimental data obtained from equilibrium, kinetic and thermodynamic studies were modelled using Freundlich model (Freundlich 1906), Langmuir model (Langmuir 1916), Dubinin-Radushkevich model (Dubinin 1960), pseudo-second order (Ho and McKay 1999), pseudo-first order (Lagergren 1898), Weber–Morris intraparticle diffusion (Weber and Morris 1963) models and Van’t Hoff equation (Ho et al. 2000) respectively, whose linear forms are mathematically written as;
where \(E\; = \;2\beta^{{{{ - 1} \mathord{\left/ {\vphantom {{ - 1} {}}} \right. \kern-0pt} {}}2}}\) and \(\varepsilon^{2} = \;\left\{ {RT\;\ln \;\left( {1{ + }\;{1 \mathord{\left/ {\vphantom {1 {C_{e} }}} \right. \kern-0pt} {C_{e} }}} \right)} \right\}^{2}\)
where \(k_{f}\) is the Freundlich constant (L/mg)1/n (mg/g), \({1 \mathord{\left/ {\vphantom {1 n}} \right. \kern-0pt} n}\) is the empirical constant for adsorption affinity, \(K_{L}\) is the Langmuir adsorption constant (L/mg), \(q_{{\max_{L} }}\) is the \(q_{e}\) for complete monolayer (mg/g), \(q_{m}\) is the Dubinin-Radushkevich theoretical monolayer saturation capacity (mg/g), \(\beta\) is the Dubinin-Radushkevich constant, \(k_{1}\) is the pseudo-first order rate constant (/min), \(k_{2}\) is the pseudo-second order rate constant (g/mg min), \(h_{ads}\) is the initial sorption rate (mg/g min), \(R\) is the universal gas constant (J/mol K), \(T\) is the, absolute temperature (K), \(q_{t}\) is the amount of metal ion adsorbed at time \(t\)(min) by the biosorbents (mg/g), \(K_{ip}\) is the Weber–Morris intraparticle diffusion constant (mg/g min1/2), \(\mathchar'26\mkern-10mu\lambda\) is the boundary layer thickness, \(\Delta H^{^\circ }\) is the enthalpy of the adsorption process (kJ/mol), \(\Delta S^{^\circ }\) is the entropy of the adsorption process (J/mol K), \(\Delta G^{^\circ }\) is the Gibb’s free energy of the adsorption process (kJ/mol), \(\varepsilon\) is the Polanyi potential and \(E\) is the adsorption energy (kJ/mol).
Results and discussion
Physicochemical analyses of PSW and PBCE
Table 1 showed that the result from proximate analysis represented the percentages of crude protein, crude fat, crude fibre, ash, moisture content and carbohydrate to be similar for PSW and PBCE respectively.
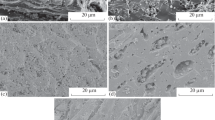
Table 2 shows the FTIR vibration signals for PSW and PBCE respectively. For PSW, the free –OH vibration stretch, –N–H vibration stretch of amines, –C–H vibration stretch of CH2 and CH3 of hemicelluloses, –S–H vibration stretch of sulphydryl, –C=O vibration stretch of ketones, lactones and ethers, –C–O vibration stretch of ketones, lactones and ethers, and –C–O vibration bend of ketones, lactones and ethers appeared at vibration frequencies of 3774, 3445, 2923, 2368, 1632, 1385 and 1037 cm−1. For PBCE, the –N–H vibration stretch of amines, –S–H vibration stretch of sulphydryl, –C=O vibration stretch of ketones, lactones and ethers, –C–O vibration stretch of ketones, lactones and ethers, and –C–O vibration bend of ketones, lactones and ethers appeared at vibration frequencies of 3434, 2357, 1632, 1366 and 1034 cm−1. The free –OH and –C–H vibration stretches of CH3 and CH2 disappeared in PBCE, which resulted from the removal of hemicelluloses and crude fat during the preparation of PBCE (Babalola et al. 2016b, c). Furthermore, Fig. 1a and b show the SEM images of PSW and PBCE. Figure 1a depicts a rough interwoven network of folded and flaky bioparticles, irregular and scattered on the biosorbent surface, while Fig. 1b represents a smooth surface, though cracked, less interwoven, less irregular and less scattered with bioparticles that look non flaky on the surface. The surface morphology of PSW might have accounted for the very high uptake of MB (\(q_{{\max_{L} }}\) = 1498.42 mg/g) over that obtained by PBCE, which was (\(q_{{\max_{L} }}\) = 403.23 mg/g) (Kumar et al. 2010; Babalola et al. 2016c).
Effect of initial solution pH Study
Solution pH is known to affect the degree of dissociation of various functional groups on biomass surface, the speciation and solubility of ions in aqueous media. Also, the solution pH of an adsorptive system exerts sharp influence on the sequestration of metals and dyes, presumably due to its effect on the surface properties of the biomass and ionisation/dissociation of the adsorbate (Deng et al. 2007; Kumar et al. 2010; Omorogie et al. 2016b; Lian et al. 2013).
The solution pH of maximum adsorption of Cr(VI), Pb(II), MB, CR by PSW and PBCE were 3.0, 6.0, 12.0, 2.0 and 3.0, 7.0, 12.0, 2.0 respectively (Fig. 2 a and b). The maximum uptake of Cr(VI) at pH value of 3.0 was the fact that from pH range 2.0–7.0, the dominant forms of Cr(VI) were Cr2O7 2− and HCrO4 − ions, and CrO4 2− at lower pH values prevailed in aqueous solution. At pH 3.0, the PSW and PBCE get more protonated, thereby reducing competition and more Cr(VI) bind to their surfaces by electrostatic attraction (Li and Bowman 2001). Furthermore, the presence of Cr2O7 2− and HCrO4 − species in solution at this pH range also enhance the greater uptake Cr(VI).
The phenomenon of complexation could take place on PSW and PBCE surfaces, especially for the carboxyl group (–C=O) or the hydroxyl group (–OH) of these biosorbents. It is understood that some functional groups probably took part in the removal of Cr(VI), Pb(II), MB and CR due to the nature of the active sites on PSW and PBCE.
At pH > 6.0, precipitation is the main mechanism of Pb(II) ion removal and this can be attributed to the formation of Pb(OH)2 precipitate. Also, the presence of the hydroxylated species at (pH ≤ 7.0) impeded further uptake of Pb(II) by PSW and PBCE (García-Rosales and Colín-Cruz 2010). This was responsible for the maximum uptake of Pb(II) ion by PSW and PBCE at pH < 7.0.
The maximum uptake of MB by PSW and PBCE at pH 12.0 was due to the fact that H+ competed less with MB for PSW and PBCE surfaces. Reverse is the case at acidic pH, the PSW and PBCE were protonated and less MB were adsorbed onto PSW and PBCE by electrostatic attraction respectively. The high removal of MB onto PSW and PBCE was due to the low proton densities on the adsorbent surfaces at pH 12.0 (Babalola et al. 2016c).
The maximum uptake of CR by PSW and PBCE was achieved at pH 2.0. The largest amounts of CR adsorbed by PSW and PBCE were expected due to an increase in the proton densities of PSW and PBCE at pH 2.0. This made the anionic CR molecules to bind easily to their surfaces (Unuabonah et al. 2007; Babalola et al. 2016c).
Effect of adsorbent dose
The amount \(q_{e}\)(mg/g) of Cr(VI), Pb(II), MB, CR adsorbed by PSW and PBCE decreased from 118.86–15.25, 355.68–18.85, 156.97–6.970, 275.00–5.42 and 149.98–16.20, 358.00–18.74, 145.83–9.78, 270.63–10.19 respectively with increase in adsorbent dose from 10 to 200 mg. This is due to decrease in specific surface area and increase in diffusion path length resulting from agglomeration or aggregation of the adsorbent particles as the dose increased (Unuabonah et al. 2007) (see Fig. 3a, b).
Effect of contact time
The amount \(q_{t}\)(mg/g) of Cr(VI), Pb(II), MB, CR adsorbed by PSW and PBCE increased from 25.5–117.15, 31.7–356.63, 7.75–163.05, 3.75–275.33 and 5.43–125.00, 46.50-359.20, 2.50-135.55, 8.15-266.25 respectively as time \(t\)(min) also increased from 1 to 120. This is attributed to the fact that the adsorption processes were fast as sorbate ions (Cr(VI), Pb(II), MB and CR) diffused from the bulk aqueous solution to the unoccupied sites on the exterior surface of PSW and PBCE. Thereafter, these active sites on PSW and PBCE exterior surfaces got filled and become saturated as the sorbate ions diffused their interior pores comprising cationic functional groups as contact time increased from 1 to 120 min (Wang and Yan 2011). Figures 4 a and b show the plots of the amount of Cr(VI), Pb(II), MB and CR adsorbed, \(q_{t}\)(mg/g) by PSW and PBCE against time \(t\)(min) respectively.
Adsorption equilibrium, kinetics and thermodynamics
Adsorption isotherm models play cogent role in determining the maximum uptake capacity of adsorbent with respect to its effectiveness, efficiency and economic viability (Unuabonah et al. 2007; Omorogie et al. 2016b). Optimisation design for the adsorption process involves the maximum performance evaluation of the sorbents for sequestering Cr(VI), Pb(II), MB and CR in this research. Various equilibrium models like Freundlich, Langmuir and Dubinin-Radushkevich isotherms were used to analyse the equilibrium data obtained from the adsorption of Cr(VI), Pb(II), MB and CR by PSW and PBCE respectively. It was observed that Langmuir isotherm best fitted the equilibrium data.
Table 3 shows a list of various adsorbents and their adsorption capacities (mg/g) in literature for Cr(VI), Pb(II), MB and CR.
For PSW, the saturated monolayer capacities, \(q_{{\max_{L} }}\), for the adsorption of Cr(VI), Pb(II), MB and CR were 196.46–208.33 mg/g, 111.36–325.73 mg/g, 83.86–1498.42 mg/g and 120.05–266.67 mg/g as temperature increased from 298 to 328 K respectively (see Table 4).
For PBCE, the saturated monolayer capacities \(q_{{\max_{L} }}\), for the adsorption of Cr(VI), Pb(II), MB and CR were 187.27–194.93 mg/g, 118.62–332.23 mg/g, 318.47–403.23 mg/g and 146.63–288.18 mg/g as temperature increased from 298 to 328 K respectively (see Table 5).
The increase in the adsorption of Cr(VI), Pb(II), MB and CR by PSW and PBCE could be attributed to increase in the rate of reaction and collision of reacting particles as temperature increased. It also indicates that the mass transfer and diffusion of sorbate from the bulk solution onto the active sites in the biosorbent was faster at higher temperatures (Lian et al. 2013).
The values of 8 ≥ E > 16 kJ/mol for the sequestration of Cr(VI) and Pb(II) by PSW indicated that these processes were chemisorptive at all temperatures. The values of E < 8 kJ/mol at 313 K (physisorption), and E > 8 kJ/mol at 298 and 328 K (chemisorption) for the removal of MB by PSW. Also, the values of E < 8 kJ/mol obtained for the sequestration of CR by PSW showed physisorption at all temperatures (see Table 4).
The values of 8 ≥ E < 16 kJ/mol for the removal of Cr(VI) by PBCE signified chemisorption. It is also interesting to note that the removal of Pb(II) by PBCE had the values of E < 8 kJ/mol, which indicated that the adsorption processes were physisorptive at all temperatures. The removal of MB and CR by PBCE had the values of 8 ≥ E < 16 kJ/mol, which were indicative of chemisorption (see Table 5).
In order to understand the controlling mechanism(s), mass transfer, intraparticle diffusion and dynamics of the adsorption process, kinetic data were fitted into pseudo-first order, pseudo-second order and Morris–Weber intraparticle diffusion kinetic models. Pseudo-second order kinetic model best fits the kinetic data (see Tables 6, 7). The pseudo-second order rate constants \(k_{2}\) for the adsorption of Cr(VI), Pb(II), MB, CR by PSW and PBCE were 5.81E−4, 3.17E−4, 1.57E−4, 0.10E−4 and 1.52E−2, 2.05E−4, 9.96E−5 and 3.37E−5 respectively. These values were smaller than those of pseudo-first order rate constants \(k_{1}\).
The Morris–Weber intraparticle diffusion kinetic model for the adsorption of Cr(VI), Pb(II), MB, CR by PSW and PBCE showed that the values of the constant \(\mathchar'26\mkern-10mu\lambda\), which were the thickness of the boundary layer were >0, and hence did not pass through the origin. This implied that other diffusion processes like pore diffusion and film diffusion, apart from intraparticle diffusion took part in the adsorption process (Babalola et al. 2016a).
Thermodynamic studies indicated that all the values of Gibb’s free energy change (\(\Delta G^{o}\)) for the adsorption of Cr(VI), Pb(II), MB and CR by PSW were positive. This suggested the non feasibility and non spontaneity of the system. The values of enthalpy change (\(\Delta H^{o}\)) for the adsorption of Pb(II), MB and CR by PSW were negative, indicating the exothermic nature of the system. But, the value of enthalpy change (\(\Delta H^{o}\)) for the adsorption of Cr(VI) by PSW was positive, indicating the endothermic nature the system. The values of entropy change (\(\Delta S^{o}\)) for the adsorption of Cr(VI), MB and CR by PSW were negative, showing decrease in the degree of chaos of the system. This signified decrease in the disorderliness of the sorbent/sorbate interface. Also, the value of entropy change (\(\Delta S^{o}\)) for the adsorption of Pb(II) was positive, showing increase in the degree of chaos of the system. This signified increase in the disorderliness of the sorbent/sorbate interface. Moreover, all the values of \(\Delta G^{o}\) for the adsorption of Cr(VI), Pb(II), MB and CR by PBCE were also positive, showing the non feasibility and non spontaneity of the system. The values of \(\Delta H^{o}\) for the adsorption of Pb(II), MB and CR by PBCE were positive, indicating that the system was endothermic. But, the value of \(\Delta H^{o}\) for the adsorption of Cr(VI) by PBCE was negative, showing that the system was exothermic. The values of \(\Delta S^{o}\) for the adsorption of Pb(II), MB and CR by PBCE were positive, showing increase in the degree of chaos of the system. But, the value of \(\Delta S^{o}\) for the adsorption of Cr(VI) by PBCE was negative, indicating decrease in the degree of chaos of the system (see Tables 8, 9). Figures 5a–d and 6a–d show the plots of the amount of Cr(VI), Pb(II), MB and CR adsorbed, \(q_{e}\)(mg/g) by PSW and PBCE against equilibrium concentration, \(C_{e}\) (mg/L) at different temperatures respectively.
The plots of the amount of a Cr(VI) adsorbed, \(q_{e}\)(mg/g) by PSW against equilibrium concentration, \(C_{e}\)(mg/L) different temperatures, b Pb(II) adsorbed, \(q_{e}\)(mg/g) by PSW against equilibrium concentration, \(C_{e}\)(mg/L) different temperatures, c MB adsorbed, \(q_{e}\)(mg/g) by PSW against equilibrium concentration, \(C_{e}\)(mg/L) different temperatures and d CR adsorbed, \(q_{e}\)(mg/g) by PSW against equilibrium concentration, \(C_{e}\)(mg/L) at different temperatures
The plots of the amount of a Cr(VI) adsorbed, \(q_{e}\)(mg/g) by PBCE against equilibrium concentration, \(C_{e}\)(mg/L) different temperatures, b Pb(II) adsorbed, \(q_{e}\)(mg/g) by PBCE against equilibrium concentration,\(C_{e}\)(mg/L) different temperatures, c MB adsorbed, \(q_{e}\)(mg/g) by PBCE against equilibrium concentration, \(C_{e}\)(mg/L) different temperatures and d CR adsorbed, \(q_{e}\)(mg/g) by PBCE against equilibrium concentration, \(C_{e}\)(mg/L) different temperatures
Conclusion
This work explored the adsorptive capacities of PSW and PBCE for the removal of Cr(VI), Pb(II), MB and CR from aqua system. Physicochemical analyses, equilibrium, kinetic and thermodynamic data established that PSW and PBCE biosorbents showed viable performance for futuristic applications in the treatment of polluted and industrial wastewaters.
References
Al-Degs YS, El-Barghouthi MI, Khraisheh MA, Ahmad MN, Allen SJ (2004) Effect of surface area, micropores, secondary micropores and mesopores volumes of activated carbons on reactive dyes adsorption from solution. Sep Sci Technol 39:97–111
Aoyama M, Kishino M, Jo T-S (2005) Adsorption of Cr(VI) on Japanese cedar bark. Sep Sci Technol 39:1149–1162
Babalola JO, Omorogie MO, Babarinde AA, Unuabonah EI, Oninla VO (2016a) Optimization of the biosorption of Cr3+, Cd2+ and Pb2+ using new biowaste: Zea mays seed chaff. Environ Eng Manag J 15:1571–1580
Babalola JO, Koiki BA, Eniayewu Y, Salimonu A, Olowoyo JO, Oninla VO, Alabi HA, Ofomaja AE, Omorogie MO (2016b) Adsorption efficacy of Cedrela odorata seed waste for dyes: non linear fractal kinetics and non linear equilibrium studies. J Environ Chem Eng 4(3):3527–3536
Babalola JO, Olowoyo JO, Durojaiye AO, Olatunde AM, Unuabonah EI, Omorogie MO (2016c) Understanding the removal and regeneration potentials of biogenic wastes for toxic metals and organic dyes. J Taiwan Inst Chem Eng 58:490–499
Barnhart J (1997) Occurrences, uses, and properties of chromium. Regul Toxicol Pharmacol 26(1):S3–S7
Campbell-Platt G (1980) Africa locust bean (Parkia species) and its West African fermented food product, dawadawa. Eco Food Nutr 9:123–131
Daneshvar N, Salari D, Aber S (2002) Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solutions by soya cake. J Hazard Mater 94:49–61
Deng B, Alan TS (1996) Surface-catalyzed Chromium(VI) reduction: reactivity comparisons of different organic reductants and different oxide surfaces. Environ Sci Technol 30:2484–2494
Deng L, Su Y, Su H, Wang X, Zhu X (2007) Sorption and desorption of Lead(II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater 143:220–225
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem Rev 60:235–266
Dupont L, Guillon E (2003) Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environ Sci Technol 37:4235–4241
El Sikaily A, Khaled A, El Nemr A, Abdelwahab O (2006) Removal of methylene blue from aqueous solution by marine green alga Ulva lactuca. Chem Ecol 22(2):149–157
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Fu Y, Viraraghavan T (2000) Removal of a dye from an aqueous solution by the fungus Aspergillus niger. Water Qual Res J Can 35(1):95–111
García-Rosales G, Colín-Cruz A (2010) Adsorption of lead by maize (Zea mays) stalk sponge. J Environ Manag 91:2079–2086
Gercel O, Gercel HF (2007) Adsorption of lead(II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem Eng J 132:289–297
Gupta GS, Shukla SP, Prasad G, Singh VN (1992) China clay as an adsorbent for dye house wastewater. Environ Technol 13:925–936
Hagos TH (1962) A revision of genius Parkia in Africa. Acta Bot Nearthandica 11:231–265
Hall LS, Krausmaman PR, Morrison ML (1991) The habitat concept and a plea for standard technology. Wildl Soc Bull 25(1):173–182
Ho YS, McKay G (1999) Batch lead(II) removal from aqueous solution by peat: equilibrium and kinetics. Trans Chem B 77:165–173
Ho YS, Ng JCY, Mckay G (2000) Kinetics of pollutant sorption by biosorbents: review. Sep Purif Methods 29:186–232
Irvine FR (1961) Woody plants of Ghana. Oxford University Press, London
Issabayeva G, Aroua MK, Sulaiman NMN (2006) Removal of lead from aqueous solutions on palm shell activated carbon. Bioresour Technol 97:350–355
Johns MM, Marshall WE, Toles CA (1998) Agricultural by-products as granular activated carbons for adsorbing dissolved metals and organics. J Chem Technol Biotechnol 71:131–140
Krishnani KK, Meng X, Christodoulatos C, Boddu VM (2008) Adsorption mechanism of nine different heavy metals onto biomatrix from rice husk. J Hazard Mater 153:1222–1234
Kumar PS, Ramalingam S, Senthamarai C, Niranjanaa M, Vijayalakshmi P, Sivanesan S (2010) Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 261:52–60
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga SVenska Vetenskapsakademiens. Handlingar Band 24:1–39
Langmuir I (1916) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li Z, Bowman RS (2001) Retention of inorganic oxyanions by organo-kaolinite. Water Res 35:3771–3776
Li K, Wang X (2009) Adsorptive removal of Pb(II) by activated carbon prepared from Spartina alterniflora: equilibrium, kinetics and thermodynamics. Bioresour Technol 100:2810–2815
Lian L, Cao X, Wu Y, Lou D, Han D (2013) Synthesis of organo-functionalized magnetic microspheres and application for anionic dye removal. J Taiwan Inst Chem Eng 44:52–60
Low KS, Lee CK, Heng LL (1993) Sorption of basic dyes by Hydrilla verticillata. Environ Technol 14:115–124
Ma H, Zhuo Q, Wang B (2009) Electro-catalytic degradation of methylene blue wastewater assisted by Fe2O3-modified kaolin. Chem Eng J 155:248–253
Mahir A, Özkan D, Mehmet D (2004) Removal of acid yellow49 from aqueous solution by adsorption. Fresenius Environ Bull 13:1112–1121
Mane VS, Mall ID, Srivastava VC (2007) Kinetic and equilibrium isotherm studies for the adsorptive removal of Brilliant Green dye from aqueous solution by rice husk ash. J Environ Manag 84:390–400
Marjanovic V, Lazarevic S, Jankovic-Castvan I, Potkonjak B, Janackovic D, Petrovic R (2011) Chromium (VI) removal from aqueous solutions using mercaptosilane functionalized sepiolites. Chem Eng J 166:198–206
Marungrueng K, Pavasant P (2007) High performance biosorbent (Caulerpa lentillifera) for basic dye removal. Bioresour Technol 98:1567–1572
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Adsorption of dyes using dead macro fungi: effect of dye structure, ionic strength and pH. Bioresour Technol 97:512–521
Minero C, Lucchiari M, Vione D, Maurino V (2005) Fe(III)-enhanced sonochemical degradation of methylene blue in aqueous solution. Environ Sci Technol 39:8936–8942
Mitra P, Sarkar D, Chakrabarti S, Dutta BK (2011) Reduction of hexa-valent chromium with zero-valent iron: batch kinetic studies and rate model. Chem Eng J 171:54–60
Namasivayam C, Arasi DJSE (1997) Removal of congo red from wastewater by adsorption onto waste red mud. Chemosphere 34:401–417
Namasivayam C, Muniasamy N, Gayathri K, Rani M, Ranganathan K (1996) Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour Technol 57:37–43
Ncibi MC, Hamissa AMB, Fathallah A, Kortas MH, Baklouti T, Mahjoub B, Seffen M (2009) Adsorptive uptake of methylene blue using Mediterranean green alga Enteromorpha spp. J Hazard Mater 170:1050–1055
Oguz E (2005) Adsorption characteristics and the kinetics of the Cr(VI) on the Thuja oriantalis. Coll Surf A Physicochem Eng Asp 252:121–128
Olu-Owolabi BI, Diagboya PN, Ebaddan WC (2012) Mechanism of Pb2+ removal from aqueous solution using a nonliving moss biomass. Chem Eng J 195:270–275
Omorogie MO, Babalola JO, Unuabonah EI, Gong JR (2012) Kinetics and thermodynamics of heavy metal ions sequestration onto novel Nauclea diderrichii seed biomass. Bioresour Technol 118:576–579
Omorogie MO, Babalola JO, Unuabonah EI, Gong JR (2014a) Solid phase extraction of hazardous metals from aqua system by nanoparticle-modified agrowaste composite adsorbents. J Environ Chem Eng 2(1):675–684
Omorogie MO, Babalola JO, Unuabonah EI, Gong JR (2014b) Hybrid materials from agrowaste and nanoparticles: implications on the kinetics of the adsorption of inorganic pollutants. Environ Technol 35(5):611–619
Omorogie MO, Babalola JO, Unuabonah EI, Gong JR (2015) New facile benign agrogenic-nanoscale titania material; remediation potential for toxic inorganic cations. J Water Proc Eng 5(1):95–100
Omorogie MO, Babalola JO, Unuabonah EI, Gong JR (2016a) Clean technology approach for the competitive binding of toxic metal ions onto MnO2 nano-bioextractant. Clean Techn Environ Policy 18(1):171–184
Omorogie MO, Babalola JO, Unuabonah EI, Song W, Gong JR (2016b) Efficient chromium abstraction from aqueous solution using a low-cost biosorbent: Nauclea diderrichii seed waste. J Saudi Chem Soc 20:49–57
Owlad M, Aroua MK, Wan Daud WMA (2010) Hexavalent chromium adsorption on impregnated palm shell activated carbon with polyethyleneimine. Bioresour Technol 101:5098–5103
Pagnanelli F, Mainelli S, Veglio F, Toro L (2003) Heavy metal removal by olive pomace: biosorbent characterization and equilibrium modeling. Chem Eng Sci 58:4709–4717
Ponnusami V, Kritika V, Madhuram R, Srivastava SN (2007) Adsorption of reactive dye using acid-treated rice husk: factorial design analysis. J Hazard Mater 142:397–403
Poopal AC, Laxman RS (2009) Studies on biological reduction of chromate by Streptomyces griseus. J Hazard Mater 169:539–545
Rawajfih Z, Nsour N (2008) Thermodynamic analysis of sorption isotherms of chromium(VI) anionic species on reed biomass. J Chem Thermodyn 40:846–851
Richard F, Bourg A (1991) Aqueous geochemistry of chromium: a review. Water Res 25:807–816
Ricordel S, Taha S, Cisse I, Dorange G (2001) Heavy metals removal by adsorption onto peanut husks carbon: characterization, kinetic study and modeling. Sep Purif Technol 24:389–401
Say R, Birlik E, Erdemgil Z, Denizli A, Ersoz A (2008) Removal of mercury species with dithiocarbamate-anchored polymer/organosmectite composites. J Hazard Mater 150:560–564
Sharma D, Forster CF (1994) A preliminary examination into the adsorption of hexavalent chromium using low-cost adsorbents. Bioresour Technol 47:257–264
Singh KK, Rastogi R, Hasan SH (2005) Removal of Cr(VI) from wastewater using rice bran. J Colloid Interface Sci 290:61–68
Sulaymon AH, Abid BA, Al-Najar JA (2009) Removal of lead, copper, chromium and cobalt onto granular activated carbon in batch and fixed-bed adsorbers. Chem Eng J 155:647–653
Tan WT, Ooi ST, Lee CK (1993) Removal of chromium (VI) from solution by coconut husk and palm pressed fibres. Environ Technol 14:277–282
Unuabonah EI, Olu-Owolabi BI, Adebowale KO, Ofomaja AE (2007) Adsorption of lead and cadmium ions from aqueous solutions by tripolyphosphate-impregnated Kaolinite clay. Coll Surf A Physicochem Eng Asp 292:202–211
Vilar VJP, Botelho CMS, Boaventura RAR (2007) Methylene blue adsorption by algal biomass based materials: biosorbents characterization and process behaviour. J Hazard Mater 147:120–132
Wang L-G, Yan G-B (2011) Adsorptive removal of direct yellow 161dye from aqueous solution using bamboo charcoals activated with different chemicals. Desalination 274:81–90
Wang S, Boyjoo Y, Choueib A, Zhu H (2005) Removal of dyes from solution using fly ash and red mud. Water Res 39:129–138
Wang XS, Li ZZ, Tao SR (2009) Removal of chromium (VI) from aqueous solution using walnut hull. J Environ Manag 90:721–729
Weber WJ, Morris CJ (1962) Removal of biological resistant pollutants from wastewater by adsorption. Pergamon Press, New York
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng ASCE 89:31–59
Wong YC, Szeto YS, Cheung WH, McKay G (2003) Equilibrium studies for acid dye adsorption onto chitosan. Langmuir 19:7888–7894
Wu C-H, Chern J-M (2006) Kinetics of photocatalytic decomposition of methylene blue. Ind Eng Chem Res 45:6450–6457
Zhu H-Y, Fu Y-Q, Jiang R, Jiang J-H, Xiao L, Zeng G-M, Zhao S-L, Wang Y (2011) Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: equilibrium, kinetic and thermodynamic studies. Chem Eng J 173:494–502
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Babalola, J.O., Bamidele, T.M., Adeniji, E.A. et al. Adsorptive modelling of toxic cations and ionic dyes onto cellulosic extract. Model. Earth Syst. Environ. 2, 1–15 (2016). https://doi.org/10.1007/s40808-016-0246-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-016-0246-z