Abstract
Background and Objective
Multimorbidity is common in hospitalised adults who are at increased risk of inappropriate prescribing including drug–disease interactions. These interactions occur when a medicine being used to treat one condition exacerbates a concurrent medical condition and may lead to adverse health outcomes. The aim of this review was to examine the association between drug–disease interactions and the risk of mortality and readmission in hospitalised middle-aged and older adults.
Methods
A systematic review was conducted on drug–disease interactions in hospitalised middle-aged (45–64 years) and older adults (≥65 years). The study protocol was prospectively registered with PROSPERO (Registration Number: CRD42022341998). Drug–disease interactions were defined as a medicine being used to treat one condition with the potential to exacerbate a concurrent medical condition or that were inappropriate based on a comorbid medical condition. Both observational and interventional studies were included. The outcomes of interest were mortality and readmissions. The databases searched included MEDLINE, CINAHL, EMBASE, Web of Science, SCOPUS and the Cochrane Library from inception to 12 July, 2022. A meta-analysis was performed to pool risk estimates using the random-effects model.
Results
A total of 563 studies were identified and four met the inclusion criteria. All were observational studies in older adults, with no studies identified in middle-aged adults. Most of the studies were at risk of bias because of an inadequate adjustment for covariates and a lack of clarity around individuals lost to follow-up. There were various definitions of drug–disease interactions within these four studies. Two studies assessed drugs that were contraindicated based on renal function, one assessed an individual drug–disease combination, and one was based on the clinical judgement of a pharmacist. There were two studies that showed an association between drug–disease interactions and the outcomes of interest. One reported that the use of diltiazem in patients with heart failure was associated with an increased risk of readmissions. The second reported that the use of medicines contraindicated according to renal function were associated with increased risk of all-cause mortality and a composite of mortality and readmission. Three of the studies (total study population = 5705) were amenable to a meta-analysis, which showed no significant association between drug–disease interactions and readmissions (odds ratio = 1.0, 95% confidence interval 0.80–1.38).
Conclusions
Few studies were identified examining the risk of drug–disease interactions and mortality and readmission in hospitalised adults. Most of the identified studies were at risk of bias. There is no universal accepted definition of drug–disease interactions in the literature. Further studies are needed to develop a standardised and accepted definition of these interactions to guide further research in this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There were few studies assessing the association between drug–disease interactions and mortality or readmissions in hospitalised adults. |
There is not a universal accepted definition of drug–disease interactions in the literature. |
Further studies are needed to develop a standardised definition of these interactions and assess whether they are associated with adverse health outcomes. |
1 Introduction
Multimorbidity, defined as the presence of two or more long-term health conditions, occurs in over 50% of older adults [1,2,3]. Multimorbidity is recognised as a contributing factor to the rising incidence of hospital admissions and mortality [4]. Hospitalised patients often have multimorbidity and are at increased risk of inappropriate prescribing (IP) including drug–disease interactions. Drug–disease interactions are common occurring in 6–30% of community-dwelling older adults [5,6,7,8] and 15% of frail older adults at hospital discharge [9]. From one study in the hospital setting, the most common drug–disease interactions were the use of first-generation calcium channel blockers in patients with congestive heart failure and the use of aspirin in patients with peptic ulcer disease [9]. Hospitalisation may represent an opportunity to rectify prescribing for adults with multimorbidity and improve health outcomes.
Drug–disease interactions typically occur when a medicine being used to treat one condition exacerbates a concurrent medical condition. Although this broad definition exists, there is no standardised criteria for drug–disease interactions as has been developed for potentially inappropriate medicines in the elderly. Drug–disease interactions can result from treatment-related medication conflicts, which are increasingly being recognised in the multimorbid population [10,11,12,13]. Although the incidence of drug–disease interactions has not been well studied, almost half of older adults with heart failure have a treatment-related medicine conflict. Examples of these include the prescription of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease or non-steroidal anti-inflammatory drugs for pain, both of which are associated with worsening heart failure [12]. Certain drug–disease interactions have been shown to be associated with adverse outcomes in the community setting. For instance, the concurrent use of corticosteroids in individuals with diabetes mellitus and chronic obstructive pulmonary disease is associated with an increased risk of diabetes-related hospitalisation [14].
One of the possible reasons for these drug–disease interactions is that the evidence underlying the presumed risks and benefits of medicines may not be generalisable to patients with multimorbidity. Guideline recommendations are generally developed from short-term randomised controlled trials in single disease states [15, 16]. Although an individual patient meta-analysis demonstrated that the treatment effect of medicines did not differ based on comorbidity, the most multimorbid adults are excluded from clinical trials and the extent to which the findings are generalisable to this growing group is unclear [17,18,19]. There is evidence from observational studies using real-world datasets that the risks and benefits of medications may differ based certain comorbidities, multimorbidity or frailty. For instance, a US observational study demonstrated that the benefits of guideline-directed medicines for multiple common chronic conditions were dependent on the comorbidities present [20]. An Australia study of older frail women with acute coronary syndrome has shown that the prescription of four guideline-recommended medicines was associated with an increased risk of falls without the expected reduction in cardiovascular events [21]. Adults with atrial fibrillation and cognitive impairment had higher rates of mortality and bleeding compared with those who were not anticoagulated. The use of antihypertensives in community-dwelling adults with frailty have been associated with an increased risk of harm with evidence suggesting that a higher blood pressure may be protective [22, 23]. Similarly, in adults with frailty, dual antiplatelet therapy has been associated with an increased risk of adverse events and statins do not appear to confer mortality benefits in a systematic review of prospective or retrospective studies [24, 25]. An improved understanding of the risks and benefits of guideline-directed medicines in the real-world setting is needed including the effect of comorbidities, multimorbidity and frailty. The findings of these studies are expected to inform prescribing decisions and guidelines.
To the best of the authors’ knowledge, there are no systematic reviews on IP assessing the association between drug–disease interactions and mortality or readmissions in hospitalised patients. This review included middle-aged adults who are the largest group of patients with multimorbidity in absolute terms and are likely to be exposed to drug–disease interactions [26,27,28]. We focussed on the hospitalised population as these individuals are expected to be at the highest risk of adverse outcomes and their admission represents an opportunity to rectify IP. The aim of this systematic review was to determine the association between drug–disease interactions and the risk of readmission and mortality in hospitalised middle-aged and older adults and whether interventions addressing drug–disease interactions alter that risk.
2 Methods
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [29]. The protocol was prospectively registered on PROSPERO (CRD42022341998).
2.1 Inclusion Criteria
2.1.1 Participants
This review considered studies involving middle-aged (45–64 years) and older (≥65 years) adults exposed to drug–disease interactions. The age ranges for middle-aged and older adults were in accordance with accepted definitions [30]. Studies were required to have at least 80% of participants meeting the criteria for middle-aged or older aged adults or where outcomes from these subgroups could be extracted. This threshold was chosen to ensure that the majority of participants fell within the target range so that our findings would be generalisable to the intended population. The use of a threshold is consistent with guidance in the Cochrane Handbook and was possible because participant age is consistently reported in these studies [31]. This same threshold has been used in previously published systematic review protocols in the area of IP [32].
2.1.2 Exposure of Interest
This review considered studies reporting on any drug–disease interaction, which was defined as a medicine being used to treat one condition with the potential to exacerbate a concurrent medical condition or were inappropriate based on the presence of a comorbid medical condition. The presence of a drug–disease interaction was assessed by two prescribers at the point of abstract screening and full-text review. In instances of uncertainty, the Australian Medicines Handbook was consulted, which is the primary source of medicine information for prescribers in Australia [33]. When the drug monograph stated that there was a precaution or contraindication to administering the drug in patients with a particular disease, then this was considered to be a drug–disease interaction. This method of adjudication is consistent with a previously published systematic review on drug–disease interactions [28]. This review did not include studies of published tools to assess for potentially inappropriate medicines, such as the Screening Tool of Older Persons’ Prescriptions (STOPP) or Beers criteria, as these include forms of IP beyond drug–disease interactions (e.g. inappropriate medicines and drug–drug interactions) that were not within the scope of this review. However, studies of potentially inappropriate medicine criteria that included the outcomes of interest for a subgroup of potentially inappropriate medicines that represented drug–disease interactions were included.
2.1.3 Outcomes
The review considered studies that reported mortality (using any measure including all-cause mortality, non-cancer mortality and cause-specific mortality) and hospital readmission (including emergency department presentations), using any measure including all-cause readmission, cause-specific readmission and readmission for medicine-related problems. Readmissions were chosen as the outcome as this review only considered studies involving hospitalised individuals. There was no minimum duration of follow-up.
2.1.4 Types of Studies
The included studies were required to have some form of accepted measurement of the risk of exposure to drug–disease interactions relative to a comparator group. The review included observational studies as well as experimental and quasi-experimental study designs such as randomised controlled trials, non-randomised trials, before-after studies and interrupted time-series studies, which reported on the risk associated with an intervention. Review articles, case reports and case series were excluded.
2.1.5 Search Strategy
A three-step search strategy was utilised to locate both published and unpublished studies. First, an initial limited search of MEDLINE (PubMed) was undertaken to identify articles on the topic. The text words contained in the titles and abstracts of relevant articles, and the index terms used to describe the articles were used to develop a full search strategy for the databases to be searched. These comprised MEDLINE (OVID), CINAHL (EBSCO), EMBASE (OVID), Web of Science, SCOPUS and the Cochrane Library. These databases were searched from inception to 12 July, 2022. The ‘grey literature’ was searched using Google Scholar. Only studies published in English were included as we did not have translation facilities to adapt the search strategy for other languages to ensure all non-English articles were captured nor screen, appraise and extract outcomes from these articles. The search terms were developed in consultation with a librarian specialising in health databases (Appendix A of the Electronic Supplementary Material [ESM]). The search strategy was adapted from Mekonnen et al. and Sirois et al. [27, 34]. All efforts were made to obtain full-text manuscripts by external requests through the university library, contacting authors and purchasing full-text articles were available.
2.1.6 Study Selection
Following the search, all identified citations were collated and uploaded into Covidence (Covidence, Melbourne, VIC, Australia) and duplicates removed. Following a pilot test, titles and abstracts were screened by two independent reviewers (JI and TT or KB) for assessment against the inclusion criteria. Potentially relevant studies were retrieved in full.
The full text of selected citations was assessed in detail against the inclusion criteria by two independent reviewers (JI and KB). Reasons for exclusion of papers were recorded. Any disagreements between the reviewers at each stage of the selection process were resolved through discussion with a third investigator (AM).
2.2 Assessment of Methodological Quality
The methodological quality was assessed using the Joanna Briggs Institute Critical Appraisal Checklists for randomised controlled trials, cohort studies, analytical cross-sectional studies and case-controlled studies [35]. This was undertaken by two independent reviewers (JI and KB). Any uncertainty was resolved through discussion with a third investigator (AM). Authors were contacted to request missing or additional data for clarification, as required. Studies with scores <50% were considered to have a high risk of bias, 50–70% were considered to have a moderate risk of bias and >70% were considered to have a low risk of bias. All studies, regardless of the results of their methodological quality, underwent data extraction and synthesis.
2.3 Data Extraction
Data was extracted from studies included in the review by one author (JI) using a pre-defined data extraction template. The data extracted included specific details about the study design, sample size, age of participants included, inclusion criteria, study duration, follow-up period, intervention where appropriate, definition used to determine drug–disease interactions and adverse outcomes (see Appendix B of the ESM). Authors of papers were be contacted to request missing or additional data as required. Once the primary data extraction was complete all authors reviewed the data extracted for each of the studies.
2.4 Data Synthesis
Studies were pooled in a statistical meta-analysis using Stata 17 (StataCorp LLC, College Station, TX, USA). Effect sizes were expressed as relative risk with 95% confidence intervals (95% CIs). Where effect estimates and standard errors were not available, they were calculated from crude data along with 95% CIs.
In a random-effects model, untransformed effect-size estimates were used to calculate the weighted mean correlation coefficient. The random-effects model was selected given the anticipated clinical heterogeneity of the studies. Heterogeneity was assessed statistically using the Cochrane Q test and I2. Where a meta-analysis was not possible, the findings were presented in narrative form. If there were more than ten studies in the meta-analysis, then a funnel plot was to be generated to assess for publication bias. It was planned to perform statistical tests for funnel plot asymmetry (Egger’s test, Begg’s test or Harbord’s test) where appropriate.
2.5 Analysis of Subgroups or Subsets
Subgroup analyses were planned where there were sufficient data in order to assess for heterogeneity. These were to investigate age groups (i.e. middle-aged and older adults), sex (i.e. male or female), comorbidities (based on organ system) and readmission type (i.e. all-cause readmission, cause-specific readmission or readmission for medication-related problem). Sensitivity analyses were planned where there were sufficient data to confirm the robustness of the findings. These were to include the method used to measure mortality (i.e. all-cause vs non-cancer mortality) and control for confounding by comorbidity (i.e. regression vs propensity score analysis) as well as the selected covariates to account for comorbidity (i.e. count of chronic medical conditions or Charlson Comorbidity Index).
2.6 Assessing Certainty in the Findings
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach for grading the certainty of evidence was applied by two authors (JI and KB). [36, 37] Observational studies were considered to have low certainty whereas randomised trials were considered to have high certainty. This certainty level was downgraded if there was risk of bias, inconsistency, indirectness or imprecision in the studies and upgraded if there was a large effect, dose response or all plausible confounding factors and biases were accounted for in the studies. This was used to determine the final level of certainty as high, moderate, low or very low.
3 Results
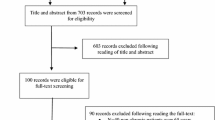
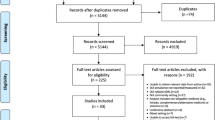
A total of 11,846 records were identified. After removing duplicates, 6318 records were screened by title and abstract for inclusion. Of these, 563 required full-text review, 559 were excluded with the most common reasons being that they did not involve drug–disease interactions (n = 205), they were in the wrong setting (n = 107), had the wrong outcomes (n = 88) or were not assessing IP (n = 45).
During the screening process, several studies were identified that initially appeared to meet the inclusion criteria but were later excluded from the analysis after the full-text review. The following studies are examples of excluded studies with explanations. Delgado et al. undertook a retrospective cohort study to estimate the effect of potentially IP on adverse health outcomes [38]. However, patients were in the primary care setting rather than hospitalised so this study was excluded because of being performed in the wrong setting. Wessinger et al. undertook a case-controlled study assessed patients using selective serotonin reuptake inhibitors in gastrointestinal haemorrhage based on the theory that this class inhibits platelet function and haemostasis [39]. However, the study did not report on the outcomes of interest, which were mortality or readmissions. Rassen et al. performed a cohort study on cardiovascular outcomes and mortality in those with ischaemic heart disease or acute coronary disease receiving clopidogrel with proton pump inhibitors [40]. However, the Australian Medicines Handbook states that “available evidence does not support a clinical effect from any interaction between clopidogrel and proton pump inhibitors”, so this was not considered a drug–disease interaction [33].
A total of four studies met the inclusion criteria and were include in this review (Fig. 1). All four were observational [41]. The samples sizes of these studies ranged from 119 to 5046 participants. There were no studies of established potentially inappropriate medicines criteria (e.g. STOPP or Beers criteria) that reported on subgroups that were exclusively drug–disease interactions. No studies were excluded because less than 80% of participants were middle-aged or older aged.
3.1 Risk of Bias
Assessment of methodological quality was variable between studies. There were two retrospective cohort studies [42, 43] and two prospective cohort studies [41, 44]. The two retrospective cohort studies had a moderate and high risk of bias. The two prospective cohort studies were at a low and moderate risk of bias (Table 1).
Of the four cohort studies, the baseline characteristics of groups exposed or not exposed to drug–disease interactions were not similar in the study by Gigante et al. [41]. It was unclear whether the exposed and unexposed groups were similar in the studies by Rodighiero et al. and O’Shaughnessy et al. [42, 44]. It was unclear whether the follow-up was complete or the strategies used to address the incomplete follow-up in the studies by Rodighiero et al. and Bingham et al. [42, 43]. The authors were contacted to clarify unclear methodological factors but no responses were received.
Only two of the four studies identified confounding factors and adjusted for covariates in their statistical analysis. These were retrospective and prospective cohort studies, respectively. Rodighiero et al. adjusted for age, sex, Charlson Comorbidity Index and procedure performed for aortic valve replacement [42]. Gigante et al. adjusted for lifestyle factors (smoking and alcohol use), polypharmacy and multiple comorbidities including hypercholesterolaemia, myocardial infarction, peripheral vascular disease, chronic liver disease, chronic kidney disease and dementia [41]. The remaining studies by Bingham et al. and O’Shaughnessy did not identify confounding factors or adjust for covariates in their statistical analysis [43, 44].
3.2 Characteristics of Included Studies
The details of the studies are outlined in Table 2. The studies identified were prospective cohort (n = 2) and retrospective cohort studies (n = 2). Studies were conducted in Canada, Italy, Ireland and the USA.
The studies included both male and female individuals (the percentages of male individuals ranged from 48 to 57% between studies) with the mean or median age from 73 and 80 years. Two of the studies were limited to older adults using cut-offs of 65 and 70 years, respectively [41, 42]. Although two studies included adults of all ages, the median age and distribution suggests that few if any middle-aged adults were included [43, 44].
Studies differed with respect to the cohort of hospitalised patients included. Gigante et al. included all hospitalised patients [41]. Bingham et al. included those referred to pharmacy for transition of care services [43]. O’Shaughnessy et al. included hospitalised patients with chronic kidney disease [44]. Rodighiero et al. included and those hospitalised and discharged alive after having aortic valve replacements [42].
Although all studies reported on exposure to drug–disease interactions, the tools used to define this type of IP varied across studies. Rodighiero et al. used the MedSafer electronic tool, which identified drug interactions and potentially inappropriate medicines considering patient specific comorbidities [42]. This included a subgroup analysis for patients taking diltiazem with heart failure. Bingham et al. assessed drug–disease interactions as identified by a pharmacist [43]. O’Shaughnessy et al. assessed non-compliance with recommendations in the Renal Drug Handbook and British National Formulary [44]. Gigante et al. assessed drugs that were contraindicated based on renal function according to clinical guidelines or the product information [41].
Three studies assessed the outcome of readmissions [41,42,43] and two assessed mortality [41, 44]. Three studies recruited patients over 6 years [41], 1 year [43] and 6 months [44], respectively. The study by Rodighiero et al. did not report the study duration. Follow-up for the outcomes varied between studies. Rodighiero et al. assessed all-cause hospital readmissions with follow-up to 30 days although it was unclear whether this was measured from the time of admission, aortic valve replacement or discharge [42]. Bingham et al. assessed hospital readmissions at 30-days following discharge [43]. O’Shaughnessy et al. assessed mortality at 1 year from the date of assessment for drug–disease interactions [44]. Gigante et al. assessed all-cause mortality, cardiovascular mortality, readmissions and cardiovascular readmissions as well as composited of death and readmission or cardiovascular death and readmission. They assessed outcomes with follow-up to 3 months from discharge between 2010 and 2016 and 12 months from discharge between 2012 and 2016 [41].
3.3 Association of Drug–Disease Interactions with Readmissions and Mortality
Two of the studies showed an association between drug–disease interactions and mortality, or readmissions [41, 42]. Rodighiero et al. performed a retrospective cohort study of adults aged ≥70 years who were discharged alive after transcatheter aortic valve replacement or surgical aortic valve replacement at two university hospitals [42]. The study found that the combination of diltiazem and heart failure conferred an increased risk of readmission at 30 days (adjusted odds ratio [OR] = 3.16, 95% CI 1.04–36.41). Bingham et al. assessed the risk of drug–disease interactions in adults referred to transition of care services who received a pharmacist review [43]. Drug–disease interactions were not associated with hospital readmissions at 30 days following discharge. O’Shaughnessy et al. assessed non-compliance with the recommendations for dosing in the Renal Drug Handbook or British National Formulary in hospitalised patients with chronic kidney disease [44]. They did not show a difference in mortality at 1 year from the date of assessment in patients who were and were not exposed to drug–disease interactions. Gigante et al. assessed the use of drugs that were contraindicated according to renal function based on clinical guidelines and the product information for the chronic conditions of hypertension, diabetes, atrial fibrillation, coronary artery disease and chronic heart failure [41]. The use of medicines that were contraindicated according to renal function was associated with an increased risk of all-cause death (adjusted OR = 1.46, 95% CI 1.11–1.94) and a composite of any death or rehospitalisation (adjusted OR = 1.29, 95% CI 1.03–1.62). Conversely, there was no association between this IP and cardiovascular death (adjusted OR = 1.25, 95% CI 0.81–1.92), cardiovascular rehospitalisation (adjusted OR = 1.13, 95% CI 0.69–1.85) or a composite of cardiovascular death or rehospitalisation (adjusted OR = 1.22, 95% CI 0.87–1.71).There were no studies that reported on outcomes for middle-aged adults exposed to drug–disease interactions.
3.4 Meta-Analysis
Three of the four observational studies were suitable for inclusion in a meta-analysis (Fig. 2). The fourth study was not included as the risk was not presented as an OR and the raw numbers were not published to calculate this. The corresponding author was contacted but the original data were not available. In the meta-analysis, there was no significant association between drug–disease interactions and hospital readmissions (OR = 1.0 95% CI 0.80–1.38) with limited heterogeneity (I2 = 0.0%, P = 0.395). However, the limited heterogeneity in the meta-analysis may be attributed to the fact that most of the weight was placed on a single study by Gigante et al. Given that there were only three studies in the meta-analysis, it was not possible to perform the planned subgroup or sensitivity analyses.
3.5 Certainty of Evidence
The certainty in the body of evidence was very low for the outcomes of readmissions and mortality. This was because the identified studies were observational rather than interventional. The certainty was downgraded from low to very low because of the risk of bias, inconsistencies in effects between studies and imprecision within studies.
4 Discussion
Despite the prevalence of multimorbidity in hospitalised adults, there were few studies that examined the risk of drug–disease interactions and mortality or readmission in these individuals. Two studies found an increased risk of readmissions or mortality in hospitalised older adults exposed to two specific types of drug–disease interactions. These were diltiazem use in patients with heart failure and the use of medicines contraindicated based on renal function. However, there was no association between drug–disease interactions and readmissions in older adults in the meta-analysis. The absence of a standardised definition for drug–disease interactions is a limitation of the literature in this field. The observational studies in the literature were at risk of bias often because of inadequate identification of and adjustment for covariates. Further research on drug–disease interactions and adverse outcomes is necessary given the rising prevalence of multimorbidity and polypharmacy in parallel with the ageing population. This will include pharmacoepidemiological studies assessing the association of drug–disease interactions with clinical outcomes as well as systematic reviews and meta-analyses in different populations including community-dwelling adults and those in the residential care setting.
Multimorbidity is common in the hospitalised population and many patients are exposed to polypharmacy because of guideline recommendations for chronic medical conditions [45]. In one review of 12 national guidelines in the UK, there were 32 potentially serious drug–disease interactions between drugs recommended for patients with multimorbidity, which were mainly related to chronic kidney disease [28]. Previous studies have demonstrated conflicts between disease-specific guidelines as well as the co-prescribing of medicines resulting in a drug–disease interaction in these patients [10,11,12,13]. In an Australian study, one third of recently hospitalised adults with diabetes had treatment-related medication conflicts including the prescription of corticosteroids and antipsychotics, which exacerbate hyperglycaemia as well as the use of non-steroidal anti-inflammatory drugs that may worsen renal function especially in the setting of diabetic nephropathy [10]. In another study of older adults dispensed an antidepressant, 87% had at least one comorbid condition that may cause a treatment-related conflict. Examples included antihypertensive agents that may increase the risk of hypotension and falls in adults with osteoporosis in addition to selective serotonin retake inhibitors, which were associated with reduced bone mineral density and an increased fracture risk [33, 46,47,48]. Similarly, in community-dwelling older adults with heart failure, almost all had a comorbidity that may cause a treatment-related conflict. For instance, those with comorbid diabetes taking metformin have an increased risk of lactic acidosis, those with glaucoma taking a topical beta-adrenoceptor antagonist may develop hypotension and bradycardia, and those taking tricyclic antidepressants have an increased risk for arrythmia and orthostatic hypotension [33, 49, 50]. Despite these studies demonstrating the prevalence of potential drug–disease interactions due to comorbidities in the community setting, there are relatively few studies assessing whether drug–disease interactions are associated with readmissions or mortality in hospitalised older adults who may be at the highest risk and where there is an opportunity to intervene to improve prescribing.
This review has identified the lack of a standardised definition for drug–disease interactions in the literature. The studies identified in this review assessed either individual drug–disease interactions or groups of interactions that shared a common pathophysiology such as reduced excretion in chronic kidney disease. Two studies identified in this review assessed drugs contraindicated based on renal function and one assessed a non-dihydropyridine calcium channel blocker used in patients with heart failure. These interactions are different from the drug–disease combinations that have previously been identified in the outpatient setting. The most common categories of diseases where drug–disease interactions occurred were cardiovascular and renal conditions consistent with a previous study of emergency department patients [51]. This is unsurprising given cardiovascular conditions are the most common group of comorbidities in both community-dwelling and hospitalised adults [52]. Patients using anti-cancer drugs might be expected to have high rates of drug–disease interactions although this was not identified in the included studies. For instance, anthracyclines are contraindicated in chronic heart failure owing to a risk of cardiotoxicity and immune checkpoint inhibitors are contraindicated in autoimmune diseases because of a risk of immune-related adverse effects [53,54,55,56]. These interactions might have not been identified by this review given the restriction to hospitalised adults as the treatment of solid organ cancers occurs almost exclusively in the outpatient setting. Hepatic impairment might also be expected to be associated with drug–disease interactions as this is a major mechanism of drug excretion, although no such studies were found.
Consensus is needed on the definition of drug–disease interactions to guide further studies in this field. These is a published methodology to determine what constitutes a drug–disease interaction by a multidisciplinary expert panel with review of published evidence and re-evaluation over time [57]. This has been used to develop a consensus-based list of clinically relevant drug–disease interactions using a Delphi consensus similar to the process used to develop the Beers or STOPP criteria [9]. However, no studies were identified in this review that assessed these interactions as a group, which is the way that the Beers and STOPP criteria have been researched [27]. However, first-generation calcium channel blockers in patients with heart failure and non-steroidal anti-inflammatory drugs in chronic kidney disease were both clinically relevant interactions on this list, which encompassed the two studies identified by review [9]. Further research is needed determine whether these drug–disease interactions as a group or as individuals are associated with adverse health outcomes including mortality, readmissions and medication-related harm [58, 59]. It is possible that novel approaches using data mining or machine learning will be able to identify drug–disease interactions using big data. This has been done to predict medication combinations association with medication-related admissions, although these approaches may identify medication combinations that occur in high-risk individuals as opposed to problematic drug–disease interactions [60].
The observational studies in this review were at risk of bias and this was one of the reasons for the low certainty in the evidence. Two of the studies were at moderate risk of bias, one was at high risk of bias and one was at low risk of bias. Bias was due to a lack of adequate adjustment of covariates, inadequate statistical power and a lack of clarity about those lost to follow-up. Ridighiero et al. assessed specific drug–disease interactions in a high-risk cohort undergoing aortic valve replacement [42]. The authors found that diltiazem use in heart failure was associated with an increased risk of all-cause hospital admissions at 30 days. This is biologically plausible as diltiazem is avoided in heart failure because of negative inotropic effects that may precipitate heart failure exacerbations. Gigante et al. also showed that the use of drugs contraindicated based on renal function were associated with a higher risk of all-cause mortality or a composite of any mortality or rehospitalisation [41]. However, it is possible that there was residual confounding in this study as adjustment was performed for various comorbidities but not demographic factors, severity of renal dysfunction or dialysis use. This is relevant because age, end-stage kidney disease and dialysis use are independent predictors of death in this population [61]. The remaining two studies by Bingham et al. and O’Shaughnessy et al. were inadequately powered, which may explain the lack of a significant association, although this was not overcome by a meta-analysis [43, 44].
Future high-quality studies are needed that address the methodological limitation of the existing literature including the use of statistical methods to address confounding, ensuring adequate power and reporting on loss to follow-up. The standard confounders in studies of medication-related outcomes are age, sex, comorbidity burden and number of medications. Comorbidities may be assessed using a count of comorbidities from a published list such as Barnett et al., the Charlson Comorbidity Index, which can be calculated from International Classification of Diseases, Tenth Revision codes from hospital coding or the Cumulative Illness Rating Scale [26, 62, 63]. The Charlson Comorbidity Index has the advantage of being associated with adverse health outcomes such as 10 year survival [64]. Additional covariates such as markers of renal and hepatic function are ideally included used as these are the main mechanisms of drug excretion and both chronic kidney disease and hepatic failure are associated with both mortality and hospital admissions. Two of the four studies used appropriate statistical techniques to control for confounding such as multivariate regression. Propensity score matching is another method to account for differences between groups by matching exposed and control participants on a variety of baseline characteristics [65, 66]. This is said to simulate a clinical trial, known as target trial emulsion, by ensuring that the selected individuals are matched. This method is well suited to large databases of routinely collected data given the large number of potential control participants for matching. However, residual confounding remains an issue as occurs in all observational studies [67]. The number of participants lost to follow-up was not well reported in the identified studies. Given these analyses are often using routinely collected data, it is difficult to identify the number of individuals who were lost to follow-up unlike a traditional registry. Authors need to report the nature of the data source where outcomes were extracted from. The risk of loss to follow-up is low when using is a universal electronic medical record, population-based dataset or national death registry. However, it may be higher in towns or states where there are multiple electronic medical records such that one does not cover the entire town or state. In the latter case, individuals may have readmissions or deaths in another system that is not captured in the study data source. Studies were not always adequately powered to detect a clinically meaningful difference in mortality or readmissions. Large population-based datasets should make it easier to adequately power studies to detect clinically important difference in outcomes such as mortality or readmissions.
Giving the rising rates of multimorbidity and polypharmacy worldwide, further studies are needed to determine whether drug–disease interactions are associated with mortality and readmissions in middle-aged and older adults. There were no studies identified that included middle-aged adults exposed to drug–disease interactions and this group has been largely excluded from research relating to IP. This is despite middle-aged adults accounting for the largest number of adults with multimorbidity in absolute terms in the UK [26]. This systematic review only examined hospitalised patients, which may have explained the lack of studies examining middle-aged adults who are more likely to receive care in the outpatient setting. While waiting for additional studies to be performed, a further systematic review on drug–disease interactions may broaden the populations to community-dwelling adults, including younger and middle-aged adults, as well as adults in residential care.
There are several limitations of this review and our methodology. First, the lack of an accepted definition of drug–disease interactions may have led to inconsistency in the screening and review process. This was mitigated for by having two reviewers for all articles, using the Australian Medicines Handbook as a reference source to identify these interactions and having a third reviewer to adjudicate on disagreements. Nevertheless, it is possible that articles that others might have considered as drug–disease interactions might not have been included based on the interpretation of the authors or the Australian Medicines Handbook precautions for the medication. Second, given the large number of articles in the initial screen, it is possible that relevant articles may have been missed. However, this was prevented by having two authors for screening and requiring a consensus before an article was included or excluded. Third, we were not able to include articles in languages other than English because of the lack of local translation services to facilitate screening, appraisal and outcome extraction. This may have led to articles not published in English being missed and may limit the generalisability of our findings to non-English speaking regions. Fourth, there was methodological and clinical heterogeneity in the studies identified that may limit the generalisability of these findings. Each study assessed a different type or group of drug–disease interactions. Although all studies looked at some combination of drugs and diseases, some assessed a single drug and disease (i.e. verapamil in chronic heart failure) whereas other looked at groups of drugs with one disease state (i.e. medication use that was contraindicated based on chronic kidney disease). This is partly owing to the lack of an accepted definition of clinically significant drug–disease interactions. Fifth, the identified observational studies are at risk of residual confounding that may have affected the results and meta-analysis. Only two of the four studies identified adjusted for confounding factors in their analysis. Of those that did adjust for covariates, one did not include age, which is a standard covariate in medication-related research. Adjustment for covariates is particularly important in this field as both medicines and the comorbidities for which they are prescribed may individually be associated with mortality and readmissions.
5 Conclusions
There were few studies assessing whether drug–disease interactions in hospitalised older adults were associated with mortality and readmissions. The studies that were identified were at risk of bias owing to the inadequate identification and adjustment for confounding factors, a lack of statistical power to detect clinically important differences and uncertainty about the number of individuals lost to follow-up. Noting these deficiencies in the literature, only two of the four identified studies found an increased risk of readmissions or mortality in hospitalised patients exposed to specific types of drug–disease interactions. However, in the three studies amenable to a meta-analysis, there was no association between drug–disease interactions and readmissions in older adults. There were no studies where hospitalised middle-aged adults were well represented despite the large number of multimorbid adults in this age group. The literature is limited by the lack of an accepted and standardised definition of drug–disease interactions. Further high-quality studies are needed to assess which types of drug–disease interactions are associated with mortality and readmission as well as whether rectifying this form of IP improves patient outcomes. A published consensus list of drug–disease interactions may be used in future studies [9]. While awaiting these studies, subsequent systematic reviews may examine these interactions in other populations including community-dwelling adults and those in residential care.
References
Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–9.
Koné Pefoyo AJ, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:1–11.
Almirall J, Fortin M. The coexistence of terms to describe the presence of multiple concurrent diseased. J Comorbidity. 2013;3:4–9.
Osanlou R, Walker L, Hughes DA, Burnside G, Pirmohamed M. Adverse drug reactions, multimorbidity and polypharmacy: a prospective analysis of 1 month of medical admissions. BMJ Open. 2022;12: e055551.
Chin MH, Wang LC, Jin L, Mulliken R, Walter J, Hayley DC, et al. Appropriateness of medication selection for older persons in an urban academic emergency department. Acad Emerg Med. 1999;6:1232–42.
Giron MS, Wang HX, Bernsten C, Thorslund M, Winblad B, Fastbom J. The appropriateness of drug use in an older nondemented and demented population. J Am Geriatr Soc. 2001;49:277–83.
Hanlon JT, Schmader KE, Boult C, Artz MB, Gross CR, Fillenbaum GG, et al. Use of inappropriate prescription drugs by older people. J Am Geriatr Soc. 2002;50:26–34.
Hailu BY, Berhe DF, Gudina EK, Gidey K, Getachew M. Drug related problems in admitted geriatric patients: the impact of clinical pharmacist interventions. BMC Geriatr. 2020;20:13.
Lindblad CI, Hanlon JT, Gross CR, Sloane RJ, Pieper CF, Hajjar ER, et al. Multidisciplinary Consensus Panel. Clinically important drug-disease interactions and their prevalence in older adults. Clin Ther. 2006;28:1133–43.
Caughey GE, Barratt JD, Shakib S. Medication use and potentially high-risk prescribing in older patients hospitalized for diabetes. Diabet Med. 2017;34:432–9.
Caughey GE, Roughead EE, Shakib S. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing. 2010;39:488–94.
Caughey GE, Roughead EE, Shakib S, Vitry AI, Gilbert AL. Comorbidity and potential treatment conflicts in the elderly with heart failure. Drugs Aging. 2011;28:1–7.
Caughey GE, Roughead EE, Vitry AI. Comorbidity in the elderly with diabetes: identification of areas of potential treatment conflicts. Diabetes Res Clin Pract. 2010;87:385–93.
Caughey GE, Preiss AK, Vitry AI, Gilbert AL, Roughead EE. Comorbid diabetes and COPD: impact of corticosteroid use on diabetes complications. Diabetes Care. 2013;36:3009–14.
Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–40.
Jadad AR, To MJ, Emara M, Jones J. Consideration of multiple chronic diseases in randomized controlled trials. JAMA. 2011;306:2670–2.
Fanning L, Ilomäki J, Bell JS, Dārziņš P. The representativeness of direct oral anticoagulant clinical trials to hospitalized patients with atrial fibrillation. Eur J Clin Pharmacol. 2017;73:1427–36.
Tinetti ME, McAvay G, Trentalange M, Cohen AB, Allore HG. Association between guideline recommended drugs and death in older adults with multiple chronic conditions: population based cohort study. BMJ. 2015;351: h4984.
Caughey GE, Inacio MC, Bell JS, Vitry AI, Shakib S. Inclusion of older people reflective of real-world clinical practice in cardiovascular drug trials. J Am Heart Assoc. 2020;9: e016936.
Tinetti ME, McAvay G, Trentalange M, et al. Association between guideline recommended drugs and death in older adults with multiple chronic conditions. BMJ. 2015;351: h4984.
Peeters G, Tett SE, Hollingworth SA, et al. Associations of guideline recommended medications for acute coronary syndromes with fall-related hospitalizations and cardiovascular events. J Gerontol A Biol Sci Med Sci. 2017;72:259–65.
Sheppard JP, Koshiaris C, Stevens R, Lay-Flurrie S, Banerjee A, Bellows BK, et al. The association between antihypertensive treatment and serious adverse events by age and frailty: a cohort study. PLoS Med. 2023;20: e1004223.
Kremer KM, Braisch U, Rothenbacher D, Denkinger M, Dallmeier D. Systolic blood pressure and mortality in community-dwelling older adults: frailty as an effect modifier. Hypertension. 2022;79:24–32.
Faridi KF, Strom JB, Kundi H, Butala NM, Curtis JP, Gao Q, et al. Association between claims-defined frailty and outcomes following 30 versus 12 months of dual antiplatelet therapy after percutaneous coronary intervention: findings from the EXTEND-DAPT Study. J Am Heart Assoc. 2023;2: e029588.
Mondal A, Li A, Edusa S, Gogineni A, Karipineni S, Abdelhafez S, et al. Does statin use in frail patients provide survival benefits? Insights from a meta-analysis. Curr Probl Cardiol. 2024;49: 102038.
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43.
Mekonnen AB, Redley B, de Courten B, Manias E. Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87:4150–72.
Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;350: h949.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Kastner M, Wilczynski NL, Walker-Dilks C, McKibbon KA, Haynes B. Age-specific search strategies for medline. J Med Internet Res. 2006;8: e25.
Chapter 3. Defining the criteria for including studies and how they will be grouped for the synthesis. Available from: https://training.cochrane.org/handbook/current/chapter-03. [Accessed 1 Jun 2024]
Sirois C, Laroche ML, Guénette L, Kröger E, Cooper D, Émond V. Polypharmacy in multimorbid older adults: protocol for a systematic review. Syst Rev. 2017;6:104.
Australian medicines handbook. Available from: https://amhonline.amh.net.au/. [Accessed 1 Jun 2024].
Sirois C, Domingues NS, Laroche ML, Zongo A, Lunghi C, Guénette L, et al. Polypharmacy definitions for multimorbid older adults need stronger foundations to guide research, clinical practice and public health. Pharmacy (Basel). 2019;7:126.
JBI manual for evidence synthesis. Available from: https://synthesismanual.jbi.global. [Accessed 1 Jun 2024].
Holger S, Suzanne H, Gordon G, Elie AA, Faruque A. The GRADE approach and Bradford Hill’’s criteria for causation. J Epidemiol Community Health. 2011;65:392.
Morgan RL, Thayer KA, Bero L, Bruce N, Falck-Ytter Y, Ghersi D, et al. GRADE: assessing the quality of evidence in environmental and occupational health. Environ Int. 2016;92–93:611–6.
Delgado J, Jones L, Bradley MC, Allan LM, Ballard C, Clare L, et al. Potentially inappropriate prescribing in dementia, multi-morbidity and incidence of adverse health outcomes. Age Ageing. 2021;50:457–64.
Wessinger S, Kaplan M, Choi L, Williams M, Lau C, Sharp L, et al. Increased use of selective serotonin reuptake inhibitors in patients admitted with gastrointestinal haemorrhage: a multicentre retrospective analysis. Aliment Pharmacol Ther. 2006;23:937–44.
Rassen JA, Choudhry NK, Avorn J, Schneeweiss S. Cardiovascular outcomes and mortality in patients using clopidogrel with proton pump inhibitors after percutaneous coronary intervention or acute coronary syndrome. Circulation. 2009;120:2322–9.
Gigante A, Proietti M, Petrillo E, Mannucci PM, Nobili A, Muscaritoli M. Renal function, cardiovascular diseases, appropriateness of drug prescription and outcomes in hospitalized older patients. Drugs Aging. 2021;38:1097–105.
Rodighiero J, McDonald E, Lee T, Piazza N, Martucci G, Langlois Y, et al. Polypharmacy in older adults after transcatheter or surgical aortic valve replacement. Eur Heart J. 2021. https://doi.org/10.1093/eurheartj/ehab724.2829.
Bingham JM, Baugham L, Hilaneh A, Tranchina K, Arku D, Eckert B, et al. Assessing the impact of an advanced clinical decision support system on medication safety and hospital readmissions in an innovative transitional care model: a pilot study. J Clin Med. 2022;11:2070.
O’Shaughnessy M, Allen N, O’Regan J, Payne-Danson E, Mentre L, Davin D, et al. Agreement between renal prescribing references and determination of prescribing appropriateness in hospitalized patients with chronic kidney disease. QJM. 2017;110:623–8.
Aggarwal P, Woolford SJ, Patel HP. Multi-morbidity and polypharmacy in older people: challenges and opportunities for clinical practice. Geriatrics (Basel). 2020;5:85.
Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588–95.
Haney EM, Chan BK, Diem SJ, Ensrud KE, Cauley JA, Barrett-Connor E, et al. Osteoporitic Fractures in Men Study Group. Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med. 2007;167:1246–51.
Richards JB, Papaioannou A, Adachi JD, Joseph L, Whitson HE, Prior JC, et al. Candian Multicentre Osteoporosis Study Research Group. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–94.
Krum H, Jelinek MV, Stewart S, Sindone A, Atherton JJ, Hawkes AL. Guidelines for the prevention, detection and management of people with chronic heart failure in Australia 2006. Med J Aust. 2006;185:549–57.
Roughead EE, Barratt JD, Ramsay E, Pratt N, Ryan P, Peck R, et al. The effectiveness of collaborative medicine reviews in delaying time to next hospitalization for patients with heart failure in the practice setting. Circ Heart Fail. 2009;2:424–8.
Goldberg RM, Mabee J, Chan L, Wong S. Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med. 1996;14:447–50.
Heart, stroke and vascular disease: Australian facts. Available from: https://www.aihw.gov.au/reports/heart-stroke-vascular-disease/hsvd-facts/contents/comorbidity-of-heart-stroke-and-vascular-disease. [Accessed 1 Jun 2024].
Boland P, Pavlick AC, Weber J, Sandigursky S. Immunotherapy to treat malignancy in patients with pre-existing autoimmunity. J Immunother Cancer. 2020;8: e000356.
Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. 2020;7:26.
Cardiac toxicity associated with anthracyclines. Available from: https://www.eviq.org.au/clinical-resources/side-effect-and-toxicity-management/cardiovascular/1667-cardiac-toxicity-associated-with-anthracyclin. [Accessed 1 Jun 2024].
Management of immune-related adverse events (irAEs). Available from: https://www.eviq.org.au/clinical-resources/side-effect-and-toxicity-management/immunological/1993-management-of-immune-related-adverse-events#cardiac-toxicity. [Accessed 1 Jun 2024].
van Tongeren JMZ, Harkes-Idzinga SF, van der Sijs H, Atiqi R, van den Bemt BJF, Draijer LW, et al. The development of practice recommendations for drug-disease interactions by literature review and expert opinion. Front Pharmacol. 2020;11:707.
Tan ECK, Sluggett JK, Johnell K, Onder G, Elseviers M, Morin L, et al. Research priorities for optimizing geriatric pharmacotherapy: an international consensus. J Am Med Dir Assoc. 2018;19:193–9.
Kwoh CK, Ibrahim SA. Rheumatology patient and physician concordance with respect to important health and symptom status outcomes. Arthritis Rheum. 2001;45:372–7.
Fahmi A, Wong D, Walker L, Buchan I, Pirmohamed M, Sharma A, et al. Combinations of medicines in patients with polypharmacy aged 65–100 in primary care: large variability in risks of adverse drug related and emergency hospital admissions. PLoS One. 2023;18: e0281466.
Heaf J, Heiro M, Petersons A, Vernere B, Povlsen JV, Sørensen AB, et al. First-year mortality in incident dialysis patients: results of the Peridialysis study. BMC Nephrol. 2022;23:229.
Toson B, Harvey LA, Close JC. New ICD-10 version of the multipurpose Australian comorbidity scoring system outperformed Charlson and Elixhauser comorbidities in an older population. J Clin Epidemiol. 2016;79:62–9.
Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–64.
Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. 2011;174:613–20.
Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
Acknowledgements
The authors acknowledge Josephine McGill (Medical Librarian, Flinders University) for assistance in designing the search strategy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Joshua M. Inglis was supported by a Research Training Program scholarship through the University of Adelaide. There is no other funding to declare for this review.
Conflicts of Interest/Competing Interests
Joshua M. Inglis, Gillian Caughey, Tilenka Thynne, Kate Brotherton, Danny Liew, Arduino A. Mangoni and Sepehr Shakib have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated during this review are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Authors’ Contributions
JI developed the systematic review protocol with support from GC, TT, DL, AM and SS. JI, TT and KB screened the articles. JI and KB reviewed the full-text articles and performed the critical appraisal. JI extracted characteristics of the studies, performed the analysis and drafted the manuscript. All authors reviewed the extracted studies, revised the manuscript for important intellectual content and approved the final manuscript prior to submission.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Inglis, J.M., Caughey, G., Thynne, T. et al. Association of Drug–Disease Interactions with Mortality or Readmission in Hospitalised Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis. Drugs - Real World Outcomes (2024). https://doi.org/10.1007/s40801-024-00432-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s40801-024-00432-3