Abstract
Introduction
The association between omeprazole and hypertension is poorly documented. The summary of product characteristics of omeprazole approved by major regulators did not mention hypertension as an adverse drug event. Triggered by a locally reported case, this study was conducted to assess the possible causal relationship between omeprazole and hypertension.
Methods
Globally reported cases of hypertension following use of omeprazole submitted to the World Health Organization global database, VigiBase, were retrieved on 5 March 2024 and analyzed descriptively. Besides this, a literature search was made to identify preclinical, clinical, and epidemiological information on the association between omeprazole and hypertension or increased blood pressure using different data sources. Relevant information, gathered from different data sources, was finally systematically organized into an Austin Bradford-Hill causality assessment framework to assess the causal relationship between omeprazole and hypertension.
Results
VigiBase indicated a total of 1043 cases of hypertension related to omeprazole from 36 different countries. In the global database, a statistical signal was triggered (IC025: 0.12) on association of omeprazole and hypertension. From the 1043 cases, 65.0% and 10.6% were reported as ‘serious’ and ‘fatal’, respectively. Hypertension resolved following withdrawal of omeprazole in 85 cases and recurred after re-introduction of the suspect drug in 14 cases. In 225 cases, omeprazole was the only suspected drug, while in 122 cases, omeprazole was the sole drug administered. When only these 122 cases were considered, 29 cases had positive dechallenge, four cases were with positive rechallenge and the median time-to-onset was 2 days. Literature search identified a possible biological mechanism and some experimental evidence that indicates omeprazole could possibly cause hypertension.
Conclusion
Currently available totality of evidence suggests there is a possible causal relationship between omeprazole and hypertension. Hence, it is recommended to monitor and report any incidence of hypertension related to omeprazole, and further epidemiological studies are recommended to corroborate the suggested causal association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Omeprazole is not known to cause hypertension. |
The possible causal relationship of omeprazole and hypertension was assessed by analyzing the WHO global database of individual case safety reports and publications. |
The available totality of evidence suggests there is a possible causal relationship between omeprazole and hypertension that warrants monitoring and reporting any incidence of hypertension related to omeprazole. |
1 Introduction
Drug-induced hypertension represents an important and modifiable cause of secondary hypertension. Many drugs are known to cause elevated blood pressure or hypertension [1]. Nevertheless, omeprazole’s effect on blood pressure is poorly documented. Except in the prescribing information of the innovator company of omeprazole approved by the United States Food and Drug Administration (FDA) [2], which reported elevated blood pressure as a post-marketing experience, the summary of product characteristics of omeprazole approved by major regulators does not mention elevated blood pressure and/or hypertension as an adverse drug event [3,4,5].
Several studies explored the association between proton pump inhibitors (PPIs), including omeprazole, and the risks of cardiovascular disorders (CVDs) [6,7,8,9,10,11,12,13]. These studies reported that the use of PPIs has been associated with increased risks of ischemic stroke and myocardial infarction. None of these studies, however, explicitly considered hypertension as an outcome measure, except in one clinical trial in which dexlansoprazole is reported to be associated with hypertension, especially at higher doses [3]. In contrast, in a large randomized COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial) study, omeprazole did not increase the risk of cardiovascular events in patients with prior cardiovascular diseases [14]. Furthermore, in a review study evaluating the safety of omeprazole, which comprised 72 publications from 1995 to 2018, hypertension was not reported as a possible side effect of omeprazole [15]. To the best of the authors’ knowledge, there is, nevertheless, a paucity of studies related to omeprazole and hypertension.
The Eritrean Pharmacovigilance Centre received a reported case of a 35-year-old female non-hypertensive patient who experienced palpitations [pulse rate: 98 beats per minute (bpm)], increased blood pressure of 160/100 mmHg, nausea, and generalized weakness 3 days following the initiation of an omeprazole 20 mg twice-daily regimen that was prescribed for peptic ulcer disease. At the time of reporting, the increased blood pressure could not be resolved even with the withdrawal of omeprazole. Triggered by this report, this study was conducted to assess the causal relationship between omeprazole and hypertension by analyzing the World Health Organization (WHO) global pharmacovigilance database and the scientific/medical literature.
2 Methods
2.1 Study Design and Data Source
A retrospective descriptive analysis of cases of hypertension associated with omeprazole reported to the WHO global database, VigiBase, and in the medical literature was carried out. VigiBase, the world’s largest pharmacovigilance database with over 37 million individual case safety reports (as of March 2024) was used as the main data source for this study [16]. The global database, which has been collecting data since 1968, currently pools safety data from 150+ countries. In addition, published articles that associated omeprazole with hypertension were searched in the medical literature and globally available databases.
2.2 Exposure and Outcome Definition
The primary exposure of interest was omeprazole or omeprazole-containing products used in all dosage forms, regardless of their dose, frequency, and duration. The primary outcome measure for this study was a reported diagnosis of hypertension following use of omeprazole or omeprazole-containing products.
2.3 Data-Mining Approach
Data were retrieved from VigiBase using an analysis tool called VigiLyze, which is regularly maintained by the Uppsala Monitoring Centre on behalf of the WHO [16]. To identify all globally reported cases of hypertension associated with omeprazole, a search was made on 5 March 2024, using ‘omeprazole’ as an active ingredient or drug substance and ‘hypertension’ as a MedDRA (Medical Dictionary for Regulatory Activities) reaction term (preferred term). Possible duplicates were eliminated using VigiMatch, a tool developed by the Uppsala Monitoring Centre to remove duplicate safety reports, which takes into account reported drugs, ADRs, patient age and sex, reaction onset, and country of origin. In order to achieve this, the database was set to ‘de-duplicate’, which automatically removes all suspected/potential duplicates from the analysis. The same search criteria were applied for both qualitative and quantitative data analysis in VigiLyze. The data mining was done by two authors, MB and MR, who have experience in data mining from VigiLyze.
2.4 Literature Search and Labeling
In an effort to explore the association between omeprazole and hypertension or increased blood pressure, literature searches were carried out in PubMed, Google Scholar, the Martindale adverse drug reactions checker [17], and SIDER side effect resources [18]. The search terms used were ‘omeprazole’ as a drug substance and ‘hypertension’ or ‘increased blood pressure’ as reaction terms. To check whether the causal association between hypertension and omeprazole is documented (labeledness), we also referred to the summary of product characteristics and/or prescribing information of omeprazole approved by major regulators such as the European Medicines Agency (EMA), the UK’s Medicines and Healthcare Products Agency, and the FDA [2,3,4,5].
2.5 Data Analysis
Retrieved data from VigiBase was exported to Excel spreadsheet 2019 for descriptive analysis. Reaction outcomes, seriousness, reason for seriousness, time-to-onset of hypertension, dechallenge and rechallenge information, patients’ demographic characteristics, dose, and other relevant information were explored from the retrieved dataset. Patients with a history of previous hypertension were excluded from the analysis, as they had already developed the outcome of interest. To explore the strength of the causal association, a two-stage subgroup analysis was also carried out. In the first stage of the subgroup analysis, only cases with a single suspect (i.e., omeprazole) were included, meaning that, cases in which omeprazole was the only suspected drug (as reported) as a possible cause of hypertension, regardless of whether any concomitant medications were taken or just omeprazole, were retrieved. Cases of hypertension suspected to be caused by the sole administration of omeprazole were further retrieved in the second stage of the subgroup analysis. In both stages of the subgroup analyses, cases with positive dechallenge and rechallenge, the number of reporting countries, sex, median age, dose, outcome, and time-to-onset of hypertension were researched.
2.6 Causality Assessment
Relevant information gathered from different data sources was systematically organized using the Austin Bradford-Hill Criteria as a causality assessment framework in aggregate form to assess the causal relationship between omeprazole and hypertension [18]. Using the Austin Bradford-Hill criteria, the strength of the association, consistency and specificity of the association, temporal relationship, experimental evidence, biological plausibility, dose-response relationship, coherence, and analogy of the association were assessed. Finally, evidence that supports the causal relationship and, on the other hand, information that supports an alternative explanation were critically reviewed.
3 Results
3.1 Reports in the WHO Global Pharmacovigilance Database, VigiBase
A total of 1,043 de-duplicated cases of hypertension associated with omeprazole were found and retrieved from VigiBase. The cases were reported between 1990 and 5 March 2024 from 36 countries across six continents with the top ten reporting countries and number of reports as follows: USA (879), France (25), UK (23), Germany (18), Mexico (12), the Netherlands (11), Australia (10), Spain (10), Canada (5), and New Zealand (5).
The majority of the cases were females and ≥ 45 years of age. Most of the cases (65.0%) were reported as ‘serious’, and the outcome was marked as ‘fatal’ in 10.6% (n = 109). Overall, from the 1043 cases, there were 85 cases with positive dechallenge and 14 cases with positive rechallenge. The information component (IC) value (IC025) was 0.03. A summary of the characteristics of the 1043 cases is depicted in Table 1. Furthermore, when the 1043 cases were stratified by age and sex, disproportionality analysis revealed a positive statistical signal with IC025 of 0.1 in females while it was negative (IC025 = − 0.1) in males. Similarly, disproportionate reporting was observed in those aged between 45 and 64 years (IC025 = 0.01).
3.2 Subgroup Analysis
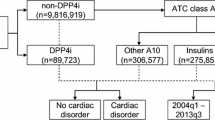
In the first stage of the subgroup analysis, after exclusion of the cases with a reported history of previous hypertension and those who had other co-suspected drugs, a total of 225 cases of hypertension associated with omeprazole were identified and included for analysis (Fig. 1). Of these 225 cases, hypertension resolved following withdrawal of omeprazole in 56 cases (positive dechallenge), and recurred after re-introduction of the suspected drug (omeprazole) in eight cases (positive rechallenge). The median time-to-onset of hypertension following use of omeprazole was 2.0 days (interquartile range (IQR): 1). Moreover, hypertension was the only reaction reported in 60 of the 225 cases with omeprazole as the only drug suspected (Table 2).
In the second stage of the subgroup analysis, only reported cases of hypertension that were suspected of being associated with the sole administration of omeprazole (n = 122 cases) were retrieved and further analyzed (Fig. 1). In this subgroup analysis, where information was available, hypertension resolved in 29 cases following withdrawal of omeprazole and recurred in four cases with re-introduction of the drug. The median time-to-reaction onset of hypertension following intake of omeprazole was 2 days (IQR 29.3). Additionally, hypertension was the only reaction reported in 41 of the 122 cases having omeprazole as a sole drug administered (Table 2).
3.3 Results of Literature Search and Labeling
Epidemiological studies that associate omeprazole with hypertension or increased blood pressure could not be identified in PubMed and Google Scholar searches. Furthermore, the association is not documented in Martindale’s adverse drug reactions checker [17] or SIDER side effect resources [18]. However, reports indicating the association between PPIs and CVDs, particularly myocardial infarction and stroke, dose-response relationship of dexlansoprazole and hypertension, possible biological mechanisms, mouse models and cultures of human endothelial cells indicating PPIs caused cardiovascular problems, including the inability of blood vessels to relax, which could indirectly lead to hypertension [6,7,8,9,10,11,12,13, 19, 20, 22] are published elsewhere and are described in the relevant sections of this document. In addition, except for the innovator company’s SmPC of PRILOSEC, approved by the FDA, which reported elevated blood pressure as a post-marketing experience, none of the SmPCs and/or prescribing information of omeprazole approved by major regulators such as the EMA and the UK’s Medicines and Healthcare Products Agency have reported elevated blood pressure and/or hypertension as an adverse drug event [3,4,5].
3.4 Results of Causality Assessment Using the Austin Bradford Hill Criteria
The existing level of evidence that supports and/or, on the other hand, provides an alternative explanation for the association is presented, as appropriate, in the nine criteria of Hill’s causality assessment framework. Except for the dose-response relationship, at least some level of evidence that favors Hill’s criteria were identified (Table 3).
4 Discussion
In the WHO global database, several cases of hypertension associated with omeprazole were identified. In the subgroup analysis, cases with a previous history of hypertension and those taking other drugs concurrently, other than omeprazole, were excluded to minimize confounders. Where time-to-onset was documented, the subgroup analysis reflected that the median time-to-onset of hypertension was shortly within 2 days following initiation of omeprazole. The fact that hypertension resolved following discontinuation of omeprazole and, again, recurred with re-introduction of omeprazole supports the causal relationship of this combination. Besides, having reports of different cases from several countries, observed by different persons, having a similar time-to-onset indicates consistency of the association, which also supports the causal relationship between omeprazole and hypertension. The differential reporting of hypertension related to omeprazole, among different countries, could be attributed to the country’s population size, maturity of their pharmacovigilance systems, and extent of utilization of the product. The reasons for disproportionate reporting of omeprazole and hypertension in females and in those aged 45–65 years is unclear, as the denominator of omeprazole users or consumption data by gender and age, as well as their clinical features, are unknown because the data are gathered by spontaneous reporting.
In 112 cases, hypertension developed with the sole intake of omeprazole, making the association very specific in terms of the exposure of interest, and the fact that a significant proportion of the cases (41/122) developed hypertension as the only reaction suggests that the association was specific in terms of the outcome of interest. On the other hand, the advanced age of the subjects of around 60 years [35, 36] and the gastrointestinal (GI) problems in these subjects, including gastroesophageal reflux disease (GERD), which are known to be associated with increased blood pressure/hypertension (though it is not known whether these GI problems cause hypertension) [37,38,39,40], could be considered as an alternative cause for hypertension in this study population. However, the consistency of about 2 days onset of action following intake of omeprazole (temporal relationship) and the positive dechallenge and rechallenge in several cases indicates a causal relationship between omeprazole and hypertension.
In the WHO global database, a statistical signal was observed for the association between omeprazole and hypertension. This means the number of cases of hypertension reported following the use of omeprazole have been higher than expected. Moreover, reports show that other PPIs, such as dexlansoprazole (Dexilant), have been previously associated with hypertension, indicating an analogy of the association [19]. Development of hypertension with the use of omeprazole might be possible, as several studies have associated PPIs with cardiovascular events [6,7,8,9,10,11,12,13] and hypertension [19]. All these findings support the causal inference between omeprazole and hypertension. In the prescribing information of the innovator company of omeprazole, approved by the FDA, it is indicated that cases of hypertension have been reported as a post-marketing experience, which further supports the a causal association [2].
The possible biological mechanism by which omeprazole could cause hypertension has been reported in several studies. In mouse models and cultures of human endothelial cells, it was found that PPIs cause cardiovascular problems, including the inability of blood vessels to relax, which could indirectly cause hypertension by reducing nitric oxide synthase [20, 22, 41]. According to these studies, it was found that PPIs suppressed the enzyme dimethylarginine dimethyl aminohydrolase (DDAH), which caused an increase in the blood levels of asymmetric dimethylarginine (ADMA), which in turn suppressed the production of another chemical messenger, nitric oxide (NO) [20]. In addition, treatment with omeprazole impairs vascular redox biology by XOR-mediated mechanisms, leading to tense vascular tissue compared to controls in rats [22]. Moreover, PPIs interference with the gastric formation of protective NO-related species from dietary nitrite and nitrate may also impair non-enzymatic formation of NO and normal cardiovascular function [23,24,25,26]. Some studies also associated PPIs with hypomagnesemia, which could elevate vascular tone and potentiates the vasoconstrictor activity of various agonists [27,28,29,30,31]. Furthermore, the high concentration of sodium in omeprazole can also be a possible mechanism for the emergence of hypertension [32]. Additionally, omeprazole-induced kidney problems such as interstitial nephritis [2, 33, 34], which in turn can result in hypertension, are another possible mechanism.
The seriousness of hypertension and the several deaths reported underscore the importance of early detection and appropriate management of patients with hypertension. Taking the risk of hypertension and other previously identified cardiovascular adverse effects related to PPIs into consideration, patients, especially older adults on omeprazole, need to be closely followed up. Moreover, PPIs need to be prescribed by a qualified healthcare professional only when their use is actually required. This is because PPIs are one of the most frequently prescribed classes of drugs worldwide, and yet about half of the prescriptions are known to be inappropriate [42,43,44]. Healthcare professionals and consumers are also advised to report any incident of hypertension or increase in blood pressure following the use of omeprazole.
4.1 Limitations
Readers should interpret the findings of this study with caution, taking the following limitations into consideration: First, hypertension is a multifactorial disease in which several variables that could contribute to this causal inference cannot be controlled or ruled out due to the retrospective nature of the study. Second, the diagnosis of hypertension could not be validated by the authors, and the probability of reversibility of the outcome of interest is unknown for a similar reason to that mentioned above. Third, the incidence rate of hypertension associated with omeprazole is not known as the cases were voluntarily reported from different parts of the world, which is an inherent limitation of spontaneous reporting of adverse drug reactions. Fourth, the causal inference is made based on the currently available level of evidence, and thus, this signal should be considered as tentative, and the direction of association/causation might be changed over time with better evidence. Hence, further experimental and epidemiological studies are recommended to substantiate the suggested causal relationship.
5 Conclusion
The strength of the association, temporality, specificity, consistency, existing possible biological mechanisms and experimental evidence, coherence, and analogies support the notion that omeprazole could be a potential cause of hypertension. Hence, it is recommended to monitor and report any incidence of hypertension or elevation in blood pressure following the administration of omeprazole, and conduct additional experimental and epidemiological studies to corroborate the suggested causal association.
References
Grossman A, Messerli FH, Grossman E. Drug induced hypertension—an unappreciated cause of secondary hypertension. Eur J Pharmacol. 2015;763:15–22.
AstraZeneca Pharmaceuticals LP. Summary of characteristics of PRILOSEC (omeprazole) Delayed-Release Capsules and PRILOSEC (omeprazole magnesium) For Delayed-Release Oral Suspension. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019810s096lbl.pdf. Accessed 24 Jan 2024.
European Medicines Agency. Summary of characteristics of OMEPRAZOLE 20mg. https://www.medicines.org.uk/emc/product/1509/smpc. Accessed 24 Aug 2024.
Medicines and Healthcare Products Regulatory Agency, May 2019. Public Assessment Report of Omeprazole (20mg). https://assets.publishing.service.gov.uk/media/5fee1e828fa8f53b74173806/Omeprazole_20mg_Gastro-Resistant_Tablets_PAR.pdf. Accessed 24 Aug 2024.
Health Products Regulatory Authority. Bayer Limited. Summary of characteristics of Losec Control 20 mg gastro-resistant tablets (omeprazole 20mg). http://www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PPA1151-007-002_07122012145052.pdf. Accessed 24 Jan 2024.
Sehested TS, et al. Long-term use of proton pump inhibitors, dose–response relationship and associated risk of ischemic stroke and myocardial infarction. J Intern Med. 2018;283(3):268–81.
Wang Y-F, et al. Proton-pump inhibitor use and the risk of first-time ischemic stroke in the general population: a nationwide population-based study. Off J Am Coll Gastroenterol ACG. 2017;112(7):1084–93.
Shah NH, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS ONE. 2015;10(6): e0124653.
Shih C-J, et al. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int J Cardiol. 2014;177(1):292–7.
Sun S, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29(2): e12926.
Juurlink DN, et al. Proton pump inhibitors and the risk of adverse cardiac events. PLoS ONE. 2013;8(12): e84890.
Turkiewicz A, et al. Revising the link between proton-pump inhibitors and risk of acute myocardial infarction—a case-crossover analysis. Eur J Clin Pharmacol. 2015;71:125–9.
Bateman D, et al. Mortality study of 18 000 patients treated with omeprazole. Gut. 2003;52(7):942–6.
Houston M, et al. Binding of xanthine oxidase to vascular endothelium: kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274(8):4985–94.
Forgerini M, Mieli S, Mastroianni PDC. Safety assessment of omeprazole use: a review. Sau Paulo Med J. 2018;136:557–70.
Uppsala Monitoring Center. WHO global individual case safety reports database. https://www.who-umc.org/vigibase/VigiBase/. Accessed 08 Mar 2023.
Martindale: The Complete Drug Reference. Pharmaceutical Press. 2023. Accessed Jan 2023.
Side Effect Resource’, http://sideeffects.embl.de, database of drugs and ADRs.
Hill A. Environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. https://doi.org/10.1177/003591576505800503.
Takeda Pharmaceuticals America, Inc. DEXILANT (dexlansoprazole) delayed-release capsules for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022287s019lbl.pdf. Accessed 24 Jan 2024.
Ghebremariam YT, et al. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation. 2013;128(8):845–53.
Pinheiro LC, et al. Omeprazole impairs vascular redox biology and causes xanthine oxidoreductase-mediated endothelial dysfunction. Redox Biol. 2016;9:134–43.
Pinheiro LC, et al. Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radic Biol Med. 2015;87:252–62.
Pinheiro LC, et al. Increase in gastric pH reduces hypotensive effect of oral sodium nitrite in rats. Free Radic Biol Med. 2012;53(4):701–9.
Amaral JH, et al. TEMPOL enhances the antihypertensive effects of sodium nitrite by mechanisms facilitating nitrite-derived gastric nitric oxide formation. Free Radic Biol Med. 2013;65:446–55.
Pinheiro LC, Amaral JH, Tanus-Santos JE. Letter by Pinheiro et al regarding article, “Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine.” Circulation. 2014;129(13):e427–e427.
Choi M-K, Bae YJ. Association of magnesium intake with high blood pressure in Korean adults: Korea national health and nutrition examination survey 2007–2009. PLoS ONE. 2015;10(6): e0130405.
Whang R, et al. Hypomagnesemia and hypokalemia in 1,000 treated ambulatory hypertensive patients. J Am Coll Nutr. 1982;1(4):317–22.
Rodríguez-Ramírez M, et al. Prevalence of prehypertension in Mexico and its association with hypomagnesemia. Am J Hypertens. 2015;28(8):1024–30.
Durlach J, et al. Magnesium and blood pressure II. Clinical studies. Magnes Res. 1992;5(2):147–53.
Efstratiadis G, Sarigianni M, Gougourelas I. Hypomagnesemia and cardiovascular system. Hippokratia. 2006;10(4):147.
Nedderman J. What is the relationship between omeprazole and blood pressure? 2023.
Hung S-C, et al. Using proton pump inhibitors correlates with an increased risk of chronic kidney disease: a nationwide database-derived case-controlled study. J Fam Pract. 2018;35(2):166–71.
Guedes JVM, et al. Omeprazole use and risk of chronic kidney disease evolution. J Plos One. 2020;15(3): e0229344.
Buford TW. Hypertension and aging. J Age Res Rev. 2016;26:96–111.
Cheng W, et al. Age-related changes in the risk of high blood pressure. J Front Cardiovasc Med. 2022;9: 939103.
Gudlaugsdottir S, et al. Hypertension is frequently present in patients with reflux esophagitis or Barrett’s esophagus but not in those with non-ulcer dyspepsia. J Eur J Internal Med. 2002;13(6):369–75.
Li Z-T, et al. The role of gastroesophageal reflux in provoking high blood pressure episodes in patients with hypertension. J J Clin Gastroenterol. 2018;52(8):685.
Shankar V, Kutty A, Annamalai N. Helicobacter pylori infection and hypertension: is there an association. J Biomed Res-India. 2012;23(4):537–9.
Sonnenberg A. Concordant occurrence of gastric and hypertensive diseases. J Gastroenterology. 1988;95(1):42–8.
Yepuri G, et al. Proton pump inhibitors accelerate endothelial senescence. Circ Res. 2016;118(12):e36–42.
Service NH. Drugs for dyspepsia. PACT centre pages. 2006.
Grant K, et al. Continuation of proton pump inhibitors from hospital to community. Pharm World Sci. 2006;28:189–93.
Batuwitage BT, et al. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J. 2007;83(975):66–8.
Acknowledgements
The authors would like to sincerely thank staff of the Ministry of Health of the State of Eritrea, Dr Habtom Kifle, as well as Pharmacists Sirak Tesfamariam and Heaven Yohannes. All persons named in the acknowledgements have given permission to be named in the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mulugeta Russom is an Editorial Board member of Drugs - Real World Outcomes, but was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. The other authors declare that they have no competing interests.
Ethics approval
Information of all cases retrieved from the WHO global database of individual case safety reports is de-identified and ethics approval is not required.
Availability of data and material
The datasets generated and analyzed during the current study are not publicly available due to agreements between contributors of data to the database used (VigiBase) and the custodian of the database. National Centers (mainly drug regulatory authorities) constituting the WHO Programme for International Drug Monitoring (PIDM) contribute data to VigiBase and the Uppsala Monitoring Centre is the custodian in its capacity as WHO Collaborating Centre for International Drug Monitoring. Some subsets of the data may be available from the corresponding author on reasonable request.
Disclaimer
The authors are indebted to the National Centers that make up the WHO Programme for International Drug Monitoring and contribute reports to VigiBase. However, the opinions and conclusions of this study are not necessarily those of the various Centers nor of the WHO or the Uppsala Monitoring Centre, Sweden. Readers should be aware that cases reported to VigiBase are of different quality and merely represent suspicious associations.
Author contributions
MB and MR contributed to the conception and planning of the work described. All the authors contributed to the study design, analysis of publications, data analysis and their interpretation. MB and MR drafted the article, and it was reviewed by the rest of the authors. Finally, all authors read and approved the final manuscript and, therefore, share collective responsibility and accountability for the manuscript.
Funding
There was no funding for this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bahta, M., Russom, N., Ghebrenegus, A.S. et al. Omeprazole and Risk of Hypertension: Analysis of Existing Literature and the WHO Global Pharmacovigilance Database. Drugs - Real World Outcomes (2024). https://doi.org/10.1007/s40801-024-00441-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s40801-024-00441-2