Abstract
Background and Objectives
Direct-acting antivirals (DAAs) offer a high rate of hepatitis C virus (HCV) eradication. However, concerns on the risk of cancer after HCV eradication remain. Our study aimed at quantifying the incidence of cancer in patients treated with anti-HCV therapies in Catalonia (Spain) and their matched controls.
Methods
This was a population-based study using real-world data from the public healthcare system of Catalonia between 2012 and 2016. Propensity score matching was performed in patients with HCV infection treated with interferon-based therapy (IFN), sequential IFN and DAA (IFN+DAA), and DAA only (DAA) with concurrent controls. We estimated the annual incidence of overall cancer, hepatocellular carcinoma, and non-liver cancer of HCV-treated patients and their corresponding rate ratios.
Results
The study included 11,656 HCV-treated patients and 49,545 controls. We found statistically significant increases in the rate of overall cancer for IFN+DAA-treated (rate ratio [RR] 1.77, 95% confidence interval [CI] 1.27–2.46) and DAA-treated patients (RR 1.90, 95% CI 1.66–2.19) and in the rate of HCC for IFN-treated (RR 1.50, 95% CI 1.02–2.22), IFN+DAA-treated (RR 3.89, 95% CI 2.26–6.69), and DAA-treated patients (RR 6.45, 95% CI 4.90–8.49) compared with their corresponding controls. Moreover, DAA-treated patients with cirrhosis showed an increased rate of overall cancer versus those without cirrhosis (RR 1.92, 95% CI 1.51–2.44).
Conclusions
Results showed that overall cancer and hepatocellular carcinoma incidence in Catalonia was significantly higher among HCV-treated patients compared with matched non-HCV-infected controls, and risks were higher in patients with cirrhosis. An increased awareness of the potential occurrence of uncommon malignant events and monitoring after HCV eradication therapy may benefit patients.
Plain Language Summary
Direct-acting antivirals (DAAs) are effective drugs for eradicating hepatitis C virus (HCV). However, concerns about the risk of cancer after HCV eradication remain. Therefore, this study aimed to compare the incidence of cancer between patients treated with anti-HCV therapies in Catalonia (Spain) and properly matched, non-HCV-infected individuals (controls).
This study was based on real-world data from the public healthcare system of Catalonia, specifically from patients with HCV infection treated with interferon-based therapy (IFN), sequential IFN and DAA (IFN+DAA), or DAA only (DAA). We calculated the incidence and rate ratios of overall cancer and hepatocellular carcinoma of HCV-treated patients.
We observed that the rate of overall cancer increased in patients receiving DAA or IFN+DAA, whereas the rate of hepatocellular carcinoma increased in all groups of HCV-treated patients. Of note, DAA-treated patients with cirrhosis showed an increased rate of overall cancer versus those without cirrhosis. Thus, a close monitoring for detection of cancer in patients after HCV eradication seems reasonable, especially in those with cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with hepatitis C virus infection undergoing treatment with direct antiviral agents after interferon treatment or as a single course showed a higher incidence of overall cancer as compared with non-infected controls. |
Having cirrhosis at the time of treatment was associated with a higher rate of cancer, while the human immunodeficiency virus co-infection was not. |
After hepatitis C virus eradication, patients may still benefit from close monitoring for early detection of cancer. |
1 Introduction
Hepatitis C virus (HCV) infection is a global health problem. According to World Health Organization estimates, in 2019, 58 million people were chronically infected by HCV, 1.5 million people had newly acquired HCV, and 290,000 died of HCV infection worldwide [1]. Hepatitis C virus can lead to cirrhosis, end-stage liver disease, and hepatocellular carcinoma (HCC), but also to extrahepatic manifestations, including non-liver cancer, such as non-Hodgkin lymphomas [2, 3].
Currently, there is no vaccine to prevent HCV infection, but it can be cured with antiviral treatment. In the 1990s, interferon (IFN)-based regimens were the only therapies against HCV, but they entailed many side effects and required close monitoring of patients [4]. In the last decade, direct-acting antivirals (DAAs) emerged as alternatives for treating patients with HCV infection, with improved efficacy and tolerability [5]. All of these anti-HCV approaches aim to cure the infection in order to avoid possible transmission to other persons but also to prevent HCV-related complications [6].
The development and recurrence of HCC after curing HCV infection has been a focus of increasing interest [7,8,9,10,11,12]. In a previous study, we reported that the risk of HCC was associated with the imaging detection of non-characterized nodules before HCV treatment initiation [13]. This association was also observed by Sangiovanni et al. [14], and both studies pointed out that HCC could appear in a different location from that of non-characterized lesions [13, 14]. However, meta-analyses on the risk of HCC recurrence have been hampered by the heterogeneity of data, and definite conclusions are lacking [15, 16].
Regarding non-liver cancer, it is well known that B-cell non-Hodgkin lymphoma is associated with HCV infection and may regress after HCV eradication [17,18,19,20]. Additionally, HCV infection of non-hepatic cells and the subsequent alteration of the immune surveillance system seem to increase the risk of non-hematological neoplasms [3]. Moreover, a French multicenter study reported that non-liver cancer was the most frequent cause of death in patients with sustained viral response or HCV eradication [21].
The perception of the benefit of antiviral treatment, potentially normalizing cancer risk, may lead to less intensive monitoring of patients after eradication therapy. It is thus important to determine whether an increased cancer risk remains in HCV-treated patients compared with uninfected people in order to ensure appropriate screening and early detection. The objective of our study was to assess the incidence of cancer—specifically, overall cancer, HCC, and non-liver cancer—in patients treated with anti-HCV therapies (with IFN-based regimens, DAAs, or both) in the public healthcare system of Catalonia, compared with appropriately matched controls.
2 Methods
2.1 Study Design and Patients
This was a retrospective population-based cohort study of patients treated for HCV in Catalonia (North-East of Spain) from 1 January, 2012, to 31 December, 2016, compared with their matched controls. Patients aged 18 years or older, diagnosed with HCV infection during the study period and without any record of cancer diagnosis or treatment on 1 January, 2012, were included in the study and classified into three cohorts: (1) patients treated with interferon with or without ribavirin (IFN-based regimens, IFN); (2) patients who received an IFN-based regimen and subsequent or concomitant DAAs (IFN+DAA); and (3) patients treated with DAAs only (DAA). Matched controls for these cohorts were individuals without evidence of HCV diagnosis prior to or during the study period and without cancer diagnosis or cancer treatment on 1 January, 2012. As DAAs became available in Catalonia in 2014, 1 January, 2012 was chosen as the starting date for selecting participants. This way, IFN-treated patients, DAA-treated patients, and their matched controls were closer in time. Data were extracted in 2018 for a period ensuring a minimum of 18 months of follow-up since the last treated patient was included in 2016.
The study protocol was approved by the Ethics Committee for Clinical Research of the Institute of Research in Primary Care (IDIAP Jordi Gol, code CEI P17/061). Patients’ informed consent was waived because of the retrospective design and the pseudo-anonymization of the data collected from electronic databases.
2.2 Data Sources
Data sources were clinical and administrative databases from Catalonia, a Spanish region with approximately 7.9 million inhabitants [22] with a universal, public, and free-of-charge healthcare system, which citizens access using an individual health card (TSI). Electronic clinical records from primary care, hospital administrative invoicing, pharmacy dispensations, and a specific registry, including drug-related clinical outcomes for particular drugs, are linkable through the TSI code of each citizen.
We used linked data from four different registries of the public healthcare system of Catalonia (CatSalut) through the Public Data Analysis for Health Research and Innovation Programme (PADRIS): the Registry of Patients and Treatments (RPT), the Information System for the Development of Research in Primary Care (SIDIAP), the database corresponding to the Primary Care Minimum Basic Data Set (PC-MBDS), and the general registry of insured citizens in Catalonia (RCA). PADRIS allows the linking of RCA demographic information for all insured patients, diagnostic data for each episode of hospitalization, and pharmacy invoicing data for outpatient medications dispensed either in community or hospital pharmacies [23]. The RPT is a therapeutic registry created for a longitudinal follow-up and assessment of clinical outcomes of specific treatments, such as those for HCV [24], and was used to identify HCV-treated patients (i.e., individuals with de novo billing data on anti-HCV treatments from hospital pharmacies within the study period and without previous cancer diagnosis or billing for cancer drugs). The SIDIAP registry contains longitudinal medical records from primary care managed by the Catalan Institute of Health (ICS), which has been using electronic health records in primary care (eCAP) since 2006, covering approximately 80% of the total Catalan population. SIDIAP includes sociodemographic characteristics, health conditions registered as International Classification of Diseases, Tenth Revision codes, clinical parameters, laboratory data, and outpatient prescriptions. The corresponding pharmacy invoice data have been available since 2005 and include information on all pharmaceutical products dispensed by community pharmacies for ICS prescriptions using the Anatomic Therapeutic Chemical Classification System codes [25]. SIDIAP was used to select control patients (i.e., individuals without evidence of HCV diagnosis, cancer diagnosis, or billing of cancer drugs before 1 January, 2012). Finally, the PC-MBDS includes diagnoses made in the primary care setting and recorded as International Classification of Diseases, Ninth Revision codes. All datasets were pseudo-anonymized in compliance with Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April, 2016 on General Data Protection Regulation and Organic Law 3/2018, of 5 December, on Protection of Personal Data and Guarantee of Digital Rights, prior to the transfer to final data management system and statistical analyses.
2.3 Control Matching Procedure
Matching criteria included age, gender, smoking habit, and geographical region according to the 36 codes grouping all primary healthcare districts (DAPs) in Catalonia. The DAPs are characterized by sociocultural aspects and access to healthcare services, and are primarily used for healthcare budgeting adjustment processes, so that they are often used as a proxy for socioeconomic status in combination with the MEDEA index [27]. MEDEA is a deprivation index based on urban census data that were used to aggregate DAPs with similar socioeconomic conditions and categorize them into quintiles, allowing clustering of DAPs with low population density in order to match subjects from areas where there were few eligible subjects or even none.
Hepatitis C virus-treated patients were matched to controls in a maximum ratio of 1:5. Matching was conducted following a two-step sequential procedure: first, using an exact restriction for gender and DAP, and then, using a propensity score procedure, which was based on the logit from a logistic regression model that included gender, age (estimated from the year of birth to index date), smoking habit, alcohol consumption, and geographical code from DAP [26], and evaluated with MEDEA index quintiles. This second step used greedy nearest neighbor matching [27] with a caliper of <0.06 distance. The matching was performed by two independent technicians who were unaware of the characteristics of the project and had no access to clinical information.
2.4 Variables and Endpoints
Sociodemographic characteristics included age, gender, and MEDEA index and were collected from the RCA. Clinical characteristics included height, weight, body mass index, smoking habit, alcohol consumption, presence of comorbidities (diabetes mellitus, HIV infection, and cirrhosis), and glomerular filtration rate as per the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, all of which were obtained from the SIDIAP registry; in addition, the type of treatment for HCV, degree of liver fibrosis (0–4) according to transient elastography, HCV genotype, HCV viral load, immunoglobulin G levels to HCV and hepatitis B virus, serum levels of albumin, bilirubin, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transferase, platelet count, prothrombin time, and International Normalized Ratio, all of which were collected from the RPT.
The incident events related to cancer diagnosis in all study participants were identified using the International Classification of Diseases, Tenth Revision codes from eCAP within the SIDIAP registry and the International Classification of Diseases, Ninth Revision codes from the PC-MBDR (Table S1 of the Electronic Supplementary Material [ESM]). We also reviewed Anatomic Therapeutic Chemical codes related to oncology drugs from hospital pharmacy billing registries to identify potentially missed incident cancer cases. The length of follow-up for HCV-treated patients was defined as the period between the index date (i.e., the first date of the billing prescription of HCV treatment) and the date of event or last available record, with a minimum follow-up of 18 months in patients without events. For patients in the control group, the index date was that of the case they were paired with, while the last follow-up date was the date of the event or the last date available in the registry.
2.5 Statistical Analysis
Categorical data were expressed as frequencies and percentages, whereas quantitative data were described as the median and interquartile range (25th–75th percentiles). Homogeneity for baseline characteristics was assessed using standardized differences (STDs), i.e., differences divided by the pooled standard deviation between each HCV-treated cohort and its matched control cohort. Proper balance of all matching covariates was pursued by using a 20% cut-off point for STDs [28]. No inferential analysis was performed to compare groups.
The cumulative incidences of cancer (overall, HCC, and non-liver cancer) and their corresponding 95% confidence intervals (CIs) were estimated using Poisson models with the natural logarithmic transformation of follow-up as offset and presented as new cancer diagnoses per 100,000 person-years of follow-up. Incidence rate ratios (RRs) and their corresponding 95% CIs in HCV-treated cohorts were calculated using the incidence of their matched control cohort as a reference. A complementary time-to-event analysis for sensitivity purposes was performed using the Kaplan–Meier method to describe the instantaneous hazard up to 36 months of follow-up. Additionally, risk increases in HCV-treated cohorts with respect to their matched controls were estimated through hazard ratios (HRs) and their 95% CIs using Cox proportional regression models. Direct comparisons between different cohorts were not planned because of inherent differences in the prevalence of cirrhosis and HIV infection, which are well-established risk factors for the development of neoplasms [29] and clinical limitations for prescribing IFN-based treatments. However, the rates of overall cancer according to the presence or absence of HIV coinfection and cirrhosis were analyzed within each HCV-treated cohort. The threshold for statistical significance was established at a two-sided alpha value of 0.05. All statistical analyses were conducted using the Statistical Analysis Systems (SAS) version 9.4 (SAS Institute, Cary, NC, USA).
3 Results
3.1 Characteristics of Study Patients
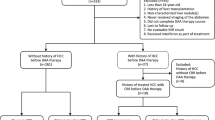
A total of 11,656 HCV-treated patients were included in the study and classified into one of the three treatment cohorts: IFN (n = 4329, 37.1%), IFN+DAA (n = 794, 6.8%), and DAA (n = 6533, 56.0%). The screened population for control sampling included 572,381 patients, of which 11,786 were excluded because of insufficient data for matching. Of the remaining potentially eligible 560,595 patients, 19,376, 3507, and 26,662 paired controls were selected for matching the IFN, IFN+DAA, and DAA cohorts, respectively, with an average of 4.25 controls per patient (Fig. 1).
Table 1 shows the main demographic and clinical characteristics of study patients. All cohorts of HCV-treated patients were evenly balanced with their matched controls regarding all matching covariates (age, gender, smoking habit, and MEDEA index). In contrast, STDs >20% were found for HIV coinfection (all cohorts) and cirrhosis (IFN+DAA and DAA cohorts). This observation accounts for the higher prevalence of HIV coinfection and cirrhosis in the HCV-treated patients of such cohorts, respectively. In addition, all cohorts showed STDs >20% regarding the body mass index, which was approximately 1 kg/m2 lower in HCV-treated patients. Moreover, IFN+DAA and DAA cohorts showed STDs >15% for diabetes, the prevalence of which was slightly higher in HCV-treated patients. Of note, HCV-treated patients in the IFN+DAA and DAA cohorts were older than those in the IFN cohort and showed a higher prevalence of diabetes, HIV, and particularly cirrhosis. Data on additional clinical variables can be found in Table S2 of the ESM.
3.2 Cancer Incidence
Table 2 shows the estimated incidence and incidence RRs of overall cancer, HCC, and non-liver cancer in all study cohorts. The highest incidences of cancer (overall, HCC, and non-liver) were found in DAA-treated patients, followed by IFN+DAA-treated patients. The overall cancer rate was 77% and 90% higher in HCV-treated patients of the IFN+DAA and DAA cohorts than in their respective controls, but did not change for patients in the IFN cohort. Regarding HCC, HCV-treated patients of the IFN, IFN+DAA, and DAA cohorts showed 50%, 289%, and 545% higher rates than their matched controls. However, the rate of non-liver cancer did not show statistically significant differences between HCV-treated patients and their matched controls in any of the study cohorts; it only showed a trend toward an increase in DAA-treated patients.
The estimated incidence and incidence RRs of overall cancer according to the presence or absence of HIV coinfection and cirrhosis are shown in Table 3. The highest incidences of overall cancer in HCV-treated patients with HIV coinfection and cirrhosis were found in the DAA cohort, followed by the IFN cohort. However, the only statistically significant difference regarding the presence of HIV coinfection was found in the IFN+DAA cohort, where HCV-treated patients showed a lower rate of overall cancer than their matched controls. The DAA cohort, where HCV-treated patients showed the highest prevalence of HIV coinfection, exhibited a similar trend, but it did not reach statistical significance. Concerning cirrhosis, its presence was associated with a higher rate of overall cancer in HCV-treated patients, but only in the DAA cohort.
3.3 Risk of Overall Cancer at the Follow-Up
As can be seen in Fig. 2, at the follow-up, all HCV-treated patients had a higher probability of cancer than their matched controls, although statistically significant differences were found only in the IFN+DAA and DAA cohorts, with risk increases of 75% (HR, 1.75; 95% CI 1.26–2.44; p = 0.0009) and 89% (HR, 1.89; 95% CI 1.64–2.17; p < 0.0001), respectively (see Fig. 1 of the ESM for stratified figures for liver and non-liver cancers). The lowest proportion of overall cancer events amongst HCV-treated patients was observed in the IFN cohort, where less than 1% of patients had advanced liver disease.
4 Discussion
In this population-based study, the overall cancer rate was significantly higher in HCV-treated patients of the IFN+DAA and DAA cohorts than in their respective controls. The highest incidences of cancer (overall, HCC, and non-liver) were found in DAA-treated patients, followed by IFN+DAA and IFN-treated patients. In addition, patients with HCV treated with IFN, IFN+DAA, and DAA showed higher HCC rates than their matched controls. However, no statistically significant differences with matched controls were observed in the rate of non-liver cancer in any of the study cohorts. The DAA cohort showed the highest estimated incidences of overall cancer in HCV-treated patients with HIV coinfection or cirrhosis, followed by those in the IFN cohort, but statistically significant differences were only found in the IFN+DAA cohort and the DAA cohort, respectively. In the IFN+DAA cohort, patients with HIV coinfection showed a lower rate of overall cancer than those without HIV coinfection. Conversely, in the DAA cohort, patients with cirrhosis showed a higher rate of overall cancer compared with patients without cirrhosis.
The core finding of the study is the higher incidence rate of overall cancer in IFN+DAA and DAA-treated patients compared with their matched controls. There may be a number of explanations for this observation. Some authors have proposed that the development or reactivation of HCC may occur after treatment. They argue that reactivation of the hepatitis B or herpes virus related to DAAs [30, 31] suggests a disruption of immune surveillance, which may allow malignant clones to emerge [32]. This may be consistent with the fact that, despite the relatively short length of follow-up, our Kaplan–Meier curves show increases in overall cancer incidence close to DAA or IFN+DAA treatment courses. In this regard, if the oncogenic hit leading to hepatic malignant transformation had already taken place at the time of the DAA therapy, [32, 33] the incidence of liver cancer cannot be reduced despite clearing of the virus, at least during the first years after eradication.
However, because of the observational design of our study, we should also consider that residual confounding and some biases may also be potential explanations for our findings [34]. First, detection bias may explain the apparent increase in the incidence rate of overall cancer in patients receiving DAAs through a more intense follow-up after initiating their therapy and increased early diagnosis of cancer. Against this idea, we should consider that the public healthcare system in Catalonia conducts population-based screening programs for breast and colon cancer [35], and also that symptom-related diagnoses of cancer are unlikely to be affected by a follow-up after HCV eradication. Moreover, the opposite might also be argued, that is, as patients cured of HCV may be assumed to be already healthy, they may be less prone to be concerned about minor symptoms and medical visits. In addition, as DAA therapies involve a shorter duration and fewer complications than IFN-based regimens, less intensive clinical care requirements would lead to less frequent visits and testing. Yet, detection bias is difficult to discard.
Second, an indication bias, determining differences in eligibility for antiviral treatment throughout the years and between treatments, may also explain part of the results. On the one hand, early IFN-based treatments had poor tolerability and were limited to subjects with poor hepatic prognosis but a good general condition. This means that those difficult-to-treat and most severe HCV-infected cirrhotic patients were not even considered for IFN therapy because of safety concerns and low efficacy rates. On the other hand, DAA treatment had better safety profiles, so that it allowed the consideration of treatment of patients in a poorer condition, including those that had already not responded to a previous treatment, and patients with cirrhosis, previous hepatic decompensation, or comorbidities. Especially when first introduced in therapeutics, DAAs were indicated in patients who already had advanced fibrosis, and thus a higher risk of complications such as cirrhosis and cancer. Actually, we saw substantial differences between treated patients, which prevented direct comparisons between HCV-treated cohorts. This was already anticipated in the design of the study, and because of that, we compared each HCV-treated cohort with their corresponding controls, rather than comparing treated cohorts between them. This way, we aimed to compare the incidence rate in the cohorts with that of the general population without known HCV infection. The external validity of our control population is supported by the fact that the overall cancer incidence in the control population agrees with that of the Spanish Association Against Cancer (AECC) registry. [36] According to this registry, 39,237 new cancer cases were diagnosed in Catalonia in 2012, corresponding to an incidence of 614 cases per 100,000 person-years, and our study shows incidences of 514.9, 710.8, and 815.3 cases per 100,000 person-years in the controls for IFN, IFN+DAA, and DAA cohorts, respectively. In addition, the selection of controls in our study took into account that oncogenic factors such as specific dietary habits or environmental conditions may vary across socioeconomic conditions and geography, so that control matching was performed considering the socioeconomic condition and geographical region using DAPs.
Third, survival bias cannot be discarded either, which could also be differential across the cohorts. On the one hand, early IFN-based treatments had poor effectivity and reached poor rates of eradication compared with DAA treatments, which not only could be offered to patients in a poor condition, but also had high effectiveness, theoretically halting the course of fibrosis to end-stage liver disease. By improving liver disease, these subjects had increasing survival that kept them at cancer risk instead of dying of liver complications or other related causes. Finally, we cannot discard that observations may be due to residual confounding or unmeasured unknown factors.
The analysis of the influence of HIV coinfection and cirrhosis on cancer risk in HCV-treated patients did not suggest an adverse effect of the former, but it identified high-risk patients in the latter. Yet, the inherent high prevalence of cirrhosis in IFN+DAA and DAA-treated patients may be an alternative explanation for the high incidence of overall cancer in these cohorts. In this regard, the prevalence of cirrhosis in patients treated with DAA or IFN+DAA regimens in our study (43.2% and 46.2%, respectively) is comparable to that observed for patients treated with DAAs in our country (40.8%) [37]. As for HIV, DAA and IFN+DAA-treated patients showed a prevalence for HIV coinfection of 42.5% and 26.1%, respectively. In 2013, in Catalonia, the percentage of patients with active HCV and a diagnosis of HIV was 69% [38]. As explained above, the indication criteria for antiviral treatment evolved over the years and may be related to the observed prevalences and risks. As IFN-based regimens had plenty of contraindications and interactions that DAA treatments did not have, the profile of patients treated with each had likely been different. However, detailed data to describe and eventually be adjusted by potential differences are not available, and the low counts of patients without HCV infection but with HIV infection or cirrhosis in control individuals did not allow a robust estimate of the stratified cancer incidence, nor was it possible to assess the incidence by cancer type.
This study has several limitations. First, it has the inherent limitations of the analyses of population registries, such as missing data and paucity of variables, to adjust for potential differences in risk factors between treated cohorts. For instance, environmental contaminants were controlled by geographical matching, and smoking habits and alcohol consumption were adjusted in the propensity model, but no reliable data for other toxics exposure were available for adjustments. Second, from a methodological standpoint, ideal control groups may consist of HCV-untreated patients. However, such patients were not identified in administrative databases, and once DAAs became available, the health authorities actively sought HCV carriers to treat them. Therefore, a very early cross-over between cohorts would have occurred. Nevertheless, the chosen approach of selecting non-HCV-infected individuals provides pragmatic information for identifying patients at high risk of cancer, who may benefit from active surveillance. Finally, this study describes the estimated incidence of cancer in patients receiving different treatments for HCV, but the data are not contemporary across cohorts. Interferon-based treatments were used up to 2015, and DAA treatments were used thereafter. Because we cannot exclude that cancer risk or detection has changed along the study period, incidences across treatment cohorts should be interpreted with caution. In fact, while cancer incidence in controls is consistent with epidemiological data, we observed differences in cancer incidence between the controls of IFN and DAA cohorts. This variability may be attributed to differences in age and unknown external factors, as well as to a selection bias because the tolerability profile of the drugs differs substantially, and DAAs can be given to older patients with more known risk factors for cancer (e.g., obesity, diabetes, or HIV infection). Therefore, despite the propensity score matching, considering the paucity of data to adjust for indication bias or other confounders, we cannot exclude that a residual confusion remains, limiting the comparability between HCV-treated and control cohorts and across HCV-treated cohorts for subgroup analyses.
Nevertheless, despite all these limitations, the large sample of patients in our study allowed us to assess the incidence of cancer in HCV-treated patients in Catalonia, to observe that the risk is higher than that of the uninfected population, and to identify those subjects with cirrhosis as being at a particular higher risk of cancer (mainly HCC), even after receiving antiviral treatment.
5 Conclusions
In summary, this population-based study in patients with HCV showed that the incidence rate of overall cancer and HCC in Catalonia was higher in patients treated with DAA and IFN+DAA than in their matched non-HCV-infected controls, although the different prevalence of key risk factors between cohorts prevents causal conclusions. While the absolute risk remains low and the benefits of treating HCV are not questioned, increased awareness of the potential occurrence of uncommon malignant events (especially HCC) after therapy with DAAs may benefit patients receiving these drugs.
Abbreviations
- AECC:
-
Spanish Association Against Cancer
- ATC:
-
Anatomic Therapeutic Chemical Classification System
- BMI:
-
Body mass index
- CatSalut:
-
Catalan Health Service
- CI:
-
Confidence interval
- DAA:
-
Direct-acting antiviral
- DAP:
-
Geographical codification of healthcare units (Direcció d’Atenció Primària)
- eCAP:
-
Electronic health records in primary care
- GDPR:
-
European Data Protection Regulation
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HR:
-
Hazard ratio
- ICD:
-
International Classification of Diseases
- ICS:
-
Catalan Institute of Health
- IDIAP:
-
Institute of Research in Primary Care (IDIAP Jordi Gol)
- IFN:
-
Interferon
- IQR:
-
Interquartile range
- MEDEA:
-
Deprivation index (Spanich acronym for “Mortality in Small Spanish Areas and Socioenconomic and Environmental Inequalities”)
- PADRIS:
-
Public Data Analysis for Health Research and Innovation Program
- PC-MBDS:
-
Primary care minimum basic data set
- PS:
-
Propensity score
- RPT:
-
Registry of Patients and Treatments
- RR:
-
Rate ratio
- SAS:
-
Statistical Analysis Systems
- SD:
-
Standard deviation
- SIDIAP:
-
System for the Development of Research in Primary Care
- STD:
-
Standardized difference
- TSI:
-
Individual health card
References
World Health Organization (WHO). Global progress report on HIV, viral hepatitis and sexually transmitted infections. accountability for the global health sector strategies 2016–2021: actions for impact. Geneva: World Health Organization (WHO); 2021. p. 2021.
Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26(3 Suppl. 1):15S-20S.
Pol S, Vallet-Pichard A, Hermine O. Extrahepatic cancers and chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2018;15(5):283–90.
Gane E. Future hepatitis C virus treatment: interferon-sparing combinations. Liver Int. 2011;31(Suppl. 1):62–7.
Meshram RJ, Kathwate GH, Gacche RN. Progress, evolving therapeutic/diagnostic approaches, and challenges in the management of hepatitis C virus infections. Arch Virol. 2022;167(3):717–36.
Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. J Viral Hepat. 2012;19(7):449–64.
van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernández-Rodríguez CM, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66(3):485–93.
Omland LH, Krarup H, Jepsen P, Georgsen J, Harritshøj LH, Riisom K, et al. Mortality in patients with chronic and cleared hepatitis C viral infection: a nationwide cohort study. J Hepatol. 2010;53(1):36–42.
El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(5):1767–75.
El Kassas M, Funk AL, Salaheldin M, Shimakawa Y, Eltabbakh M, Jean K, et al. Increased recurrence rates of hepatocellular carcinoma after DAA therapy in a hepatitis C-infected Egyptian cohort: a comparative analysis. J Viral Hepat. 2018;25(6):623–30.
Guarino M, Sessa A, Cossiga V, Morando F, Caporaso N, Morisco F, et al. Direct-acting antivirals and hepatocellular carcinoma in chronic hepatitis C: a few lights and many shadows. World J Gastroenterol. 2018;24(24):2582–95.
Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(Suppl. 1):139–45.
Mariño Z, Darnell A, Lens S, Sapena V, Díaz A, Belmonte E, et al. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: relevance of non-characterized nodules. J Hepatol. 2019;70(5):874–84.
Sangiovanni A, Alimenti E, Gattai R, Biganzoli E, Colombo M, Lampertico P. Undefined/non-malignant hepatic nodules are associated with early occurrence of HCC in DAA-treated patients with HCV-related cirrhosis. J Hepatol. 2020;73(3):593–602.
Sapena V, Enea M, Torres F, Celsa C, Rios J, Rizzo GEM, et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: an individual patient data meta-analysis. Gut. 2022;71(3): 593604.
Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, et al. Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology. 2019;156(6):1683-92.e1.
Hermine O, Lefrère F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347(2):89–94. https://doi.org/10.1056/NEJMoa013376. PMID: 12110736.
Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, et al. Role of anti-hepatitis C virus (HCV) treatment in lymphoma: a multicenter Italian experience. J Clin Oncol. 2005;23(3):7–12.
Kawamura Y, Ikeda K, Arase Y, Yatsuji H, Sezaki H, Hosaka T, et al. Viral elimination reduces incidence of malignant lymphoma in patients with hepatitis C. Am J Med. 2007;120(12):1034–41.
Marie MJ, Canioni D, Driss H, Alric L, Cacoub P, Suarez F, et al. Antiviral therapy is associated with a better survival in patients with hepatitis C virus and B-cell non-Hodgkin lymphomas, ANRS HC-13 lympho-C study. Am J Hematol. 2015;90(3):197–203.
Allaire M, Nahon P, Layese R, Bourcier V, Marcellin P, Guyader D, et al. Extrahepatic cancers are the leading cause of death in patients achieving hepatitis B virus control or hepatitis C virus eradication. Hepatology. 2018;68(4):1245–59.
Idescat. Statistical yearbook of Catalonia. Population on 1 January. Provinces.
Burgun A, Kuchinke W, Van ST, Cunningham J, Lettieri E. Health data for public health: towards new ways of combining data sources to support research efforts in Europe. IMIA Yearb Med Informatics. 2017;26:235–40.
Roig Izquierdo M, Prat Casanovas MA, Gorgas Torner MQ, Pontes GC. Registry of patients and treatments of hospital medicines in Spain: 10 years of clinical data. Med Clin (Barc). 2020;154(5):185–91.
Bolíbar B, Fina Avilés F, Morros R, Garcia-Gil del MM, Hermosilla E, Ramos R, et al. SIDIAP database: electronic clinical records in primary care as a source of information for epidemiologic research. Med Clin (Barc). 2012;138(14):617–21.
Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21.
Rosenbaum PR. Optimal matching for observational studies. J Am Stat Assoc. 1989;84(408):1024–32.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
Reid E, Suneja G, Ambinder RF, Ard K, Baiocchi R, Barta SK, et al. Cancer in people living with HIV, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(8):986–1017.
Collins JM, Raphael KL, Terry C, Cartwright EJ, Pillai A, Anania FA, et al. Hepatitis B virus reactivation during successful treatment of hepatitis C virus with sofosbuvir and simeprevir. Clin Infect Dis. 2015;61(8):1304–6.
Perelló CS, Fernández-Carrillo C, Carlota LM, Hernández-Conde M, Llerena S, Crespo J, et al. Reactivation of herpesvirus in patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol. 2016;14(11):1662–6.
Reig M, Boix L, Mariño Z, Torres F, Forns X, Bruix J. Liver cancer emergence associated with antiviral treatment: an immune surveillance failure? Semin Liver Dis. 2016;37(02):109–18.
Manjili MH. The inherent premise of immunotherapy for cancer dormancy. Cancer Res. 2014;74:6745–9.
Stürmer T, Wang T, Golightly YM, Keil A, Lund JL, Funk MJ. Methodological considerations when analysing and interpreting real-world data. Rheumatology (Oxford). 2020;59(1):14–25.
Ribes J, Pareja L, Sanz X, Mosteiro S, Escribà JM, Esteban L, et al. Cancer diagnosis in Catalonia (Spain) after two years of COVID-19 pandemic: an incomplete recovery. ESMO Open. 2022;7(3): 100486.
Asociación Española Contra el Cancer (AECC). Informe dinámico: incidencia. Enero 2022. Available from: https://observatorio.contraelcancer.es/informes/informe-dinamico-incidencia. Accessed 15 May 2024.
Secretaria General de Sanidad y Consumo. Informe de situación del plan estratégico para el abordaje del a hepatitis C crónica presentado al consejo interterritorial del SNS. Madrid; 2017. Available from: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatitisC/PlanEstrategicoHEPATITISC/docs/informeSituacionPEAHCPresentadoCISNS_Jun2017.pdf. Accessed 10 Jul 2023.
Centre d’Estudis Epidemiològics sobre les Infeccions de Transmissió Sexual i Sida de Catalunya. Sistema integrat de vigilància epidemiològica de la SIDA/VIH/ITS a Catalunya. Barcelona: Agència de Salut Pública de Catalunya; 2015. Available from: https://hdl.handle.net/11351/3418. Accessed 10 Jul 2023.
Acknowledgments
The authors thank Marta Roig, PhD, for helping with the selection of relevant databases and interpretation of parameters within the Registry of Treatments and Patients, Eduard Hermosilla and Lluis Martínez for their help in data extraction, and Marta Pulido, MD, for editing the manuscript and editorial assistance. The authors also thank i2e3 Procomms, especially Alba Rebollo, for providing medical writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Grant support was received from the Spanish National Health Ministry (National Strategic Plan Against Hepatitis), Instituto de Salud Carlos III (PI18/00768, PI15/00145, PI18/0358), Spanish Association Against Cancer (AECC) [PI044031], CERCA Programme/Generalitat de Catalunya, and World Wide Cancer Research (Association for International Cancer Research) 16-0026.
Conflicts of Interest/Competing Interests
José Ríos has received educational/training fees from Amgen, AstraZeneca, Boehringer Ingelheim, Janssen-Cilag, Novartis, and Lilly. Zoe Mariño has received consultancy fees from Gilead, AbbVie, Alexion, Orphalan, and Deep Genomics, speaker fees from Gilead and AbbVie, and research grants from Gilead. Víctor Sapena has received travel grants from Bayer and consultancy fees from Leo Pharma. Jordi Bruix has been a consultant for Arqule, Bayer-Shering Pharma, Novartis, BMS, BTG-Biocompatibles, Eisai, Kowa, Terumo, Gilead, Bio-Alliance, Roche, AbbVie, MSD, Sirtex, Ipsen, Astra-Medimmune, Incyte, Quirem, Adaptimmune, Lilly, Basilea, Nerviano, and Sanofi, has received research/educational grants from Bayer, and lecture fees from Bayer-Shering Pharma, BTG-Biocompatibles, Eisai, Terumo, Sirtex, and Ipsen. Xavier Forns acted as an advisor for Gilead and received a grant from AbbVie. María Reig has received consultancy fees from Bayer, BMS, Roche, Ipsen, AstraZeneca, and Lilly, lecture fees from Bayer, BMS, Gilead, and Lilly, and research grants from Bayer and Ipsen. Ferran Torres has received fees for the Data and Safety Monitoring Board from Basilea Pharmaceutica International and ROVI, and educational/training fees from Janssen and Ferrer. Rosa Morros and Caridad Pontes have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The study protocol was approved by the Ethics Committee for Clinical Research of the Institute of Research in Primary Care (IDIAP Jordi Gol, code CEI P17/061).
Consent to Participate
Patients’ informed consent was waived because of the retrospective design and the pseudo-anonymization of the data collected from electronic databases.
Consent for Publication
Not applicable.
Availability of Data and Material
Study data are available from the author (JR) upon request.
Code Availability
Not applicable.
Authors’ Contributions
JR, JB, RM, MR, FT, and CP: concept and design of the study. ZM, VS, XF, RM, MR, and CP: data collection. JR, VS, and FT: statistical analysis. JR, JB, CP, and FT: interpretation of results. JR: drafting of the initial manuscript. ZM, VS, JB, XF, RM, MR, FT, and CP: draft review for important intellectual content. All authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ríos, J., Sapena, V., Mariño, Z. et al. Incidence of Liver and Non-liver Cancers After Hepatitis C Virus Eradication: A Population-Based Cohort Study. Drugs - Real World Outcomes (2024). https://doi.org/10.1007/s40801-024-00437-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s40801-024-00437-y