Abstract
Background
Patients with chronic thromboembolic pulmonary hypertension (CTEPH) in countries with limited resources have, to date, been poorly represented in registries.
Objective
This work assesses the epidemiology, diagnosis, hemodynamic and functional parameters, and treatment of CTEPH in Russia, Kazakhstan, Turkey, Lebanon, and Saudi Arabia.
Methods
A prospective, cohort, phase IV, observational registry with 3-year follow-up (n = 212) in patients aged ≥ 18 years diagnosed with CTEPH was created. Clinical, hemodynamic, and functional parameters were obtained at an initial visit, follow-up visits, and a final visit at the end of 3 years’ observation or end of follow-up. Data were recorded on electronic case report forms. Parameters evaluated included 6-minute walking distance (6MWD), use of pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA), pulmonary hypertension (PH)-targeted therapy, and survival. All statistical analyses were exploratory and descriptive, and were performed in the overall population.
Results
The most common symptoms were typical of those expected for CTEPH. Almost 90% of patients underwent right heart catheterization at diagnosis or initial study visit. In total, 66 patients (31%) underwent PEA before the initial visit; 95 patients (45%) were considered operable, 115 (54%) were inoperable, and two (1%) had no operability data. Only 26 patients (12%) had been assessed for BPA at their initial visit. PH-targeted therapy was documented at diagnosis for 77 patients (36%), most commonly a phosphodiesterase type 5 inhibitor (23%). Use of PH-targeted therapy increased to 142 patients (67%) at the initial visit, remaining similar after 3 years. Use of riociguat increased from 6% of patients at diagnosis to 38% at 3 years. Between baseline and end of observation, results for patients with paired data showed an increase in 6MWD. Survival at the end of observation was 88%.
Conclusions
These data highlight the current diagnosis and management of CTEPH in the participating countries. They show that early CTEPH diagnosis remains challenging, and use of off-label PH-targeted therapy is common. ClinicalTrials.gov: NCT02637050; registered December 2015.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
The authors performed a non-interventional registry study of the diagnosis and treatment of 212 patients with chronic thromboembolic pulmonary hypertension (CTEPH) in Russia, Kazakhstan, Turkey, Lebanon, and Saudi Arabia over 3 years. |
Almost 90% of patients underwent right heart catheterization at diagnosis or initial study visit, while other diagnostic techniques were performed less frequently, possibly reflecting limited resources. |
These results show that early CTEPH diagnosis remains challenging in countries with limited resources. |
1 Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by obstruction of pulmonary arterial vessels by organized thromboembolic material; a CTEPH cumulative incidence of up to ~ 9% has been reported following an acute pulmonary embolism (PE) event [1]. The pathophysiological mechanisms underlying CTEPH are complex; they include changes to the pulmonary vascular bed such as remodeling and microvasculopathy, which increase pulmonary vascular resistance (PVR); this in turn drives right ventricular (RV) responses to the increased RV afterload [1, 2]. With progressive disease, the RV remodels to adapt to the increased afterload until it has no further capacity to do so. From this point maladaptive remodeling occurs; this eventually results in RV dysfunction, which can progress to right heart failure and be fatal [3,4,5,6,7,8,9,10,11]. The symptoms of CTEPH are similar to those of other types of pulmonary hypertension (PH), and therefore a specific sequence of tests is required to make the diagnosis [8, 12,13,14,15]. Following echocardiography to estimate the probability of PH, a ventilation/perfusion (V/Q) scan is the recommended screening test for CTEPH [8, 12,13,14,15], followed by confirmation of the diagnosis and disease severity using right heart catheterization (RHC). Depending on the patient and resources available, other imaging tests such as (computed tomography) pulmonary angiography and cardiac magnetic resonance imaging may be used for imaging of pulmonary vessel lesions and morphology to assess RV structure and function and to evaluate eligibility for pulmonary endarterectomy (PEA) [1, 2, 8, 12,13,14,15].
PEA is the treatment of choice for CTEPH as it is potentially curative, with a short-term mortality < 3% if performed in expert centers [6, 14,15,16,17,18,19,20,21]. For patients with non-operable or persistent/recurrent CTEPH, the oral soluble guanylate cyclase (sGC) stimulator riociguat [22, 23] and the subcutaneous prostacyclin analog (PCA) treprostinil [24] are the only targeted medical treatments approved in Europe [6, 15, 25,26,27]. Therapies licensed for the treatment of pulmonary arterial hypertension—PCAs other than treprostinil, endothelin receptor antagonists (ERAs), and phosphodiesterase type 5 inhibitors (PDE5is)—are often prescribed off-label to patients with CTEPH [6, 28] despite the absence of clear benefits [29,30,31,32,33,34]. For patients with inoperable CTEPH, balloon pulmonary angioplasty (BPA) is another treatment option [6, 15], although it should be performed only in experienced, high-volume CTEPH centers [35, 36]. Medical therapy is frequently given before BPA to stabilize the patient or improve hemodynamics before the procedure and is generally continued afterward. While medical therapy in conjunction with BPA has not been systematically evaluated, the ancillary extension to the RACE study suggests that this option is feasible [37].
The diagnosis and management of CTEPH have been assessed in the International CTEPH Registry in Canada and Western/Central European countries [7, 19, 38], while the worldwide prospective CTEPH Registry encompasses the USA, Japan, Taiwan, and many countries in Europe, including the Russian Federation [39]. The US CTEPH Registry has also reported results [40], and a retrospective observational single-center study in the Netherlands assessed clinical outcomes of macitentan therapy in patients with non-operated CTEPH or with residual PH after PEA [41]. The Russian National Registry reported demographic and clinical characteristics of 727 newly diagnosed patients with PH including 206 with CTEPH [42]. The Systematic Prospective Follow Up for Better Understanding of Clinical Characteristics of Patients with Pulmonary Hypertension Disease (SAUDIPH) registry in Saudi Arabia reported preliminary results from patients with CTEPH [43]. Most patients had advanced disease (World Health Organization functional class III or IV) at baseline, suggesting that diagnosis was often delayed, but patients were diagnosed and managed according to international guidelines, and cumulative probability of survival at 1 year was 96.6%. The first PH registry in the United Arab Emirates (UAE) recently reported the baseline characteristics of 34 patients with CTEPH [44]. Other than these reports, there are few available registry CTEPH data for other Eastern European countries or the Middle East. The CTEPH registry reported here was designed to assess data regarding CTEPH epidemiology, the use of diagnostic techniques at baseline, initial visit, and during the study, the use of PEA-, BPA-, and PH-approved medical therapies, and clinical parameters at diagnosis and under treatment in Russia, Kazakhstan, Turkey, Lebanon, and Saudi Arabia. The aim was to improve understanding of patient management and medical resources in these countries. Here, we present the baseline and 3-year follow-up data.
2 Methods
This was an international, prospective cohort, phase IV observational registry (NCT02637050) with all decisions on diagnostic- and treatment-related procedures made at the discretion of the attending physician according to their medical practice. Data collection started on 3 March 2016 and was completed on 3 July 2020. All participating study sites were expert PH centers. Site selection criteria were based on the potential for patient enrollment, familiarity with research quality standards such as good pharmacovigilance practice, and availability of dedicated resources. The observation period was 3 years from study inclusion or until withdrawal, death, transfer to another physician, or loss to follow-up.
2.1 Inclusion and exclusion criteria
Enrolled patients were ≥ 18 years of age and diagnosed with CTEPH, regardless of current treatment, with a mean pulmonary artery pressure ≥ 25 mmHg at rest and a pulmonary arterial wedge pressure ≤ 15 mmHg as measured by RHC. Confirmation of CTEPH diagnosis by at least one segmental perfusion defect in V/Q scan, or pulmonary artery obstruction observed by multidetector CT pulmonary angiography or conventional pulmonary cineangiography, was required for inclusion. Patients with suspicion or diagnosis of sub-acute PE were required to have ≥ 3 months of anticoagulation therapy before diagnosis of CTEPH. Patients diagnosed with CTEPH < 6 months before the initial visit and who did not receive PH-targeted therapy were classified as incident. Patients who did not meet these criteria were defined as prevalent. Patients with an underlying medical disorder that could have jeopardized the safety of the patient or their compliance in the study or could have resulted in an anticipated life expectancy of < 6 months were excluded.
2.2 Endpoints and Assessments
The primary objective of this registry was to evaluate the clinical course of CTEPH through assessment of clinical, hemodynamic, and functional parameters in routine medical practice. These parameters included 6-minute walking distance (6MWD), PVR, cardiac index (CI), mean pulmonary artery pressure, and World Health Organization functional class (WHO FC) between initial and final visit. The secondary objective was to collect additional disease-related data:
-
Baseline demographics and characteristics
-
o
Signs and symptoms, clinical subtypes of CTEPH, risk factors for CTEPH, prior PE/venous thromboembolism (VTE), concomitant diseases, and medication
-
o
-
Mode of diagnosis and follow-up for CTEPH
-
Timespan between onset of symptoms and CTEPH diagnosis, use of imaging studies, and other tests.
-
Treatment at time of diagnosis and afterwards, including use of PH-targeted therapies. PH-targeted therapy was defined as PDE5is, ERAs, PCAs, sGC stimulators, prostacyclin agonists, tyrosine kinase inhibitors, or calcium channel blockers (CCBs), classified by World Health Organization Drug Dictionary Drug Record number and Anatomic Therapeutic Chemical code. The number of patients with monotherapy or combination PH therapy at initial visit was recorded. Combination PH therapy at initial visit was further classified as sequential combination therapy or upfront (initial) combination therapy. Sequential combination therapy was defined as a second or third PH-targeted drug added over time until the time of initial visit. Upfront (initial) combination therapy started with a combination of at least two PH-targeted drugs at the same time or within a few days, with no further PH-targeted drug added until the time of initial visit
-
PEA eligibility and outcomes
-
BPA eligibility
-
Long-term outcomes
-
o
WHO FC, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), survival (all-cause mortality), number of healthcare professional visits, length of hospitalization due to CTEPH, and CTEPH-related complications
-
o
Data were obtained at initial visit, follow-up visits, and a final visit at the end of 3 years’ observation or end of follow-up. All data were collected during routine clinic visits: the study or its documentation did not affect routine treatment in any way. Historic data (demographic and clinical characteristics) were collected from medical records if available, or by interviewing the patient. The variables documented at initial visit, follow-up visits, and final visit are presented in the Online Resource, Supplementary Table 1.
At all participating centers, data were collected via electronic case report forms (eCRFs) for standardization, using an electronic data capture system. All data underwent medical review for plausibility, consistency, and completeness, and to identify potential issues that could have affected the robustness of the collected study data or the progress of the study. Data for adverse events (AEs) and serious adverse events (SAEs) occurring during treatment with any drug provided by the study sponsor were collected at every visit and documented on the eCRF. AEs and SAEs occurring while patients were receiving drugs from manufacturers other than the study sponsor were reported to the manufacturer in accordance with local regulations. AE data are not presented in this report, as patients receiving sponsor drugs might not have been representative of the entire study population.
2.3 Statistical Analysis
All statistical analyses were exploratory and descriptive and were performed in the overall population. The study was not designed to confirm or reject pre-defined hypotheses. No formal sample size calculation was performed. Since CTEPH is a rare disease and may be underdiagnosed in the countries represented in the registry, the number of patients to be recruited was set to 200 to ensure that in each country, patients could be enrolled within the planned recruiting period; the exact number of patients for each country was not specified. Follow-up data were collected at 3 years after the initial visit or at the most recent visit with available documentation (last documented visit). Missing or implausible data were queried, and the data were validated. Where available, data were analyzed at each visit, by 3-month intervals, and at the last visit. Rules for dealing with missing or partial information were implemented to avoid excluding subjects with missing or partially complete data, as shown in the Online Resource, pages 1–3.
All variables were analyzed descriptively: categorical variables by frequency tables (absolute and relative frequencies) and continuous variables by sample statistics [i.e., number of observations, mean, standard deviation (SD), minimum, median, quartiles, and maximum]. Continuous variables were described by absolute value and as change from initial visit to analysis time point, if applicable. All mortality data were evaluated using Kaplan–Meier methods with patients alive at the end of the study censored at their last visit date. Annual hospitalization rates, defined as the number of hospitalizations divided by the person-years of follow-up, were calculated per patient-year.
Institut Dr. Schauerte GbR, Munich, Germany had full access to all the registry study data and takes responsibility for its integrity and the data analysis.
3 Results
3.1 Study Population
Data presented here are 3-year follow-up data with a cut-off of 10 September 2020. Between March 2016 (start of data collection) and June 2017 (end of enrollment), 234 patients were enrolled in the registry from 25 study centers: 10 in Turkey, 8 in the Russian Federation, 3 in Saudi Arabia, 2 in Kazakhstan, 1 in Kyrgyzstan, and 1 in Lebanon. Two sites—one in Kazakhstan and one in Kyrgyzstan—were terminated due to ongoing inactivity.
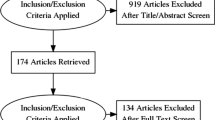
Patient disposition is presented in Fig. 1.
In total, 146 patients (69% of the overall population) met all the formal inclusion criteria. A further 66 patients (31%) did not meet these criteria, as they had data in the eCRF at the time of diagnosis or initial visit that were inconsistent with either pulmonary capillary wedge pressure (PCWP) ≤ 15 mmHg or mean pulmonary artery pressure (mPAP) ≥ 25 mmHg (50 patients, 24%) and/or they had no confirmation of CTEPH by imaging (23 patients, 11%). However, these patients were included in the study because hemodynamic data consistent with CTEPH were obtained by other tests, other imaging studies were available, or the explanation for RHC data outside the formal criteria was accepted by the investigator. In total, 22 patients were excluded, mainly because of lack of follow-up information and/or protocol violations. Consequently, 212 patients were included in the 3-year follow-up (Russian Federation, n = 89, 42%; Turkey, n = 57, 27%; Saudi Arabia, n = 54, 25%; Kazakhstan, n = 11, 5%; Lebanon, n = 1, < 1%). Of the 212 patients, 31 (15%) were incident and 181 (85%) were prevalent. The median (range) observation time was 990 (45−1378) days. Demographics for the patients included in the 3-year follow-up are presented in Table 1.
3.2 Patient Characteristics and Diagnostic Procedures
CTEPH risk factors, symptoms, hemodynamics, and functional class of the 212 patients included in the analysis are presented in Table 2.
At the time of diagnosis, the most common symptoms were shortness of breath (92%), fatigue (42%), and edema (35%). At the initial visit, the most common documented risk factors for CTEPH were VTE (42%) and antiphospholipid antibody syndrome (14%), and the most common symptoms were shortness of breath (73%), fatigue (39%), and chest discomfort (25%). A history of VTE was documented at the initial visit in 145 patients (68%). (Investigators provided data on VTE as part of the medical history and separately as a risk factor for CTEPH. The difference between these two results suggests that investigators did not always consider VTE a risk factor for CTEPH.)
At diagnosis, the mean (SD) value for mPAP was 52.7 (16.3) mmHg, PVR was 10.5 (7.8) WU, cardiac index was 2.3 (0.7) L/min/m2, and PCWP was 11.7 (5.4) mmHg, and the majority of patients were in WHO FC III (40%) (Table 2).
Baseline characteristics of the patients who did not meet the formal diagnostic entry criteria as outlined above (n = 66) were generally similar to those who did meet the entry criteria (n = 146) (Online Resource, Supplementary Table 2). However, the subgroup who did not meet the criteria tended to live further from the specialist center, had a lower prevalence of VTE at initial visit, and had higher baseline NT-proBNP levels than patients who met the criteria.
Diagnostic tests documented are presented in Table 3. The most common imaging tests used to confirm CTEPH diagnosis were V/Q scintigraphy only and V/Q scintigraphy plus pulmonary angiography and CT angiography (Table 4). In total, 23 patients (11%) did not undergo any diagnostic imaging. Despite the inclusion criteria mandating RHC, 24 patients (11%) did not undergo RHC at the time of diagnosis or initial visit.
At diagnosis, 102 patients underwent V/Q scintigraphy, which showed a defect in 94 patients (92%). At the initial visit, 48 patients had a V/Q scan, with defects observed in 45 patients (94%). At diagnosis and initial visit, 70 and 49 patients, respectively, underwent pulmonary angiography; 38 (54%) and 30 (61%), respectively, had findings consistent with CTEPH. At diagnosis and initial visit, 139 and 68 patients, respectively, underwent CT angiography, showing findings consistent with CTEPH in 75 patients (54%) and 38 patients (56%), respectively (Table 5). A total of 91 patients and 36 patients underwent CT at diagnosis and initial visit, respectively, showing findings consistent with CTEPH in 54 patients (59%) and 16 patients (44%) (Table 5). Echocardiographic parameters at the time of diagnosis and initial visit are presented in the Online Resource, Supplementary Table 3.
3.3 Operability Assessment and Surgical Intervention
At the time of initial visit, 66 patients (31%) had undergone PEA. The mean (SD) time between CTEPH diagnosis and PEA was 312.5 (421.3) days (data available for 63 patients). At initial visit, 95 patients (45%) were considered operable, while 115 (54%) were considered inoperable, and two patients (1%) had no operability data. During the 3-year follow-up, 27 patients (13%) underwent PEA; two of these patients (7%) died in hospital.
At initial visit, 26 patients (12% of the population) had been assessed for BPA eligibility and during the study, 37 patients (17%) were assessed for BPA. Patients were assessed for BPA up to five times during the study, and therefore a total of 47 patients (22%) were assessed for this procedure by study end.
3.4 Reasons for Inoperability
Of the 115 patients who were considered inoperable at the initial visit, the most common reason for inoperability was comorbidities (n = 49; 43%), followed by distal disease (n = 39; 34%), and inability to operate (non-patient-related factors) (n = 27; 23%).
3.5 Medical Treatment
At diagnosis, PH-targeted medical therapy (i.e., calcium channel blocker, PCA, PDE5i, or sGC stimulator) was documented for 77 patients (36%). The most commonly recorded therapy was a PDE5i, while fewer than 10% of patients received an sGC stimulator, ERA, CCB, or PCA (Fig. 2a). At the initial visit, PH-targeted therapy was documented for 142 patients (67%). Compared with diagnosis, the use of PH-targeted therapies, especially sGC stimulators, increased between diagnosis and initial visit, with little change thereafter (Fig. 2a). Use of PH-targeted monotherapy increased between diagnosis and initial visit and declined thereafter, while use of combination therapy increased throughout the study (Fig. 2b). The most commonly used combinations were PDE5i plus ERA, ERA plus sGC stimulator, and inhaled PCA plus sGC stimulator (Fig. 2c). Daily doses of PH-targeted therapy at initial visit are presented in the Online Resource, Supplementary Table 4. Median duration of PH-targeted monotherapy at the initial visit ranged from 75 days in patients receiving macitentan to 718 days in patients receiving inhaled iloprost.
a Percentage of patients receiving PH-targeted therapies (multiple treatments were possible at one timepoint), b percentage of patients receiving monotherapy or combination therapy, c combination therapies received. Data are expressed as a percentage of the total population (n = 212). Patients receiving only CCB or tyrosine kinase inhibitor are not shown. Monotherapy at initial visit: sildenafil (21%); tadalafil (< 1%); macitentan (2%); bosentan (1%); iloprost (2%); riociguat (21%). CCB calcium channel blocker, ERA endothelin receptor antagonist, PCA prostacyclin analog, PDE5i phosphodiesterase type 5 inhibitor, PH pulmonary hypertension, sGC soluble guanylate cyclase
PH-targeted therapies in patients with confirmed operability status are presented in the Online Resource, Supplementary Table 5. At initial visit a higher proportion of inoperable patients (88/115; 77%) was documented as receiving PH-targeted therapy compared with operable patients (42/95; 44%). The most commonly used therapies at baseline were riociguat in inoperable patients and PDE5i in operable patients. At 3 years, 21 of 29 inoperable patients (72%) and 11 of 18 operable patients (61%) were documented as receiving PH-targeted therapy. The most commonly used therapy at 3 years was riociguat in both inoperable and operable patients.
In total, 97 patients (46%) were receiving anticoagulation at diagnosis, compared with 168 (79%) at initial visit and 110 (52%) at study end. The most commonly documented antithrombotic agents at the time of diagnosis were warfarin (n = 64; 30%) and rivaroxaban (n = 22; 10%). This was also true at initial visit (48% and 24% of patients, respectively) and study end (30% and 18% of patients, respectively).
3.6 Balloon Pulmonary Angioplasty
At initial visit, 6 (23%) of the 26 patients assessed for BPA eligibility had undergone BPA. During the study, 23 patients (11%) underwent BPA, including 2 patients (1%) who had also undergone BPA at initial visit, resulting in 27 patients (13%) who underwent BPA in total.
3.7 Clinical Outcomes
Clinical parameters were analyzed descriptively and no formal statistical analyses were performed. On the basis of all available data at initial visit and last documented visit, 6MWD increased, the distribution of WHO FC showed a trend toward improvement, NT-proBNP and PVR decreased, and CI showed no change (data not shown). Paired data for patients with data available at both timepoints (Table 6) indicated an increase in 6MWD by 32 ± 130 m (n = 78) and an increase in NT-proBNP of 269 ± 2279 ng/mL (n = 45) from initial to last documented visit. There were too few patients with paired data for CI or PVR (n = 3 and 2, respectively) to permit meaningful analysis. Changes in vital signs from initial visit to last observation were small and unlikely to be clinically relevant.
3.8 Hospitalization Due to CTEPH or CTEPH Complications
During the observation period, complete data were available for 130 hospitalizations occurring in 72 patients, most of whom (44/72) had one admission, but one had eight admissions. The mean (SD) duration of hospitalization was 13.2 (14.5) days, and the overall hospitalization rate was 0.226 per patient-year, with a tendency to decrease during the study (from 0.322 per patient-year in 2016 to 0.103 per patient-year in 2020).
3.9 Survival Analysis
The survival analysis for all-cause mortality showed an end-of-observation survival rate of 88% with a mean ± standard error survival time of 975.1 ± 13.6 days (Fig. 3).
4 Discussion
This prospective registry assessed the demographics, clinical characteristics, diagnostic techniques, operability assessments, and treatment of patients with CTEPH in Russia, Kazakhstan, Turkey, Lebanon, and Saudi Arabia. The study design allowed trends in the study population for the diagnosis of CTEPH and its real-life clinical management over time to be evaluated in these countries. Although approximately 31% of patients did not meet the formal entry criteria, they were included in the study because other evidence for CTEPH was available. Baseline characteristics for the subgroup of patients who met the formal entry criteria and those with inclusion criteria confirmed via other information were generally similar; therefore, the latter patients were included to reflect the situation in the countries covered by the registry, in which cost constraints can restrict the availability of diagnostic tests.
Our results show that almost 90% of patients underwent RHC at diagnosis or initial study visit. PEA was widely used in the centers participating in this study: almost one-third of patients had undergone this procedure before the initial visit. By contrast, only 12% of patients had been assessed for BPA at their initial visit, suggesting underuse of this procedure, despite over half of the patients (54%) being considered inoperable. Use of PH-targeted therapy increased from diagnosis to the initial visit, remaining similar thereafter. Survival at the end of observation was 88%.
The patients in this registry were younger than those in some other registries, including the International CTEPH Registry (Europe and Canada) [45], the US CTEPH Registry [40], the worldwide prospective registry [39], the single-center study in the Netherlands [41], or the UAE Registry [44]. They were also younger than other studies, including the Giessen PH registry in Germany [46], the COMPERA (Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension) registry [47], a study from Germany which included patients from COMPERA and three German referral centers [48], and a large cohort study in Canada [49]. By contrast, the mean age at diagnosis of patients with CTEPH in the SAUDIPH Registry was approximately 40 years, reflecting the young age of the overall Saudi population [43]. Compared with these registries, the distribution of WHO FC at initial visit was more favorable in the current study, whereas the mean pulmonary artery pressure, PVR, right atrial pressure, and PCWP were higher. Compared with the Russian National Registry [42], the patients in the current study had a similar mean age, a more favorable distribution of WHO FC, and a higher mean RAP and PCWP, but it should be noted that the Russian National Registry recruited newly diagnosed patients.
In the current study, 68% of patients presented with a history of a previous VTE. This was a slightly lower proportion than in the International CTEPH Registry (81% in operable and 72% in non-operable patients) [45], the German study (76%) [48], COMPERA (72%) [47], the single-center study in the Netherlands (78% in clinically inoperable patients; 80% in technically inoperable patients or those with residual PH after PEA) [41], the UAE Registry (78% of patients had a history of PE) [44], or the Russian National Registry (53% and 27.2% of patients had a history of deep vein thrombosis or PE, respectively) [42]. Despite guideline recommendations stating that patients with CTEPH should receive lifelong anticoagulation therapy [6, 15], only 79% and 52% of patients were documented as receiving anticoagulation at initial visit and study end, respectively. This may be a result of underreporting. Other risk factors previously identified for CTEPH, such as antiphospholipid antibody syndrome, splenectomy, and elevated plasma factor VIII levels [1] were also observed in the current study although the latter was reported at a lower incidence than previously reported; ventriculoatrial shunt and infected pacemaker were not observed as risk factors. The most common symptoms at diagnosis and initial visit (e.g., shortness of breath, fatigue, and edema) were typical of those expected for patients presenting with CTEPH [8]. These symptoms were also reported in the Russian National Registry, which noted that symptoms were detected more frequently at the time of diagnosis verification than at onset of CTEPH [42]. All comparisons between registries should be made with caution because of differences in study design and entry criteria.
The most commonly used diagnostic or imaging tests were CT angiography, V/Q scintigraphy, RHC, and CT, used in 40%, 35%, 34%, and 32% of patients, respectively, at diagnosis, and 75%, 64%, 93%, and 50% of patients, respectively, at some point. Most patients also underwent electrocardiography and echocardiography at least once. CTEPH diagnosis and management guidelines recommend V/Q scintigraphy for patients with an intermediate or high probability of PH [15]. Possible explanations for the underuse of this technique include unavailability of equipment or clinician preference for other methods. This was a study of real-world diagnosis and treatment in countries where resources may be limited, and where not all imaging studies may be available for CTEPH diagnosis. For example, V/Q scintigraphy was used as the sole imaging method for diagnostic confirmation in 38 patients, despite it being more appropriate as a screening tool. This was permitted in the study protocol to reflect routine practice in the participating countries. Where patients did not meet formal diagnostic criteria, however, the diagnosis of CTEPH was confirmed on a case-by-case basis after discussion with the investigators. In addition, as shown in the Online Resource, patients who did not meet formal entry criteria differed little from those who did.
The challenges of diagnosing CTEPH and other forms of PH in settings with limited resources are substantial, and patients often present with late-stage disease, resulting in high mortality rates [50, 51]. Given the lack of adequate diagnostic tools and the non-specific nature of the symptoms, patients with PH may be diagnosed with heart failure, other cardiorespiratory conditions, or even tuberculosis [50]. The situation may be further complicated by lack of awareness of CTEPH among physicians and patients, particularly where resources for healthcare education are devoted to more prevalent conditions [51]. The proportion of patients with PH who have CTEPH has been reported as 16% in Egypt [52], 5% in Kenya [53], and 2% in the Pan-African Pulmonary Hypertension Cohort (PAPUCO) Registry [54]. These observations suggest that the diagnosis and management of CTEPH may be especially challenging in the African continent.
Our results showed a mean delay of 2.2 years between presenting symptoms and confirmed diagnosis of CTEPH, whereas the International CTEPH Registry reported a 14-month delay [7]. The longer delay in our study suggests that early diagnosis of CTEPH remains a challenge in the countries included, possibly because of a low clinical suspicion of CTEPH, underuse of guideline-recommended diagnostic tests, or diagnostic misclassification. The proportions of patients with V/Q defects (92% at diagnosis and 94% at initial visit) appear slightly lower than in the International CTEPH Registry, which reported that 99% of patients had an abnormal perfusion scan [19]. In the present study, findings consistent with CTEPH were observed at initial visit by pulmonary angiography in 61% of evaluated patients, and on CT scan in 44%, compared with 63% of patients having proximal lesions on angiography and 60–77% of patients having abnormalities visible on a CT scan in the international registry [7].
Almost all patients were evaluated for PEA eligibility at the initial visit as recommended by treatment guidelines [15]. PEA was, however, often delayed, with only 66 of 95 operable patients having undergone PEA at the initial visit, with a mean delay of almost a year between diagnosis and surgery.
PDE5is were the most commonly used PH-targeted agents at diagnosis and initial visit and were used in 23% of patients at 3-year follow-up, despite not being licensed for the treatment of CTEPH. The wide use of PDE5is may be partly a reflection of their familiarity [including use in the treatment of pulmonary arterial hypertension (PAH)] and low cost. The use of riociguat increased from 6% of patients at diagnosis to 38% at 3-year follow-up. This observation suggests increasing acceptance of riociguat by physicians, but it lags behind the German study, in which 81% of patients who received medical therapy were treated with riociguat [48]. The lower use of riociguat in the countries included in the registry may be partly a result of cost constraints or less experience with its use. By the end of the study, 21% of patients were receiving combination medical therapy. Such use is consistent with other CTEPH registries, which also recorded use of combination regimens in some patients [39, 40, 45, 55]. In the current study, PH-targeted therapies were used more frequently in inoperable patients than operable patients, and riociguat was the most frequently used of these agents in inoperable patients at initial visit (41% of patients) and 3 years (55% of patients), and also in operable patients at 3 years (44%). The use of PH-targeted therapies in the operable group suggests that off-label use is common in the countries included in the registry.
Median doses of PH-targeted therapy were generally in line with recommendations for patients with PAH, although the median daily dose of inhaled iloprost (monotherapy, 60 μg; combination therapy, 80 μg) was higher than the recommended dose range of 30–45 μg [56] , and the dose range for sildenafil monotherapy (20−120 mg/day) exceeded the recommended maximum of 60 mg/day [57]. The median riociguat monotherapy dose (6.0 mg) was lower than that reported for patients with CTEPH in the EXPosurE Registry RiociguaT in patients with PH (EXPERT) registry (7.5 mg) [58], but riociguat is individually dosed, guided by systolic blood pressure and signs and symptoms of hypotension [25, 26].
Use of BPA was limited during the study, with only 13% of patients undergoing this procedure, possibly reflecting lack of experience with BPA, or insufficient resources. In addition, at the time this registry was performed, BPA had only a Class IIb, Level of evidence C recommendation [59]. For comparison, the German study reported use of BPA in 13% of patients overall and in 25% of non-operated patients [48].
The Russian National Registry also reported low use of PEA and BPA (32% and 7% of patients, respectively) [42], although the patients in this study were newly diagnosed, and these results do not exclude these techniques being employed later.
The early mortality rate during the observation period (7%) in our study, albeit based on small absolute numbers, was higher than that generally reported at expert CTEPH centers (< 3%) [6]. Comparison of overall results between initial and last documented visit (regardless of treatment) suggested improvements in 6MWD, WHO FC, NT-proBNP, and PVR over time, although no formal statistical analyses were performed. These findings should be viewed with caution because of the small numbers of patients with data, particularly for PVR and CI, at their last documented visit. This reflects clinical practice, in which invasive assessments are not performed without a clear indication. As with all observational studies, there is potential for selection bias, with patients undergoing repeat assessment only if their condition is thought to be worsening. While the levels of NT-proBNP for all patients with data declined from diagnosis to 3-year follow-up, the paired data indicated an increase over the same period. The latter result may be a statistical artifact. The single-center study in the Netherlands reported improvements in 6MWD and NT-proBNP in patients with CTEPH treated with macitentan (mostly in combination with riociguat or sildenafil) [41], but in view of differences in study design and patient population, caution is advised if comparing these results with our study.
The estimated overall survival at approximately 3 years in the current study (88%) is higher than that reported by the Giessen registry (77%) [46] or the older ASPIRE registry (71%) [20], but comparable with that of the operated patients in the International Registry (89%) [45] and the Spanish Registry of Pulmonary Hypertension (REHAP) (91%) [55], and medically treated patients assessed as low risk in the COMPERA registry (92%) [47]. CTEPH registries have consistently shown a higher survival in operated than non-operated patients [40, 45, 46, 55, 60], and therefore the patients undergoing PEA in our study may have favorably influenced the survival rate.
This study has a number of limitations, many of which are typical of registry studies. First, the number of patients with data available (212 overall) was modest, with very few recruited from Kazakhstan (n = 11) or Lebanon (n = 1). Therefore, the data may not be representative of the overall CTEPH population in these countries. The study recruited a heterogeneous population from diverse countries and the overall results might not reflect practice or outcomes in individual countries. For example, in the SAUDIPH Registry all patients underwent RHC for the diagnosis of PH [43]. In addition, some baseline data were collected retrospectively, and some data (e.g., risk factors, PH-targeted therapies, and clinical parameters) are missing due to incomplete data collection and/or collation, or loss of information between center referrals. Missing data were particularly limiting for 6MWD, NT-proBNP, hemodynamics, and RV function. This is partly a reflection of the non-interventional study design, which did not demand any investigations beyond those used in routine practice in each center. Clinicians would not be expected to repeat invasive procedures such as RHC unless clinically indicated, generally if a patient’s condition is thought to be worsening. This may lead to selection bias for patients with repeated invasive assessments. Similarly, while information on use of diagnostic imaging was recorded, no images were collected. Furthermore, many patients were diagnosed with CTEPH without confirmatory imaging, which may reflect the limited healthcare resources available in some countries included in the registry. Another limitation is the potential for bias to be introduced by the inclusion of patients with different disease management pathways, particularly related to the operability status and the use of therapy, which could skew the results associated with the management of CTEPH. In addition, no data on the use of medical therapies other than PH-targeted agents and anticoagulants were collected. For the reasons explained above, AE data are not included in this report. This could be considered a limitation, but the current study was not a drug registry and was not designed to evaluate the safety of specific treatments.
Finally, all statistical analyses were exploratory and descriptive, and therefore caution is needed when interpreting the data. As there was no predefined hypothesis for this study, no statistical tests were done, and comparisons with other studies are for information only.
5 Conclusions
This registry highlights the current practice and management of patients with CTEPH in Russia, Kazakhstan, Turkey, Lebanon, and Saudi Arabia. The findings demonstrate an association between CTEPH diagnosis and a history of VTE. However, early diagnosis of CTEPH remains a challenge, as demonstrated by a mean delay of 2.2 years from onset of symptoms to diagnosis and the incomplete application of the recommended diagnostic process. It is likely that this is related to the limited healthcare resources available in low- and middle-income countries (with the exception of Saudi Arabia, where diagnosis and management guidelines for CTEPH are followed strictly, all treatments are available, and the probability of survival is high as shown by the SAUDIPH Registry [43]). Most patients were evaluated for PEA eligibility and treated with off-label PH-targeted treatment or riociguat, but few were referred for BPA evaluation. Analyses from this registry, as well as comparisons with international registries, highlight the importance of providing the resources to give patients with CTEPH full access to recommended diagnostic tests and treatments.
References
Ruaro B, Baratella E, Caforio G, Confalonieri P, Wade B, Marrocchio C, et al. Chronic thromboembolic pulmonary hypertension: an update. Diagnostics (Basel). 2022;12(2):235. https://doi.org/10.3390/diagnostics12020235.
Marchetta S, Verbelen T, Claessen G, Quarck R, Delcroix M, Godinas L. A comprehensive assessment of right ventricular function in chronic thromboembolic pulmonary hypertension. J Clin Med. 2022;12(1):47. https://doi.org/10.3390/jcm12010047.
Lang I. Chronic thromboembolic pulmonary hypertension: a distinct disease entity. Eur Respir Rev. 2015;24(136):246–52. https://doi.org/10.1183/16000617.00001115.
Robbins IM, Pugh ME, Hemnes AR. Update on chronic thromboembolic pulmonary hypertension. Trends Cardiovasc Med. 2017;27(1):29–37. https://doi.org/10.1016/j.tcm.2016.05.010.
Simonneau G, Torbicki A, Dorfmuller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143): 160112. https://doi.org/10.1183/16000617.0112-2016.
Delcroix M, Torbicki A, Gopalan D, Sitbon O, Klok FA, Lang I, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;57:2002828. https://doi.org/10.1183/13993003.02828-2020.
Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973–81. https://doi.org/10.1161/circulationaha.110.015008.
Kim NH, Delcroix M, Jaїs X, Madani MM, Matsubara H, Mayer E, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(1):1801915. https://doi.org/10.1183/13993003.01915-2018.
Humbert M, Guignabert C, Bonnet S, Dorfmuller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53(1):1801887. https://doi.org/10.1183/13993003.01887-2018.
Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D92–9. https://doi.org/10.1183/13993003.01915-2018.
Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J. 2013;41(2):462–8. https://doi.org/10.1183/09031936.00049312.
Frost A, Badesch D, Gibbs JSR, Gopalan D, Khanna D, Manes A, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53(1):1801904. https://doi.org/10.1183/13993003.01904-2018.
Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143): 160108. https://doi.org/10.1183/16000617.0108-2016.
Hoeper MM, Madani MM, Nakanishi N, Meyer B, Cebotari S, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Lancet Respir Med. 2014;2(7):573–82. https://doi.org/10.1016/S2213-2600(14)70089-X.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022;30:2200879. https://doi.org/10.1183/13993003.00879-2022.
Jenkins D. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24(136):263–71. https://doi.org/10.1183/16000617.00000815.
Jenkins D, Madani M, Fadel E, D’Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension (CTEPH). Eur Respir Rev. 2017;26(143): 160111. https://doi.org/10.1183/16000617.0111-2016.
Freed DH, Thomson BM, Berman M, Tsui SS, Dunning J, Sheares KK, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(2):383–7. https://doi.org/10.1016/j.jtcvs.2009.12.056.
Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–10. https://doi.org/10.1016/j.jtcvs.2010.11.024.
Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, et al. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J. 2012;39(4):945–55. https://doi.org/10.1183/09031936.00078411.
Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the UK National cohort. Circulation. 2016;133:1761–71. https://doi.org/10.1161/CIRCULATIONAHA.115.019470.
Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29. https://doi.org/10.1056/NEJMoa1209657.
Simonneau G, D’Armini AM, Ghofrani HA, Grimminger F, Jansa P, Kim NH, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4(5):372–80. https://doi.org/10.1016/S2213-2600(16)30022-4.
Sadushi-Kolici R, Jansa P, Kopec G, Torbicki A, Skoro-Sajer N, Campean IA, et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): a double-blind, phase 3, randomised controlled trial. Lancet Respir Med. 2019;7(3):239–48. https://doi.org/10.1016/S2213-2600(18)30367-9.
Bayer AG. Adempas EU summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/adempas-epar-product-information_en.pdf. Accessed 2 Nov 2023.
Bayer AG. Adempas US prescribing information. 2021. https://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf. Accessed 2 Nov 2023.
SciPharm Sàrl. Trepulmix. EU summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/trepulmix-epar-product-information_en.pdf. Accessed 2 Nov 2023.
Gall H, Preston IR, Hinzmann B, Heinz S, Jenkins D, Kim NH, et al. An international physician survey of chronic thromboembolic pulmonary hypertension management. Pulm Circ. 2016;6(4):472–82. https://doi.org/10.1086/688084.
Sheth A, Park JE, Ong YE, Ho TB, Madden BP. Early haemodynamic benefit of sildenafil in patients with coexisting chronic thromboembolic pulmonary hypertension and left ventricular dysfunction. Vascul Pharmacol. 2005;42(2):41–5. https://doi.org/10.1016/j.vph.2004.11.005.
Ghofrani HA, Schermuly RT, Rose F, Wiedemann R, Kohstall MG, Kreckel A, et al. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2003;167(8):1139–41. https://doi.org/10.1164/rccm.200210-1157BC.
Jaїs X, D’Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52(25):2127–34. https://doi.org/10.1016/j.jacc.2008.08.059.
Hoeper MM, Kramm T, Wilkens H, Schulze C, Schafers HJ, Welte T, et al. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest. 2005;128(4):2363–7. https://doi.org/10.1378/chest.128.4.2363.
Ghofrani HA, Simonneau G, D’Armini AM, Fedullo P, Howard LS, Jaïs X, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5(10):785–94. https://doi.org/10.1016/S2213-2600(17)30305-3.
Kramm T, Eberle B, Guth S, Mayer E. Inhaled iloprost to control residual pulmonary hypertension following pulmonary endarterectomy. Eur J Cardiothorac Surg. 2005;28(6):882–8. https://doi.org/10.1016/j.ejcts.2005.09.007.
Lang I, Meyer B, Ogo T, Matsubara H, Kurzyna M, Ghofrani HA. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160119. https://doi.org/10.1183/16000617.0119-2016.
Ghofrani HA, Kim NH. Medical and interventional therapies for inoperable CTEPH: a necessary combination? Lancet Respir Med. 2022;10(10):926–7. https://doi.org/10.1016/S2213-2600(22)00227-2.
Jaїs X, Brenot P, Bouvaist H, Jevnikar M, Canuet M, Chabanne C, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med. 2022;10(10):961–71. https://doi.org/10.1016/S2213-2600(22)00214-4.
Klok FA, Barco S, Konstantinides SV, Dartevelle P, Fadel E, Jenkins D, et al. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: results from the European CTEPH Registry. Eur Respir J. 2018;52(6):1801687. https://doi.org/10.1183/13993003.01687-2018.
Guth S, D’Armini AM, Delcroix M, Nakayama K, Fadel E, Hoole SP, et al. Current strategies for managing chronic thromboembolic pulmonary hypertension: results of the worldwide prospective CTEPH Registry. ERJ Open Res. 2021;7(3):00850–2020. https://doi.org/10.1183/23120541.00850-2020.
Kerr KM, Elliott CG, Chin K, Benza RL, Channick RN, Davis RD, et al. Results from the United States chronic thromboembolic pulmonary hypertension registry: enrollment characteristics and 1-year follow-up. Chest. 2021;160(5):1822–31. https://doi.org/10.1016/j.chest.2021.05.052.
van Thor MCJ, Ten Klooster L, Snijder RJ, Mager JJ, Post MC. Long-term real world clinical outcomes of macitentan therapy in chronic thromboembolic pulmonary hypertension. Respir Med. 2020;167: 105966. https://doi.org/10.1016/j.rmed.2020.105966.
Chazova IY, Martynyuk TV, Valieva ZS, Gratsianskaya SY, Aleevskaya AM, Zorin AV, et al. Clinical and instrumental characteristics of newly diagnosed patients with various forms of pulmonary hypertension according to the Russian National Registry. Biomed Res Int. 2020;2020:6836973. https://doi.org/10.1155/2020/6836973.
Aldalaan AM, Saleemi SA, Weheba I, Abdelsayed A, Hammainen P, Aleid MM, et al. Chronic thromboembolic pulmonary hypertension in Saudi Arabia: preliminary results from the SAUDIPH registry. ERJ Open Res. 2020;6(2):00218–2019. https://doi.org/10.1183/23120541.00218-2019.
Saleh K, Khan N, Dougherty K, Bodi G, Michalickova M, Mohammed S, et al. The first pulmonary hypertension registry in the United Arab Emirates (UAEPH): clinical characteristics, hemodynamic parameters with focus on treatment and outcomes for patients with group 1-PH. J Clin Med. 2023;12(5):1996. https://doi.org/10.3390/jcm12051996.
Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D’Armini AM, Snijder R, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2016;133:859–71. https://doi.org/10.1161/CIRCULATIONAHA.115.016522.
Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, et al. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;S1053–2498. https://doi.org/10.1016/j.healun.2017.02.016.
Delcroix M, Staehler G, Gall H, Grünig E, Held M, Halank M, et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur Respir J. 2018;52(5):1800248. https://doi.org/10.1183/13993003.00248-2018.
Kramm T, Wilkens H, Fuge J, Schafers HJ, Guth S, Wiedenroth CB, et al. Incidence and characteristics of chronic thromboembolic pulmonary hypertension in Germany. Clin Res Cardiol. 2018;107(7):548–53. https://doi.org/10.1007/s00392-018-1215-5.
Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, et al. Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11(2):e003973. https://doi.org/10.1161/CIRCOUTCOMES.117.003973.
Dzudie A, Dzekem BS, Ojji DB, Kengne AP, Mocumbi AO, Sliwa K, et al. Pulmonary hypertension in low- and middle-income countries with focus on sub-Saharan Africa. Cardiovasc Diagn Ther. 2020;10(2):316–24. https://doi.org/10.21037/cdt.2019.07.06.
Idrees M, Butrous G, Mocumbi A, Sastry B, Ibrahim A, Alobaidallah K, et al. Pulmonary hypertension in the developing world: local registries, challenges, and ways to move forward. Glob Cardiol Sci Pract. 2020;2020(1):e202014. https://doi.org/10.21542/gcsp.2020.14.
Soliman YA, Elkorashy R, Kamal E, Ismail MS, El-Hinnawy YH, Yamamah HG, et al. Pulmonary hypertension registry: a single-center experience in Egypt. Egypt J Chest Dis Tuberc. 2020;69(3):596–603. https://doi.org/10.4103/ejcdt.ejcdt_197_19.
Ngunga M, Mansur Abeid A, Mohamed J, Barasa A. Long-term outcomes and factors associated with mortality in patients with moderate to severe pulmonary hypertension in Kenya. Glob Heart. 2020;15(1):6. https://doi.org/10.5334/gh.384.
Thienemann F, Dzudie A, Mocumbi AO, Blauwet L, Sani MU, Karaye KM, et al. The causes, treatment, and outcome of pulmonary hypertension in Africa: insights from the Pan African Pulmonary Hypertension Cohort (PAPUCO) Registry. Int J Cardiol. 2016;221:205–11. https://doi.org/10.1016/j.ijcard.2016.06.242.
Escribano-Subias P, Del PR, Roman-Broto A, Domingo Morera JA, Lara-Padron A, Elias HT, et al. Management and outcomes in chronic thromboembolic pulmonary hypertension: from expert centers to a nationwide perspective. Int J Cardiol. 2016;203:938–44. https://doi.org/10.1016/j.ijcard.2015.11.039.
Bayer AG. Ventavis (iloprost). Summary of product characteristics. 2013. https://www.ema.europa.eu/en/documents/product-information/ventavis-epar-product-information_en.pdf. Accessed 2 Nov 2023.
Pfizer. Revatio (Sildenafil) highlights of US prescribing information. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021845s011,022473s004,0203109s002lbl.pdf. Accessed 2 Nov 2023.
Ghofrani HA, Gomez Sanchez MA, Humbert M, Pittrow D, Simonneau G, Gall H, et al. Riociguat treatment in patients with chronic thromboembolic pulmonary hypertension: final safety data from the EXPERT registry. Respir Med. 2020;178:106220. https://doi.org/10.1016/j.rmed.2020.106220.
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75. https://doi.org/10.1183/13993003.01032-2015.
Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177(10):1122–7. https://doi.org/10.1164/rccm.200712-1841OC.
Acknowledgements
Medical writing services provided by Richard Murphy, PhD, of Adelphi Communications Ltd, Macclesfield, UK, were funded by Bayer AG, Berlin, Germany in accordance with Good Publication Practice guidelines. The authors would like to thank Nergis Karacan of Bayer AG, who developed the study protocol and was initially responsible for the study, and their colleagues from Bayer AG, Institut Dr. Schauerte, and Adelphi Communications Ltd for their contributions and support throughout the project.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
Funding for this study was provided by Bayer AG, Berlin, Germany, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Conflict of interest
HGÖ has consultancy relationships, received lecture honoraria, and/or has received research funding from Actelion Pharmaceuticals Ltd, Bayer, and GlaxoSmithKline. MI has received financial honoraria for lectures from Actelion, AstraZeneca, Bayer AG, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Pfizer Inc., and research grants from Actelion, AstraZeneca, and Pfizer Inc. MS has received financial honoraria for lectures from Actelion and Bayer AG. DZ has received payments and travel funding for overseas lectures from Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, and Pfizer. NT is an employee of Bayer AG, Berlin, Germany. KV is an employee of Bayer AG, Wuppertal, Germany. KH, SK, and IS are employees of Institut Dr. Schauerte, Munich, Germany, which was contracted by Bayer AG to develop the eCRF and data capture system and were involved in the collection and analysis of the data. All other authors have nothing to declare.
Data availability statement
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations and Pharmaceutical Research and Manufacturers of America principles for responsible clinical trial data sharing, pertaining to scope, time point, and process of data access. Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and European Union as necessary for doing legitimate research. This commitment applies to data on new medicines and indications that have been approved by the European Union and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to do further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Ethics approval
Approval from appropriate independent ethics committees (ECs) and institutional review boards (IRBs) was obtained from all participating centers. The ECs, IRBs, and dates of approval are listed in Supplementary Table 6. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Consent to participate
All patients provided informed, written consent.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
The study protocol and eCRF were developed by Bayer AG. NT oversaw amendments to the protocol and eCRF following discussions with the investigators. HGÖ was the principal investigator throughout the study. HGÖ, BA, MAD, AC, GD, MI, EK, HK, VL, TM, NM, MAM, BM, GO, AO, ZPÖ, HS, NS, MS, LT, TT, HY, DZ, and AA recruited patients to the study, were responsible for all investigations and treatment decisions according to their clinical judgement and practice, agreed on the refinements to the eCRF, and provided the data to the capture system accordingly. All authors developed and revised the manuscript for important intellectual content at every stage, including interpretation of the data and all phases of manuscript preparation, and they read and approved the final version for submission. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Institut Dr Schauerte managed the electronic data capture system and collected the data. KH, SK, IS, and KV analyzed the results. IS was the project manager for the study at Institut Dr Schauerte.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Öngen, H.G., Akdeniz, B., Düzenli, M.A. et al. Diagnosis and Treatment Patterns of Chronic Thromboembolic Pulmonary Hypertension in Russia, Kazakhstan, Turkey, Lebanon, and Saudi Arabia: A Registry Study. Drugs - Real World Outcomes 11, 149–165 (2024). https://doi.org/10.1007/s40801-023-00407-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-023-00407-w