Abstract
Background and objectives
Nirmatrelvir/ritonavir was administered orally to manage mild to moderate symptoms of COVID-19 in adult patients. The objectives of this study were to (i) evaluate the cost-effectiveness of prescribing nirmatrelvir/ritonavir within 5 days of a COVID-19 illness in order to avert hospitalization within a 30-day period in the Malaysia setting; (ii) determine how variations in pricing and hospitalization rates will affect the cost-effectiveness of nirmatrelvir/ritonavir.
Methods
The 30-day hospitalization related to COVID-19 was determined using 1 to 1 propensity score-matched real-world data in Malaysia from 14 July 2022 to 14 November 2022. To determine the total per-person costs related to COVID-19, we added the cost of drug (nirmatrelvir/ritonavir or control), clinic visits and inpatient care. Incremental cost-effectiveness ratio (ICER) per hospitalization averted was calculated.
Results
Our cohort included 31,487 patients. The rate of hospitalization within 30 days was found to be 0.35% for the group treated with nirmatrelvir/ritonavir, and 0.52% for the control group. The nirmatrelvir/ritonavir group cost an additional MYR 1,625.72 (USD 358.88) per patient. This treatment also resulted in a reduction of 0.17% risk for hospitalization, which corresponded to an ICER of MYR 946,801.26 (USD 209,006.90) per hospitalization averted.
Conclusion
In Malaysia, where vaccination rates were high, nirmatrelvir/ritonavir has been shown to be beneficial in the outpatient treatment of adults with COVID-19 who have risk factors; however, it was only marginally cost effective against hospitalization for healthy adults during the Omicron period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A targeted approach involves recommending nirmatrelvir/ritonavir within the first 3 days of illness or for patients with comorbidities, with priority consideration for those with risk factors. |
Consideration should be given to the cost incurred by the healthcare system for using nirmatrelvir/ritonavir to avert COVID-19 hospitalizations. |
1 Introduction
Nirmatrelvir/ritonavir (PaxlovidTM) was the first oral antiviral agent to receive an emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for the treatment of mild to moderate COVID-19 patients [1]. This EUA was based on the EPIC-HR trial “Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients”, which demonstrated the efficacy of nirmatrelvir/ritonavir in reducing Covid-19 hospitalization or death to approximately 89% over 28 days [2]. Real-world evidence (RWE) analysis shows that the drug’s effectiveness was lower than in the clinical trial (between 56 and 88% reduction in hospitalization or death rate) [3,4,5]. This difference was largely due to the patient’s vaccination status and differences in COVID-19 variants during the study period. Given the reported reduction in effectiveness, there is a need to reassess the use of nirmatrelvir/ritonavir and its cost-effectiveness in public healthcare systems, especially for low- and middle-income countries.
In Malaysia, nirmatrelvir 300 mg/ritonavir 100 mg twice daily for 5 days has been prescribed for high-risk adult patients who tested positive for COVID-19, with mild to moderate symptoms within 5 days of illness in the public primary healthcare clinics.
Several economic and cost-effectiveness studies have been conducted, most of which have yielded positive results supporting the use of nirmatrelvir/ritonavir, especially in elderly and high-risk patients [6,7,8]. However, these studies were conducted from the perspective of developed countries or territories such as the USA, South Korea, and Hong Kong [6,7,8]. The input parameters used in these assessments are based on healthcare practices, infrastructure, financing models, and locally negotiated drug prices [9]. Hence, these results may not be directly applicable or generalizable to other countries with different healthcare systems and economic environments.
Therefore, it is important to evaluate the cost-effectiveness of nirmatrelvir/ritonavir based on real-world data in Malaysia, a middle-income country, to obtain accurate evidence to better inform policymakers about the use of this drug. The primary objective of this study was to evaluate the cost-effectiveness of outpatient prescribing of nirmatrelvir/ritonavir to avert hospitalization within 30 days of patients presenting to the primary care clinic in Malaysia with a confirmed diagnosis of COVID-19 within 5 days of illness onset. The evaluation includes the entire population receiving either nirmatrelvir/ritonavir or standard of care (control) during the study period, as well as stratified by different age groups. Our secondary objective was to determine how changes in pricing and hospitalization rates would affect the cost-effectiveness of nirmatrelvir/ritonavir.
2 Methods
2.1 Ethical Considerations
This study was registered in the National Medical Research Register and approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID-22-00938-2YN).
2.2 Data Source
Electronic medical records from Malaysia’s MySejahtera’s eCOVID system that registers patient encounters and antiviral prescription for COVID-19 patients were obtained from 647 public primary healthcare clinics across the country. The MySejahtera’s eCOVID system is an electronic system used in the public primary healthcare clinics throughout Malaysia in which the attending doctor will document all case details in the electronic case notes for every COVID-19 patient seen in the clinic. This platform is created to manage COVID-19 patients effectively by collecting patients’ demographics, medical histories, medications, clinical symptoms, progress notes, lab/test results, risk exposure, etc. into one integrated application. The data extracted from the eCOVID system included information on age, gender, prescription of nirmatrelvir/ritonavir, and text extraction from electronic case notes that documents COVID-19 symptoms, presence and type of comorbidities, and day of illness. The data were then linked to the Ministry of Health Malaysia registry of all COVID-19-related hospitalization.
2.3 Population
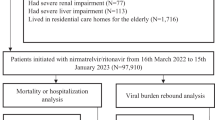
The study included all COVID-19 patients who visited 647 public primary healthcare clinics for treatment of COVID-19-related symptoms from 14 July, 2022 to 14 November 2022 as this is the period when nirmatrelvir/ritonavir was available in all public healthcare clinics throughout Malaysia. The study was conducted in the predominant BA.4, BA.5 and XBB Omicron period in Malaysia [10]. The index date was the date of the first public primary healthcare clinic encounter. Patients were included in the study if they had complete information, had an onset of illness within 5 days, or had symptoms related to COVID-19 (e.g., cough, fever, lethargy, vomiting, sore throat, headache, muscle or body aches, etc.) [11]. Patients who were critically ill (Clinical Stage 5 critically ill with or without other organ failures), required supplemental oxygen, or hospitalised as planned, were excluded from the study (see Online Supplementary Material (OSM) Fig. S1)
2.4 Exposure and Outcomes
The treatment group consisted of COVID-19 patients initiated with nirmatrelvir/ritonavir, whereas the control group comprised those not initiated with nirmatrelvir/ritonavir. The control group was given one bottle of diphenhydramine, 10 tablets of chlorpheniramine, 10 tablets of paracetamol, and 10 tablets of bromhexine for symptomatic treatment. The primary outcome was 30-day hospitalization.
The probabilities of hospitalization within 30 days from the date of first public primary healthcare clinic visit was obtained from the 1 to 1 propensity score-matched control group comparing to nirmatrelvir/ritonavir. The compiled dataset was obtained from the eCOVID register that recorded the information on COVID-19 patients being seen in the public primary healthcare clinic and COVID-19-related hospitalization register. A comprehensive description of the methods and analysis of effectiveness of nirmatrelvir/ritonavir is detailed elsewhere [12].
2.5 Costs
Direct medical cost was estimated in this analysis. Hospitalization cost for COVID-19 was obtained from Malaysian Diagnosis-Related Groups (Malaysian DRG), which is the casemix used by Ministry of Health Malaysia hospitals [13]. Total costs per-person were calculated as the sum of the medical cost related to the treatment of COVID-19. Medical costs were estimated by adding the cost of medications, clinic visit, and inpatient care multiplied by length of stay during the study period. All costs are reported in 2022 Malaysian Ringgit (MYR) and US dollars (USD) (1 USD = 4.53 MYR) [14]. The details of the input parameters used are shown in Table 1.
2.6 Cost-Effectiveness Analysis
The analysis were conducted from the perspective of the Ministry of Health as the payer for healthcare in Malaysia and only focused on patients’ first visit to the clinic. Since we had access to patient-level data, we did not use decision trees that relied on aggregated secondary data. Instead, we utilized real-world data on treatment effectiveness, following the method established by Abraham et al. [8] to more accurately capture the cost-effectiveness in the local context. After 1:1 propensity score matching by age, sex, date of registration in the public primary healthcare clinic, day of illness during encounter, vaccination status, and comorbidities with exact matching on date of registration in the public primary healthcare clinic, the number of patients was 10,483 for each group (OSM Table S1) [12].
For the cost-effectiveness analysis, we calculated the incremental cost-effectiveness ratio (ICER) of hospitalization averted. Global discounting for both costs and effectiveness were not applied because the study duration was less than 1 year, i.e. 30 days. We stratified the study population into two age groups (18–64 years, 65 years and above) to reflect the potential benefit of prescribing nirmatrelvir/ritonavir to elderly and non-elderly COVID-19 individuals. We performed subgroup analysis using four major comorbidities (hypertension, diabetes mellitus, respiratory disease, and cardiovascular disease) to examine specific comorbidities that may benefit from the prescription of nirmatrelvir/ritonavir. Additional subgroup analysis was conducted by vaccination status and day of illness. This was done to identify patients with particular comorbidities for whom the use of nirmatrelvir/ritonavir would be more cost-effective, as well as the optimum day and vaccination status of COVID-19.
One-way sensitivity analysis were performed by varying drug cost (− 100 to 100% changes from one course of nirmatrelvir/ritonavir (MYR1,132.50/USD250) and varying the hospitalization rates (0–8.00%) to simulate different severity levels of the COVID-19 strain. A probabilistic sensitivity analysis using 1000 simulations was further performed to account for parameter uncertainties in cost and effectiveness. Costs are considered as gamma distributed and effectiveness is considered as beta distributed. We do not have a formally established willingness-to-pay (WTP) threshold. Therefore, we used WTP to avert hospitalization comparing to different thresholds in the WTP acceptability curve, a comparable methodology by Savririnka [13]. Data wrangling and descriptive statistics were performed using SAS 9.4 (SAS Institute, Cary, NC, USA), whereas Microsoft Excel VBA was used in the calculation of probabilities and conducting economic evaluation (cost-effectiveness analysis and sensitivity analysis).
3 Results
The overall cohort included 31,487 patients. During our study period, hospitalization rates for both nirmatrelvir/ritonavir group and the control group were low, at 0.35% and 0.52%, respectively (Table 1).
Costs per person were significantly higher in the nirmatrelvir/ritonavir group, primarily due to higher drug cost compared with the control group. No significant differences were found in the mean length of stay in hospital for the nirmatrelvir/ritonavir group versus the control group (3.9 ± 5.9 vs. 3.7 ± 4.7, p = 0.06). Total average cost per patient in the treatment group was MYR9,093 (USD2,007), which was 21.8% higher compared to the control group, which registered an average total cost of MYR7,467 (USD1,648) per patient (Table 2).
Table 3 shows that nirmatrelvir/ritonavir cost an additional MYR1,626 per patient (USD359) and reduced the per patient hospitalization rate by 0.17% compared to control subjects. The corresponding ICER was MYR946,801 (USD209,007) per hospitalization averted with a number needed to treat (NNT) of 588.2.
Based on our data, the overall probability of hospitalization for people aged 65 years and above is higher than for people aged 18–64 years. However, the average length of stay in hospital in both age groups was not significantly different between the nirmatrelvir/ritonavir group and the control group (p > 0.05). The ICER of the nirmatrelvir/ritonavir group compared to the control group was MYR1.19 million (USD262.8 thousand) and MYR1.17 million (USD257.4 thousand), respectively, per hospitalization averted in the 18- to 64-years group and in the age group 65 years and above. The corresponding NNTs between age groups 18–64 years and 65 years and above are 493 and 714, respectively.
Our one-way sensitivity analysis on the change in drug price of nirmatrelvir/ritonavir is presented in Fig. 1. In the absence of a national threshold value, we calculated the ICER based on the change in drug price between the reference level and 100% in both directions. Fig. 2 highlights the ICER value based on the change in the control group’s hospitalization rate due to a more virulent COVID-19 strain, assuming that the hospitalization rate of patients treated with nirmatrelvir/ritonavir is held constant. ICER decreases exponentially as the hospitalization rate in the control group increases compared to the treatment group.
Results of subgroup analysis by comorbidities showed that the average length of stay in hospital was not significantly different among the four main comorbidities (hypertension, diabetes, respiratory disease, and cardiovascular disease) (p > 0.05) (OSM Table S2). The ICER for hospitalization averted for groups having hypertension, diabetes, or respiratory disease as a comorbidity showed a lower ICER compared to the overall population. In the cardiovascular disease group, hospital stay for the nirmatrelvir/ritonavir group was reduced, contributing to cost savings of MYR1,582 (USD349) for the nirmatrelvir/ritonavir group, thus producing a negative ICER of MYR149,054 (USD32,904) per hospitalization averted (OSM Table S4). The ICER per hospitalization averted was highest for respiratory disease (MYR731,342/USD161,444), followed by diabetes (MYR641,630/USD141,640) and hypertension (MYR20,806/USD4,593).
Based on the subgroup analysis by vaccination status, the ICER per hospitalization averted by the nirmatrelvir/ritonavir group compared with the control was fivefold lower in the partially vaccinated subgroup compared with the fully vaccinated/received booster group (ICER in partially vaccinated group: MYR203,666 (USD44,959); ICER in fully vaccinated group: MYR1,01 million (USD 223,772) (OSM Table S5). When stratified by day of illness, nirmatrelvir/ritonavir was more costly and less effective when administered on days 4 and 5 of illness.
In the probabilistic sensitivity analysis, the simulated ICERs are concentrated in the right quadrant of the cost-effectiveness plane (Fig. 3). This implies that nirmatrelvir/ritonavir is more clinically effective, but there is a large difference in cost. Therefore, the decision to use nirmatrelvir/ritonavir depends on whether the price of the drug can be reduced and the WTP to avert one COVID-19-related hospitalization. Based on the cost-effectiveness acceptability curve, the probability of nirmatrelvir/ritonavir being cost-effective was 42% for a WTP per hospitalization averted of MYR10,000. When the WTP per hospitalization averted increased to MYR50,000, the probability of nirmatrelvir/ritonavir being cost-effective was 55.7%. The probability of nirmatrelvir/ritonavir being cost-effective increased to 80% when the WTP per hospitalization averted increased to MYR150,000 (Fig. 4).
4 Discussion
Using nationally representative data, we found that the overall hospitalization rate within 30 days among high-risk adults with COVID-19 seen in the public primary healthcare clinic was low (0.44%).
Our study is the first to examine both the effectiveness and the cost effectiveness of nirmatrelvir/ritonavir in Malaysia during the Omicron period, reflecting the actual use of this drug in the population. The results of our study showed that the ICER per hospitalization averted comparing nirmatrelvir/ritonavir and control group was high. Using national casemix data from the Ministry of Health, the average cost of 4 days COVID-19 hospitalization was MYR8,084 (USD178.40). In contrast, the ICER per hospitalization averted for nirmatrelvir/ritonavir was MYR946,801 (USD209,007), indicating that the Ministry of Health Malaysia would have to pay 117 times more to avoid one hospitalization. Likewise, Ponce et al. found that it would cost the US Department of Defence $0.5 million to avert one hospitalization [16].
Considering the current low hospitalization rate and high vaccination coverage in Malaysia against the COVID-19 virus, the widespread use of nirmatrelvir/ritonavir to the general population would cause a significant increase in the cost of care, while providing minimal tangible benefits. Based on the average length of stay in real-world data, we found that the ICER for nirmatrelvir/ritonavir versus control group was dominant in patients with cardiovascular disease (more effective marginally and less costly by RM 1,519). This implies that the use of nirmatrelvir/ritonavir is cost saving in patients with cardiovascular disease.
It should be noted that our cost-effectiveness results depend primarily on the ability of nirmatrelvir/ritonavir to prevent hospitalization and the cost of the drug. For example, if the probability of hospitalization exceeds 1.3%, the ICER to avert one hospitalization is reduced by MYR100,000 (USD22,075). As part of better utilization of healthcare resources, the drug price would need to reduce by at least 95% to have an ICER value equivalent to the cost of 3–4 days’ hospital stay for COVID-19 patients. Our findings suggests that the adult population with no or only one risk factor may not be prioritized for nirmatrelvir/ritonavir use due to the significant financial implications, especially if COVID-19 is no longer considered a pandemic. Instead, a targeted policy approach should be adopted to prescribe nirmatrelvir/ritonavir, especially in countries such as Malaysia where the government heavily subsidizes treatment costs in public facilities. This targeted approach aims to benefit mild to moderately ill patients while ensuring hospital beds are available for those who need them most. Strategies such as negotiating drug prices and optimizing patient selection criteria can help reduce the financial burden. For example, nirmatrelvir/ritonavir may be prioritized for individuals with two or more risk factors (elderly, people with comorbidities living in remote areas where access to health care is difficult, etc.). This is similar to countries like Canada and Australia, where nirmatrelvir/ritonavir cost is reimbursed only for people with two or more comorbidities, elderly with comorbidities, or reside in long-term care facilities or in rural or remote communities [17, 18]. Furthermore, our results suggest that prescribing nirmatrelvir/ritonavir to partially vaccinated patients may be cost effective, similar to the results shown in some published studies [13, 17, 19]. Additionally, the drug may be cost-effective if administered within the first three days of illness, suggesting that nirmatrelvir/ritonavir may be beneficial when administered early in the course of disease. One of the policy implications from these findings is nirmatrelvir/ritonavir should be prescribed to patients who are partially vaccinated or suffering early from the disease. Hence, our study provides valuable information to help policymakers, especially those in low- and middle-income countries with limited resources, in deciding whether to expand the reimbursement/prescribing strategies for nirmatrelvir/ritonavir.
4.1 Strengths and Limitations
Our study had several strengths. This is one of the few economic evaluation studies of nirmatrelvir/ritonavir that utilized real-world data from a nationally representative cohort, during the dominance period of Omicron BA.4, BA.5, and XBB subvariants in Malaysia. However, our study does have some limitations. Firstly, the short duration of the study prevented us from assessing the potential cost-effectiveness of nirmatrelvir/ritonavir in reducing the risk of long covid. Secondly, due to the high vaccination rate, we were unable to evaluate the cost-effectiveness of nirmatrelvir/ritonavir in the unvaccinated group. Thirdly, we excluded severe patients (2369 individuals with symptomatic COVID with pneumonia who required supplemental oxygen, are critically ill, or are planned for admission) as they are not indicated for nirmatrelvir/ritonavir. We assumed that all COVID-19 patients prescribed nirmatrelvir/ritonavir adhered to and completed their treatment. The numbers of intensive care admissions and deaths were very small, which prevented a meaningful assessment of the cost-effectiveness of nirmatrelvir/ritonavir treatment to avert these outcomes in our analysis. Additionally, the effectiveness and economic evaluation assessment was based solely on the Malaysian population and was influenced by the dynamics of the COVID-19 situation. Therefore, the generalisability of the results varies greatly as our understanding of COVID-19 treatment evolves as new evidence emerges and the patient spectrum varies significantly in different countries and regions. However, it is important to consider factors such as the cost of healthcare delivery, COVID-19 vaccination rates, and healthcare financing models in context before adoption of our study findings for policies. In addition, future studies should look at the long-term cost-effectiveness of nirmatrelvir/ritonavir in preventing long COVID.
5 Conclusions
Nirmatrelvir/ritonavir showed limited cost-effectiveness in averting hospitalization for adults in the Omicron period. Based on current pricing and hospitalization rates, nirmatrelvir/ritonavir should be prioritized for adults with risk factors for developing severe COVID-19 illness and stand to benefit most from a cost-effectiveness perspective. Priority should be given to adults with cardiovascular comorbidities, partial vaccination status, and illness onset of not more than 3 days.
References
Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization for PAXLOVID™. 2021; https://www.fda.gov/media/155050/download. Accessed 15 Oct 2023.
Hammond J, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022. https://doi.org/10.1056/NEJMoa2118542.
Lai CC, et al. The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. Viruses. 2022. https://doi.org/10.3390/v14081706.
Tian F, Chen Z, Feng Q. Nirmatrelvir–ritonavir compared with other antiviral drugs for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2023;95(4): e28732. https://doi.org/10.1002/jmv.28732.
Wen W, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54(1):516–23. https://doi.org/10.1080/07853890.2022.2034936.
Jo Y, et al. Model-based cost-effectiveness analysis of oral antivirals against SARS-CoV-2 in Korea. Epidemiol Health. 2022;44: e2022034. https://doi.org/10.4178/epih.e2022034.
Kai Yeung, et al., Special Assessment of Outpatient Treatments for COVID-19, M.C.E.P.A. Council, Editor. 2022, Institute for Clinical and Economic Review.
Wai AK-C, et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Region Health Western Pacific. 2023. https://doi.org/10.1016/j.lanwpc.2022.100602.
Beinfeld M, et al. Oral treatments for outpatient COVID-19: effectiveness and value. J Manag Care Spec Pharm. 2022;28(8):903–9. https://doi.org/10.18553/jmcp.2022.28.8.903.
Our World in Data. SARS-CoV-2 Variants in analyzed sequences, Malaysia. 2023 https://ourworldindata.org/grapher/covid-variants-area?time=2022-07-04..2023-01-02&country=~MYS. Accessed October 15, 2023.
Ministry of Health Malaysia. Clinical Management of Confirmed COVID-19 Case in Adult and Paediatric. https://covid-19.moh.gov.my/garis-panduan/garis-panduan-kkm/ANNEX-2E-CLINICAL-MANAGEMENT-OF-CONFIRMED-COVID-19-31052022.pdf. Accessed January 15, 2023
Low EV, et al. Real world nirmatrelvir-ritonavir outpatient treatment in reducing hospitalization for high-risk COVID-19 patients during Omicron BA.4, BA.5 and XBB subvariants dominance in Malaysia: a retrospective cohort study. Int J Infect Dis. 2023;135:77–83. https://doi.org/10.1016/j.ijid.2023.08.003.
Savinkina A, et al. Population-Level Strategies for Nirmatrelvir/Ritonavir Prescribing-A Cost-effectiveness Analysis. Open Forum Infect Dis. 2022;9(12): ofac637. https://doi.org/10.1093/ofid/ofac637.
Medical Development Division, Malaysis DRG 2022. 2022.
Bank Negara Malaysia. Exchange Rate for USD/MYR (Middle Rate from July 2022 to Dec 2022). 2022 March 31, 2023]; Available from: https://www.bnm.gov.my/exchange-rates.
Ponce DM, Kitchen LK, Devlin JJ. Cost-benefit analysis of novel antiviral ritonavir in the active duty U.S. military population. Military Med. 2022;187(9–10):274–5. https://doi.org/10.1093/milmed/usab552.
Department of Health and Aged Care. Updated eligibility for oral COVID-19 treatments. 2023 https://www.health.gov.au/health-alerts/covid-19/treatments/eligibility. Accessed October 15, 2023.
Canada's Drug and Health Technology Agency. Nirmatrelvir and Ritonavir (Paxlovid) for Mild to Moderate COVID-19. 2022 October 20, 2023]; https://www.cadth.ca/nirmatrelvir-and-ritonavir-paxlovid-mild-moderate-covid-19. Accessed November 1, 2023.
Zhang W, et al. Cost-effectiveness of Paxlovid in reducing severe COVID-19 and mortality in China. Front Public Health. 2023. https://doi.org/10.3389/fpubh.2023.1174879.
Acknowledgements
We thank the Director General of Health Malaysia for his permission to publish this article. We would also like to express our gratitude to all study personnel, the National COVID-19 surveillance and healthcare professionals in Malaysia for their invaluable efforts in managing the COVID-19 patients. Without their tireless work, this project would not have been feasible. Additionally, we extend our appreciation to Prof. Asrul Akmal Shafie and Dr. Lim Ka Keat for their valuable contributions as external reviewers for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Ministry of Health Malaysia Research Grant for Communicable Diseases (NIH/800-3/2/2 Jilid 13 (182)). The funders had no role in the design and conduct of the study or the collection, management, analysis, and interpretation of the data.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Availability of Data and Materials
The data that support the findings of this study are available upon reasonable request, and with protocol approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia.
Ethics Approval
This study was registered in the National Medical Research Register and approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID-22-00938-2YN), Oct 21, 2022.
Author Contributions
Conceptualization, methodology, formal analysis, and investigation: Ee Vien Low, Hoon Shien Teh, Nicholas Yee Liang Hing. Writing – original draft preparation: Ee Vien Low, Hoon Shien Teh, Nicholas Yee Liang Hing. Writing – review and editing: All authors; Funding acquisition: Ee Vien Low. Resources: Kalaiarasu M. Peariasamy, Shahanizan Mohd Zin, Faizah Muhamad Zin, Maheshwara Rao Appannan, Samha Bashirah Mohamed Amin. Supervision: Kalaiarasu M. Peariasamy. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication. All authors read and approved the final version of the manuscript.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Low, E., Teh, H., Hing, N.Y.L. et al. Economic Evaluation of Nirmatrelvir/Ritonavir Among Adults Against Hospitalization During the Omicron Dominated Period in Malaysia: A Real-World Evidence Perspective. Drugs - Real World Outcomes 11, 299–308 (2024). https://doi.org/10.1007/s40801-024-00427-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-024-00427-0