Abstract
Background
This study aimed to understand treatment patterns, acute healthcare use, and cost patterns among adults with treatment-resistant depression (TRD) who completed induction treatment with esketamine nasal spray in the United States (US). Per label, induction is defined as administration twice a week for 4 weeks, after which maintenance is started on a weekly basis for 4 weeks, and thereafter, patients are treated weekly or bimonthly.
Methods
Adults with one or more esketamine claim (index date) on or after March 5, 2019 were selected from Optum’s de-identified Clinformatics® Data Mart Database (January 2016–June 2022). Before the index date, patients had evidence of TRD and ≥ 12 months of continuous insurance eligibility (baseline period). Patients with eight or more esketamine treatment sessions were included in the main cohort. A subgroup included patients with one or more baseline mental health (MH)-related inpatient (IP) admission or emergency department (ED) visit (i.e., prior acute healthcare users). Treatment patterns were described during the follow-up period (index date until earliest of end of insurance eligibility or data); acute healthcare (i.e., IP and ED) resource use and costs (2021 US dollars) were reported during the baseline and follow-up periods.
Results
Of the 322 patients in the main cohort, 111 comprised the subgroup of prior acute healthcare users. During the follow-up period, mean time from index date to eighth esketamine session was 73.2 days in the main cohort and 78.8 days in the subgroup (per label, 28 days). Further, 75.2% of the main cohort and 73.9% of the subgroup completed four or more esketamine maintenance sessions following induction. In the main cohort, mean all-cause acute healthcare costs per patient per month (PPPM) decreased from baseline ($837) to follow-up ($770). Similar reductions were observed for mean MH-related acute healthcare costs PPPM (baseline $648, follow-up $577). In the subgroup, mean all-cause acute healthcare costs PPPM also decreased (baseline $2323, follow-up $1423), driven by mean MH-related acute healthcare costs PPPM (baseline $1880, follow-up $1139). Mean all-cause acute healthcare use per ten patients per month remained largely stable from baseline to follow-up in the main cohort (IP days: baseline 2.24, follow-up 2.13; ED visits: baseline 1.33, follow-up 1.45) and decreased in the subgroup (IP days: baseline 6.38, follow-up 4.56; ED visits: baseline 2.58, follow-up 2.41). Trends in mean MH-related acute healthcare use were similar.
Conclusion
Patients generally required more time than label recommendation to complete esketamine induction treatment, and most went on to have 12 or more esketamine sessions. Completion of induction treatment correlated with reductions in mean all-cause and MH-related acute healthcare costs. Larger reductions were seen in the subgroup of prior acute healthcare users.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this real-world study, most patients with treatment-resistant depression who completed induction (eight sessions) with esketamine went on to complete at least four maintenance sessions. |
Acute healthcare costs trended lower after start of esketamine, particularly for patients with prior mental health-related acute healthcare use. |
These findings suggest that esketamine may be associated with certain economic benefits among patients who are able to overcome access barriers. |
1 Introduction
Treatment-resistant depression (TRD) represents a form of major depressive disorder (MDD) that is defined as inadequate response to two or more antidepressant courses of adequate dose and duration [1]. TRD affects 1.1% of the adult population in the United States (US) and is associated with over $40 billion in healthcare, unemployment, and productivity costs annually [1]. Additionally, patients with TRD incur significantly higher healthcare resource use (HRU) and costs relative to patients with MDD and no TRD [2], highlighting the large economic burden associated with TRD.
Treatment of TRD requires different approaches. These may include administering a different antidepressant class, augmenting treatment with a non-antidepressant medication, or delivering other intervention techniques such as electroconvulsive therapy (ECT) and transcranial magnetic stimulation (TMS) [3]. While treatments with traditional antidepressants typically take several weeks to demonstrate effect, the novel nasal spray esketamine, in contrast, can provide relief from depressive symptoms as soon as 2–4 h after the first dose [3,4,5]. Esketamine was approved in 2019, in combination with an oral antidepressant, for the management of TRD in adults based on clinical trial evidence demonstrating improved depressive symptoms and decreased risk of relapse after short- and long-term treatment [6,7,8]. In real-world clinical practice, a recent study demonstrated that esketamine was effective in alleviating symptoms of depression and anxiety, without major safety concerns [11]. In addition, given its rapid onset, esketamine has also shown some benefit during psychiatric emergencies in patients with MDD, including TRD and MDD with suicidal ideation, where treatment options are currently limited [4, 9, 10].
Despite the evidence of treatment benefits, some access barriers may hinder esketamine treatment initiation or adherence. For instance, access to esketamine through health insurance in the US is commonly subject to prior authorization, and a recent study showed that pharmacy claims for the initial session of esketamine were declined nearly 50% of the time [12]. Moreover, once esketamine is approved for payment by a health plan, each treatment administration must take place in a certified treatment center due to the potential for esketamine abuse, misuse, and adverse outcomes resulting from the potential for treatment-induced dissociation, sedation, and respiratory depression.[13, 14] Treatment is administered twice a week during the induction phase, weekly during the first 4 weeks of the maintenance phase, and weekly or bimonthly thereafter. The patient must be observed for at least 2 h after administration, and driving or operating heavy machinery is restricted until the following day [14]. As such, previous real-world studies have reported that only 50–60% of patients with TRD initiated on esketamine complete the induction phase of eight treatment sessions, and 35–40% complete the first maintenance phase of four additional treatment sessions following induction; among those who complete induction, the rate of completing the first four maintenance sessions was 75% [12, 15]. Notably, in the clinical trial setting, completion rates for both the induction and the initial maintenance sessions are higher, above 90% [16].
A recent real-world descriptive study indicated that mental health (MH)-related inpatient (IP) and emergency department (ED) costs trended lower in the 6 months following initiation of esketamine relative to before [15]. Notably, this study included all patients treated with esketamine, regardless of the number of sessions completed. Given that the degree of improvement in depressive symptoms has been shown to be correlated with the number of completed esketamine treatment sessions [11], it is possible that the economic benefit may be similarly increased if patients complete the induction phase. In turn, the current study was conducted to assess the change in HRU and costs among patients with TRD who completed induction with esketamine. An additional focus was placed on prior acute healthcare users to provide an opportunity to understand the benefit of esketamine among patients with severe disease who require more intensive MH care.
2 Methods
2.1 Data Source
Optum’s de-identified Clinformatics® Data Mart Database (January 1, 2016–June 30, 2022) was used. The database includes approximately 15–19 million annual covered lives, primarily representing patients with commercial insurance coverage (aged 0–65 years) and some patients with Medicare coverage (aged ≥ 65 years); however, age is capped at 90 years. The data span all US census regions and include administrative health claims along with demographics, socioeconomic characteristics, and insurance eligibility information, as well as medical and prescription drug claims. Socioeconomic information (e.g., race, income, education) through proprietary algorithms that leverage Census data, modeled data, and links at the individual level to external data [17,18,19]. Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act. Therefore, review by an institutional review board was not required.
2.2 Study Design
A retrospective observational design was used. The intake period spanned from March 5, 2019 (i.e., esketamine approval date for TRD in the US) to June 30, 2022, and the index date was defined as the date of the first claim of esketamine within the intake period. The baseline period comprised the 12-month period before the index date, while the follow-up period spanned the index date until the earliest of end of data or continuous health plan eligibility.
2.3 Study Population and Sample Selection
The main study population included patients with TRD who completed the induction phase of esketamine (i.e., had eight or more esketamine treatment sessions), herein referred to as the induction completer cohort. Patients were included in the study if they met the following inclusion criteria (Fig. 1 for study design): (1) had evidence of TRD, defined as the initiation of a line of antidepressant therapy of adequate dose after changing two different lines of adequate dose and duration within the same major depressive episode (MDE) (the definitions of TRD and MDE align with previously published work [12, 15]); (2) had a first claim for esketamine (see Online resource 1 in the electronic supplementary material for a list of codes) during the intake period and eight or more esketamine treatment sessions; (3) were aged ≥ 18 years as of the index date; (4) had ≥ 12 months of continuous insurance eligibility before the index date; and (5) had one or more claim with a diagnosis for MDD (see Online resource 1 for a list of codes) during the MDE that included the index date.
Study design. ED emergency department, IP inpatient, MDD major depressive disorder, MDE major depressive episode, MH mental health, TRD treatment-resistant depression. 1Patients were excluded if they had ≥ 2 claims on separate days during the baseline period with a diagnosis for bipolar disorder, psychosis, schizophrenia, schizo-affective disorder, or other non-mood psychotic disorders. 2See Online resource 1 for a list of codes
Patients were excluded if they had two or more claims on separate days during the baseline period with a diagnosis for bipolar disorder, psychosis, schizophrenia, schizo-affective disorder, or other non-mood psychotic disorders (see Online resource 1 for a list of codes).
A subgroup of patients with one or more MH-related acute healthcare visit (i.e., IP admission or ED visit) during the baseline period was analyzed separately, herein referred to as the prior acute healthcare user subgroup.
2.4 Study Measures and Outcomes
Patient sociodemographic characteristics were described during the baseline period.
Treatment patterns and esketamine use were described based on pharmacy and medical claims during the follow-up period, where an esketamine treatment session was defined as all esketamine claims occurring on the same date. Dose of esketamine during the treatment sessions was reported based on National Drug Code (NDC) and Healthcare Common Procedure Coding System (HCPCS) codes for esketamine pharmacy and medical claims (see Online resource 1 for a list of codes for different doses).
All-cause and MH-related HRU and medical costs were described during the baseline and follow-up periods and included acute healthcare (i.e., ED visits and IP days), reported per ten patients per month, and outpatient (OP) visits, reported per patient per month (PPPM). MH-related HRU and costs were defined based on medical claims with International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes between F01 and F99. Medical costs were reported based on medical claims from a payer’s perspective and inflated to 2021 US dollars (USD).
2.5 Statistical Analysis
Continuous variables were described with means, standard deviations, and medians, while binary variables were described with frequencies and proportions. All results were descriptive; no statistical comparisons were conducted.
3 Results
3.1 Patient Sociodemographic Characteristics
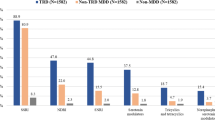
Among 500 patients with TRD initiated on esketamine (see Online resource 2 for sample selection), 322 (64.4%) completed the induction phase and were included in the induction completer cohort. The mean age was 48.7 years, 62.1% were female, and most patients (72.0%) had commercial insurance coverage (Table 1). Anxiety disorders were the most common Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) comorbidity, affecting 78.9% of patients, followed by sleep-wake disorders (39.8%) and neurodevelopmental disorders (23.3%). The most common specialized MH services used by the induction completer cohort included psychiatrist (85.1%), psychotherapy (76.4%), psychiatric nurse (22.4%), and TMS (10.2%).
Among the induction completer cohort, 111 patients had one or more MH-related acute healthcare visit during the baseline period and were included in the prior acute healthcare user subgroup. The mean age was 46.4 years, 69.4% were female, and 73.9% had commercial insurance coverage (Table 1). As in the induction completer cohort, the majority of patients within this subgroup reported anxiety disorders (91.9%), followed by sleep-wake disorders (41.4%) and neurodevelopmental disorders (23.4%). The most common specialized MH services used by the prior acute healthcare user subgroup included psychiatrist (88.3%), psychotherapy (76.4%), psychiatric nurse (23.4%), and intensive OP psychiatric program (10.8%).
3.2 Esketamine Use
Over a mean follow-up of 15.1 months, patients in the induction completer cohort had a mean of 23.1 esketamine treatment sessions. Over a mean follow-up of 16.1 months, patients in the prior acute healthcare user subgroup had a mean of 22.0 esketamine treatment sessions. Most patients initiated esketamine at a dose of 56 mg (75.8% in the induction completer cohort and 72.1% in the prior acute healthcare user subgroup). By the eighth session, nearly all patients were receiving 84 mg (81.7% in the induction completer cohort and 86.5% in the prior acute healthcare user subgroup).
The mean time to complete induction was 73.2 days in the induction completer cohort and 78.8 days in the prior acute healthcare user subgroup (per label, the induction phase should be completed in 28 days, with twice-per-week administration [14]). In both the induction completer cohort and the prior acute healthcare user subgroup, half of all patients completed the induction phase within approximately 40 days. The median time between esketamine sessions varied between 5 and 6 days until the eighth session (Fig. 2). The median time between each of the maintenance sessions remained consistent at 7 days until the 13th session, in line with label recommendations [14].
The proportion of patients who transitioned to maintenance phase was 92.2% in the induction completer cohort and 88.3% in the prior acute healthcare user subgroup (Fig. 3). Additionally, 75.2% and 73.9%, respectively, completed four or more maintenance sessions.
3.3 Antidepressant and Other Psychiatric Medication Use
During the follow-up period, patients had a mean of 2.1 unique antidepressants in the induction completer cohort and 2.2 unique antidepressants in the prior acute healthcare user subgroup. Close to one-third of patients in the induction completer cohort and over one-third of patients in the prior acute healthcare user subgroup had three or more unique antidepressants during the follow-up period. The most frequently used antidepressants were bupropion (37% in the induction completer cohort and 33.3% in the prior acute healthcare user subgroup) and trazodone (23.6% in the induction completer cohort and 28.8% in the prior acute healthcare user subgroup).
During the follow-up period, 44.1% of the induction completer cohort and 51.4% of the prior acute healthcare user subgroup received second-generation antipsychotics. The most frequently used antipsychotic agent was aripiprazole (20.2% in the induction completer cohort and 25.2% in the prior acute healthcare user subgroup).
3.4 Healthcare Resource Utilization
With respect to acute healthcare use, the mean number of all-cause IP days remained similar during the baseline and follow-up periods in the induction completer cohort and decreased by almost 2 days per ten patients per month during the follow-up period in the prior acute healthcare user subgroup (Fig. 4). In both the cohort and subgroup, almost all all-cause IP days were MH-related during the baseline and follow-up periods. The mean number of all-cause and MH-related ED visits remained similar during the baseline and follow-up periods in the cohort and subgroup, with the variation between the two periods not exceeding 0.21 visits per ten patients for any component (Fig. 5).
As expected, given that esketamine must be administered by a health professional in a certified treatment center (i.e., OP setting), the mean number of all-cause OP visits increased from baseline to follow-up by almost 2 PPPM in the induction completer cohort (3.29–5.06) and by approximately 1 PPPM in the prior acute healthcare user subgroup (4.24–5.47), driven by the increase in the mean number of MH-related OP visits (2.14–4.02 visits PPPM in the induction completer cohort and 2.81–4.35 visits PPPM in the prior acute healthcare user subgroup; Online resource 3).
3.5 Medical Costs
Mean all-cause acute healthcare costs (i.e., IP and ED combined) decreased numerically by $67 PPPM from baseline to follow-up in the induction completer cohort and by $900 PPPM in the prior acute healthcare user subgroup (Fig. 6). This decrease was entirely driven by a reduction in MH-related acute healthcare costs in the induction completer cohort, while in the prior acute healthcare user subgroup, 82.3% of the decrease in all-cause costs was attributed to MH-related costs.
Similar to OP days, OP costs were expected to increase from baseline to follow-up since they captured the medical costs of esketamine treatment. Accordingly, mean all-cause OP costs increased numerically by $1130 PPPM from baseline to follow-up in the induction completer cohort and by $948 PPPM in the prior acute healthcare user subgroup (Online resource 3). This increase was predominantly driven by increases in MH-related OP costs.
4 Discussion
In this retrospective, real-world study, most patients with TRD who completed esketamine induction required more time than the label recommendation, and 75.2% of all patients proceeded to complete at least four maintenance sessions following induction. While OP visits and costs increased expectedly when patients started esketamine treatment (in an OP setting), all-cause and MH-related acute healthcare costs trended lower after esketamine initiation for patients who completed esketamine induction, particularly among those with prior MH-related acute healthcare use.
There is scarce literature evaluating changes in HRU and costs after initiation of esketamine for TRD in the US. One recent claims-based study by Joshi et al. described medical costs among patients with TRD, before and after the start of esketamine treatment, but all initiators were included, regardless of the number of sessions completed [15]. Nevertheless, Joshi et al. found that MH-related acute healthcare costs (IP and ED) per patient per 6 months decreased from $4088 pre-esketamine to $3531 post-esketamine initiation (2020 USD) [15]. This decrease is slightly larger than that observed in the current study, potentially because Joshi et al. only included commercially insured (2.6% with Medicare Supplemental) patients, whereas the present study included 28.0% of patients with Medicare coverage, and as a result, the mean age of patients in the present study was about 4 years higher.
In contrast to costs, there is ample prior evidence demonstrating the clinical effectiveness of esketamine for the treatment of TRD [11, 20, 21]. One real-world study of patients with TRD receiving esketamine at an OP psychiatric clinic found that improvement in depression severity, as measured by Patient Health Questionnaire (PHQ-9) score, correlated with the number of completed esketamine sessions, highlighting the importance of continued treatment [11]. While clinical outcomes were not assessed in the current study, 75.2% of patients completed at least four maintenance sessions after the induction phase, suggesting that esketamine was effective, given that maintenance doses should only be administered after evidence of therapeutic benefit during induction [14]. Furthermore, the observed reduction in acute healthcare costs was driven by decreased MH-related costs, which may be associated with the improvement of depressive symptoms due to esketamine, especially among patients with a history of acute healthcare for MH prior to esketamine initiation.
The particularly large reduction in costs observed among patients with prior acute healthcare use suggests that esketamine treatment may be associated with heightened benefit in patients with more severe TRD. Indeed, in the phase 3 clinical trial of esketamine for TRD, the subgroup of patients with three or more previous treatment failures (potentially representing more severe disease) experienced a larger decrease in Montgomery-Åsberg Depression Rating Scale (MADRS) score with esketamine relative to the overall population [8]. Additionally, in the claims-based study of TRD, Joshi et al. observed generally larger reductions in acute healthcare costs after esketamine initiation in the subgroups of patients with comorbidities, the presence of which is associated with more severe depression [15, 22, 23]. These findings demonstrate the potential for esketamine to be particularly beneficial among patients with severe TRD, though the clinical and economic improvements observed in the overall TRD populations as well [8, 15] suggest that esketamine should also be considered at any stage of TRD.
In contrast to typical antidepressants, which can take several weeks to reach full efficacy [5], esketamine may provide rapid relief from depressive symptoms as soon as 2–4 h after the first administration [4]. Furthermore, a correlation has been observed between the number of completed esketamine treatment sessions and the degree of clinical improvement, highlighting the importance of completing the induction phase [11]. However, among the total population of patients with TRD initiated on esketamine in this study, only 64.4% completed the induction phase, while in prior claims-based studies, this proportion ranged from 47.6 to 61.3% [12, 15]. Though some patients may discontinue treatment due to recognized adverse events, additional factors that may explain why patients do not complete the induction phase have been discussed in previous literature and include rejected insurance claims for esketamine (e.g., coverage issues), limited availability of local treatment centers, transportation challenges (e.g., patients are not allowed to drive until the day following administration), patient and caregiver time requirements (e.g., patients must be monitored for 2 h following administration), and lack of patient motivation [12, 16, 24]. Of note, these barriers to esketamine access may have contributed to the longer time to complete induction observed in the current study (mean of 73.2 days) relative to the label recommendation (28 days) [14]. Similarly, Joshi et al. also reported a longer time to complete induction in their claims-based analysis (mean of 56.9 days) [15]. As such, fostering collaboration between payers, providers, and patients may play a crucial role in addressing and alleviating some of these access barriers.
4.1 Limitations
The study findings should be considered in the context of some limitations. The analysis presented in this paper is descriptive. Therefore, changes in HRU and costs from baseline to follow-up should be interpreted with caution, as no formal statistical analysis was performed. Relatedly, time-varying factors other than esketamine treatment initiation may have contributed to changes in HRU and costs. In addition, this study did not describe the evolution of monthly acute HRU and costs over time. Future research could explore these trends to provide a more comprehensive understanding. Since the study population included patients with commercial or Medicare coverage, the findings are not generalizable to patients with other types of insurance coverage or without health insurance. In addition, TRD was defined based on pharmacy claims for antidepressants; however, pharmacy claims do not guarantee that the medication dispensed was taken as prescribed and do not capture medications dispensed over the counter or as samples. Moreover, since the dates of the esketamine pharmacy claims may not have corresponded to the dates of esketamine administration, the actual time between esketamine treatment sessions may have been slightly different. Lastly, as with any claims-based study, the claims data may have contained billing inaccuracies or omissions in coded procedures and diagnoses.
5 Conclusions
In this real-world study, completion of induction treatment with esketamine correlated with a reduction in acute healthcare costs, particularly among patients with a history of acute healthcare use for MH prior to esketamine initiation. Most patients who completed the induction phase went on to have four or more maintenance sessions, further suggesting treatment benefits among those able to overcome access barriers to esketamine.
References
Zhdanava M, Pilon D, Ghelerter I, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. 2021;82(2).
Li G, Zhang L, DiBernardo A, et al. A retrospective analysis to estimate the healthcare resource utilization and cost associated with treatment-resistant depression in commercially insured US patients. PLoS ONE. 2020;15(9): e0238843.
Voineskos D, Daskalakis ZJ, Blumberger DM. Management of treatment-resistant depression: challenges and strategies. Neuropsychiatr Dis Treat. 2020;16:221–34.
Wang SM, Kim NY, Na HR, et al. Rapid onset of intranasal esketamine in patients with treatment resistant depression and major depression with suicide ideation: a meta-analysis. Clin Psychopharmacol Neurosci. 2021;19(2):341–54.
Cheng Q, Huang J, Xu L, et al. Analysis of time-course, dose-effect, and influencing factors of antidepressants in the treatment of acute adult patients with major depression. Int J Neuropsychopharmacol. 2020;23(2):76–87.
US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic 2019 [cited 2023 September 5]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified
Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2019;76(9):893–903.
Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428–38.
Pompili M, Sarli G, Erbuto D, et al. Clinical experiences with intranasal esketamine for major depressive disorder resistant to treatment and with a psychiatric emergency: case presentations. Int Clin Psychopharmacol. 2023;38(3):195–200.
Ionescu DF, Fu D-J, Qiu X, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2020;24(1):22–31.
Brendle M, Ahuja S, Valle MD, et al. Safety and effectiveness of intranasal esketamine for treatment-resistant depression: a real-world retrospective study. J Comp Eff Res. 2022;11(18):1323–36.
Teeple A, Zhdanava M, Pilon D, et al. Access and real-world use patterns of esketamine nasal spray among patients with treatment-resistant depression covered by private or public insurance. Curr Med Res Opin. 2023;3:1–8.
Kim J, Farchione T, Potter A, et al. Esketamine for treatment-resistant depression—first FDA-approved antidepressant in a new class. N Engl J Med. 2019;381(1):1–4.
US Food and Drug Administration. SPRAVATO® (esketamine) prescribing information. Titusville: Janssen Pharmaceuticals, Inc.; 2023.
Joshi K, Pilon D, Shah A, et al. Treatment patterns, healthcare utilization, and costs of patients with treatment-resistant depression initiated on esketamine intranasal spray and covered by US commercial health plans. J Med Econ. 2023;26(1):422–9.
Zaki N, Chen L, Lane R, et al. Long-term safety and maintenance of response with esketamine nasal spray in participants with treatment-resistant depression: interim results of the SUSTAIN-3 study. Neuropsychopharmacology. 2023:1–9.
LaRosa AR, Claxton J, O’Neal WT, et al. Association of household income and adverse outcomes in patients with atrial fibrillation. Heart. 2020;106(21):1679–85.
Optum, Inc. Retrospective database analysis 2013 [cited 2023 October 2]. https://www.optum.com/content/dam/optum/resources/productSheets/Retrospective-Database-Analysis.pdf
Zhang Y, Amin S, Lung KI, et al. Incidence, prevalence, and risk factors of infectious uveitis and scleritis in the United States: a claims-based analysis. PLoS ONE. 2020;15(8): e0237995.
Martinotti G, Vita A, Fagiolini A, et al. Real-world experience of esketamine use to manage treatment-resistant depression: a multicentric study on safety and effectiveness (REAL-ESK study). J Affect Disord. 2022;15(319):646–54.
Samalin L, Rothärmel M, Mekaoui L, et al. Esketamine nasal spray in patients with treatment-resistant depression: the real-world experience in the French cohort early-access programme. Int J Psychiatry Clin Pract. 2022;26(4):352–62.
Steffen A, Nubel J, Jacobi F, et al. Mental and somatic comorbidity of depression: a comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry. 2020;20(1):142.
Ziobrowski HN, Leung LB, Bossarte RM, et al. Comorbid mental disorders, depression symptom severity, and role impairment among Veterans initiating depression treatment through the Veterans Health Administration. J Affect Disord. 2021;1(290):227–36.
Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039–40.
Acknowledgements
Medical writing support was provided by a professional medical writer, Christine Tam, MWC, an employee of Analysis Group, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding source
This study was funded by Janssen Scientific Affairs, LLC, a Johnson & Johnson company.
Previous presentations
Part of the material in this article was presented at the American Society of Clinical Psychopharmacology (ASCP) Annual Meeting, on May 30–June 2, 2023, as a poster presentation.
Competing interests
Kruti Joshi and Cindy Chen are employees of Janssen Scientific Affairs, LLC, a Johnson & Johnson company, and are stockholders of Johnson & Johnson. Maryia Zhdanava, Aditi Shah, Arthur Voegel, and Dominic Pilon are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, a Johnson & Johnson company, which funded the development and conduct of this study and article. Lisa Harding reports the following details of compensated affiliation or involvement in an organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this article: Janssen Neuroscience Advisory Panel on Mood Disorders and Speaker Bureau; Compass Pathways Advisory Panel on Mood Disorders; Takeda Pharmaceuticals Advisory Panel on Mood Disorders.
Author contributions
MZ, AS, AV, and DP contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. KJ, CC, and LH contributed to study conception and design and data analysis and interpretation. All authors reviewed and approved the final content of this article.
Ethics statements
Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act of 1996; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4).
Data/code availability
The datasets generated and analyzed during the current study are not publicly available because they were used pursuant to a data use agreement. The data are available through requests made directly to Optum.
Consent to participate and consent for publication
Not applicable; our study was not a clinical trial and did not include patient identifiable information.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Harding, L., Joshi, K., Zhdanava, M. et al. Treatment Patterns, Acute Healthcare Resource Use, and Costs of Patients with Treatment-Resistant Depression Completing Induction Phase of Esketamine in the United States. Drugs - Real World Outcomes 11, 209–219 (2024). https://doi.org/10.1007/s40801-024-00425-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-024-00425-2