Abstract
Background
Use of the direct oral anticoagulant rivaroxaban has strongly increased in Europe since its market approval for non-valvular atrial fibrillation in 2011. Patients characteristics of rivaroxaban initiators may have changed over time but this has not been investigated so far.
Objective
We aimed to describe time trends of patient baseline characteristics among new rivaroxaban users with non-valvular atrial fibrillation from 2011 to 2016/17 in two European countries.
Methods
We used data from Germany (German Pharmacoepidemiological Research Database) and the Netherlands (PHARMO Database Network). We included new rivaroxaban users with (i) a first dispensing between 2011 and 2016/17, (ii) ≥ 2 years of age, and (iii) a diagnosis of non-valvular atrial fibrillation and described their baseline medication and comorbidity prior to starting rivaroxaban stratified by year of inclusion.
Results
Overall, 130,652 new rivaroxaban users were included during the study period (Germany: N = 127,743, the Netherlands: N = 2909). The sex ratio and median age remained relatively stable over time. The proportion of patients without prior use of oral anticoagulants before initiation of rivaroxaban increased in both countries between 2011 and 2016/17 (Germany: from 51 to 76%, the Netherlands: from 57 to 85%). In Germany, we observed a relative decrease by 27% in the proportion of new rivaroxaban users with a history of ischemic stroke and by 18% in the proportion with a transient ischemic attack at baseline. No such a pattern was observed in the Netherlands. The proportion of patients with heart failure at baseline showed a three-fold increase in the Netherlands, while there was a relative decrease by 12% in Germany.
Conclusions
Patient characteristics of new rivaroxaban users with non-valvular atrial fibrillation changed between 2011 and 2016/17, but changes differed between countries. These patterns have methodological implications. They have to be considered in the interpretation of observational studies comparing effectiveness and safety of oral anticoagulants, especially regarding potential bias due to unmeasured confounding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since 2011, the proportion of new RVX users with atrial fibrillation being naive to oral anticoagulants has substantially increased in Germany and the Netherlands. |
In both countries this was accompanied by a trend towards a lower prevalence of comorbidities among new RVX users, but the trend was more pronounced in Germany. |
These patterns have to be considered when comparing the results from observational studies using data from different countries or different years, especially regarding potential bias due to unmeasured confounding. |
1 Introduction
Direct oral anticoagulants (DOACs) have become an important therapeutic alternative to vitamin K antagonists (VKAs) in the prevention of stroke and systemic embolism among patients with non-valvular atrial fibrillation (nvAF). Randomised controlled trials (RCTs) and observational studies investigating the safety and effectiveness of DOACs have shown non-inferiority to warfarin, the most commonly used VKA worldwide [1, 2]. Additionally, data from observational studies investigating DOACs in comparison to other VKAs (e.g., acenocoumarol or phenprocoumon) suggested also non-inferiority to these VKAs [3,4,5]. The main advantage of DOACs over VKAs is that DOACs do not require routine monitoring for potential dose adjustments as their pharmacokinetic properties are more predictable.
Rivaroxaban (RVX), the first DOAC with a once-daily dose regimen, has had an increasing share of DOAC prescriptions since its market approval and is one of the most commonly used DOACs in Europe [6,7,8,9]. In view of the high number of RVX users, it is important to get a better understanding of their demographic and clinical characteristics, particularly of those that are relevant to assess effectiveness and safety outcomes. There are several reasons why these characteristics may have changed over time and vary between countries. First, the number of available DOACs has increased over time (dabigatran approved in 2011, RVX in 2011, apixaban in 2012 and edoxaban in 2015), which may have had an impact on the decision whom to prescribe which DOAC. Second, the temporal patterns in the market introduction of individual DOACs as well as the marketing strategies may have varied between countries and thus may have influenced relevant factors, e.g., prescribing behaviour, which may have led to variation in risk profiles of DOAC users between countries. Finally, the increasing knowledge from observational studies confirming RCT findings on DOACs as well as the changes in antithrombotic treatment guidelines in favour of DOACs [10] may have had an impact on the decision to prescribe DOACs rather than VKAs. Also differences in the reimbursement of DOACs between healthcare systems may cause such differences.
Recognizing variation in risk profiles of DOAC users over time is important for careful interpretation and comparison of studies assessing the effectiveness or safety of different DOACs or DOACs and VKAs. For example, persons who started RVX therapy in 2011 might differ from persons initiating it in later years regarding relevant confounding factors. Given that in most database studies information in this regard is suboptimal, the role of residual confounding possibly leading to channelling bias requires careful consideration in case risk profiles have changed over time.
To elaborate further on this topic, we studied and compared demographic and clinical characteristics of patients with nvAF over time, starting therapy with RVX between 2011 and 2017 in two European countries.
2 Methods
We conducted our analysis in the context of a post authorization safety study (PASS) on RVX [11]. We used data from Germany (German Pharmacoepidemiological Research Database, short GePaRD) and the Netherlands (PHARMO Database Network; here: Out-patient Pharmacy Database linked to General Practitioner Database) for this analysis. Both data sources have been described in detail elsewhere and in the supplement table 1 in Online Resource 1 [11].
2.1 Study Population and Study Design
We included patients initiating therapy with RVX between 1 December 2011 and 31 December 2016 for GePaRD and 31 December 2017 for PHARMO and with a diagnostic code for atrial fibrillation and flutter (ICD10: I48 for GePaRD and PHARMO, and for PHARMO ICD9: 42731 and 42732 as wells as ICPC: K78) any time before initiating RVX therapy. Initiation was defined as no previous record of RVX during the patient’s entire database history. Patients with codes for mitral stenosis or mechanical heart valves were excluded if recorded any time before or at the time of initiating RVX. Furthermore, patients were excluded if they had an initial treatment with VKA during the study period (definition as for RVX) before they initiated RVX therapy, had been enrolled in the respective database for less than 12 months, were younger than two years of age, or were older than the upper age limit defined for the respective database (Germany: 100 years of age; the Netherlands: 105 years of age).
The date of the first dispensing of RVX was defined as the cohort entry date. We assessed baseline medication use in the 90 days prior to cohort entry, and past medical events and comorbidities any time prior to cohort entry. New RVX users were categorized as naive or non-naive, defining naive patients as those with no dispensing of any other oral anticoagulant any time before the cohort entry date.
2.2 Assessment of Baseline Medication and Medical Conditions
At baseline, we considered antiplatelet drugs, non-steroidal anti-inflammatory drugs (NSAIDs), antiarrhythmic drugs, antihypertensive agents, diuretics, statins, anti-diabetic agents, oral steroids, proton pump inhibitors, selective serotonin reuptake inhibitors and antibiotics. We also assessed whether drugs classified as strong inhibitors of either cytochrome P450 3A4 or P-glycoprotein or strong inducers of cytochrome P450 3A4 were dispensed.
Furthermore, we considered relevant past cardiovascular events including ischemic stroke, transient ischemic attack (TIA), venous thromboembolism (deep vein thrombosis, pulmonary embolism), myocardial infarction and main bleedings (intracranial, gastrointestinal and urogenital), as well as chronic diseases such as hypertension, heart failure, coronary artery disease, and diabetes mellitus. For classification of the stroke and bleeding risk, we calculated the CHA2DS2VASc score and the modified HAS-BLED score as described in the supplement table 2 and 3 in the Online Resource 2.
2.3 Data Analysis
We described the characteristics of new RVX users using frequency counts and percentages for categorical variables and medians with interquartile range (IQR) and range, and means with standard deviation for continuous variables, e.g., age. To assess trends in patient characteristics over time, we stratified the analyses by year of cohort entry. The analyses were conducted for each database separately. The statistical analyses were performed with the statistical software SAS 9.4 (SAS Institute, Cary, NC, USA).
3 Results
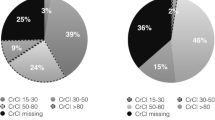
A total of 130,652 (52% male) new RVX users with nvAF were included in the cohorts (Germany: N = 127,743, and the Netherlands: N = 2909). The median age was 71 years in the Netherlands and 75 years in Germany (minimum age during the study period: 19 years in the Netherlands, 17 years in Germany). The age distribution remained stable over time in Germany, whereas the proportion of old patients (≥ 80 years) increased in the Netherlands (Fig 1).
The proportion of new RVX users classified as naive, i.e., with no previous dispensing of any oral anticoagulant drug before RVX initiation, increased from 51% in 2011/2012 to 76% in 2016 in Germany (Fig 2). A similar pattern was observed for the Netherlands (increase from 57 to 85%).
Proportion of new RVX users among patients with nvAF naive* to oral anticoagulation (OAC) by calendar year in the German Pharmacoepidemiological Research Database [GePaRD] (Germany [GER]) and PHARMO (the Netherlands [NL]). *Naive patients were defined as those with no previous dispensing of any oral anticoagulant drug recorded before RVX initiation. **2011 data (December only) and 2012 data summarized. For GePaRD, only data until 2016 available
Baseline comorbidity and medication among new RVX users at cohort entry, i.e., time of the first RVX prescription, are shown in Table 1 for Germany and in Table 2 for the Netherlands. The proportion of new RVX users with a history of ischemic stroke or TIA, respectively, decreased over time in Germany (relative decrease by 27 and 18%, respectively) while in the Netherlands no clear pattern could be observed. The prevalence of heart failure and diabetes mellitus decreased in Germany (relative decrease by 12 and 7%, respectively). In the Netherlands, the prevalence of heart failure increased (relative increase by 210%), whereas no clear trend could be observed for the prevalence of diabetes mellitus. As shown in Fig. 3, the median stroke risk assessment score CHA2DS2-VASc remained stable over the study period in Germany at a level of 5 (except for a decrease in the median between 2015 and 2016 from 5 to 4) and altered between a level of 2 and 3 in the Netherlands.
Boxplots: CHA2DS2-VASc score of new RVX users among patients with nvAF at baseline by calendar year in the German Pharmacoepidemiological Research Database [GePaRD] (Germany [GER]) and PHARMO (the Netherlands [NL]). *2011 data (December only) for GePaRD and PHARMO not shown. CHA2DS2-VASc score: congestive heart failure (1 Point); hypertension (1 Point); aged ≥ 75 years (2 Points); diabetes mellitus (1 Point); stroke/transient ischemic attack (2 Points); vascular disease (1 Point), aged 65–74 years (1 Point), female sex (1 Point)
Regarding factors relevant to bleeding risk, we observed a relative decrease in the prevalence of a history of intracranial bleeding by 29% over the years in Germany, whereas in the Netherlands it was at a low level throughout the study period (≤ 1%). The prevalence of a history of gastrointestinal bleedings showed a relative decrease by 27% in Germany, while it showed no clear time trend in the Netherlands. The bleeding risk assessment score HAS-BLED remained stable over the study period in each country, but varied between Germany (median 3) and the Netherlands (median 1).
With respect to medication dispensed within 90 days before or on the day of RVX initiation, we observed a decrease in the prevalence of antiarrhythmic drug use over the years in both countries (Germany: from 14 to 8%; the Netherlands: from 12 to 6%). The prevalence of NSAIDs use decreased by two percentage points in Germany (from 17 to 15%) and by five percentage points in the Netherlands (from 10 to 5%). The prevalence of SSRI use over the years was about 3–4% in Germany and increased from 0 to 4% in the Netherlands. The prevalence of oral corticosteroid use increased in the Netherlands (from 2 to 9%), whereas it remained relatively stable in Germany (~ 7%). The proportion using drugs classified as strong inhibitors of CYP3A4 or P-glycoprotein inhibitors decreased in both countries (11 vs. 7% in Germany; 8 vs. 6% in the Netherlands). Overall, the share of patients using CYP3A4 inducers remained stable and consistently low (< 2%).
4 Discussion
In this observational study including 130,652 new RVX users with nvAF from two European countries, we identified changes in clinical characteristics of new RVX users over time. For several factors, the patterns of changes over time differed between countries. In Germany, baseline prevalences of history of ischemic stroke and TIA, strong indicators for the recurrent stroke risk, decreased between 2011/12 and 2016, while there was no clear trend in the Netherlands. Furthermore, the baseline proportion of patients having a history of intracranial bleeding decreased in Germany. This is an important determinant given a history of bleeding may increase the risk of major bleeding, a main safety concern with oral anticoagulation [12]. On the other hand, we observed a substantial increase in baseline use of oral corticosteroids, which are known to increase the risk of gastrointestinal bleeding, in the Netherlands but not in Germany [13]. The proportion of patients using antiarrhythmic medication at baseline has decreased in Germany and the Netherlands. In both countries, there was an increase of the proportion of new RVX users being naive to oral anticoagulants.
While there are many studies evaluating safety and effectiveness of DOACs individually and as a group, there are only a few studies investigating changes in patterns and user characteristics over time and these existing studies either considered DOACs as a group [6, 14, 15] or focused on trends and treatment pathways rather than on clinical risk profiles of users of individual DOACs [16, 17]. To the best of our knowledge, there is no study comparing time trends between countries nor with focus on new RVX users with nvAF. Loo et al. provided an overview of temporal changes between 2009 and 2015 regarding baseline characteristics of patients newly prescribed DOACs, regardless of the indication, based on data from the UK Clinical Practice Research Datalink [6]. Camm et al. investigated patterns in antithrombotic therapy between 2010 and 2015 among patients with newly diagnosed nvAF and ≥ 1 additional stroke risk factor using data from the Global Anticoagulant Registry FIELD-Atrial Fibrillation (GARFIELD-AF) [14]. Huiart et al. investigated patterns of use of DOACs versus VKA in the treatment of nvAF in France between 2011 and 2015 using data provided by the French National Health Insurance System [15]. In accordance with our findings these studies underline that patient characteristics of new DOAC users changed over time [6, 14, 15]. Camm et al. pointed out that patients treated shortly after DOAC introduction were more likely to suffer from underlying diseases such as coronary artery disease and diabetes, which is largely in line with our observations [14]. Another study investigated time trends in prescribing oral anticoagulants (differentiating between VKA and DOACs) and treatment pathways among patients with AF in routine clinical practice in Belgium, France, Germany, the United States, and the United Kingdom, but this study did not focus on changes in patient characteristics [16].
Regarding demographic characteristics, the median age of patients in our study ranged from 69 to 75 years across the two countries and years, and patients were more often male than female. The latter is consistent with the ROCKET AF pivotal trial population, in which 60% of patients were male. The median age in the trial was 73 years [18]. In both participating countries and in each study year the proportion of patients with heart failure and diabetes mellitus was smaller than in the RCT population where it was 63 and 40%, respectively.
There are various reasons that may explain the observed changes in patient characteristics over time and the differential trends between countries. The higher prevalence of comorbidities such as ischemic stroke and TIA in early adopters of RVX in 2011/12 in comparison to later years observed in Germany may be due to “channelling” of the new drug to patients in whom the prior treatment lacked effectiveness or possibly after a bleeding event. Interestingly, this pattern was not observed in the Netherlands. The importance of “channelling” in Germany is supported by the high proportion of persons switching from another oral anticoagulant to RVX treatment. The increasing proportion of OAC naive new RVX user in Germany and the Netherlands likely reflects changes in stroke prevention guidelines [19, 20]. For Sweden, a different pattern has been described: Almost all new RVX users were naive to oral anticoagulants in the first years following market introduction and this proportion slightly decreased over time [21]. This differential pattern seems plausible given that in Sweden warfarin has been the only oral anticoagulant recommended for patients with nvAF by the Swedish Drug and Therapeutics Committee until 2015, whereas DOACs were suggested as alternatives for selected patients only [22].
Our finding regarding time trends in patient characteristics of new RVX users has methodological implications for the interpretation of existing observational studies as well as for the planning of new observational studies comparing effectiveness and safety of DOAC to VKA or individual DOACs. These studies typically include patients initiating DOAC therapy in various calendar years since 2011. The study from Paschke et al., using German outpatient claims data, for example, included persons initiating DOACs between 2010 and 2017, the study from Hohnloser et al., also using German claims data, included patients initiating DOACs between 2013 and 2015 [5, 7]. The studies came to different conclusions regarding the effectiveness and safety of RVX as compared to VKA. According to our study, the prevalence of underlying risk factors of outcomes of interest as well as the proportion of treatment-naive patients (i.e., no VKA treatment before) in RVX users included in these studies over the years may have been rather heterogeneous and it is not clear to which extent adjustment for measured confounding was sufficient to balance the groups. In addition, time-related biases could have played a role in these studies.
Another finding of our study deserving attention is the fact that we observed hardly any changes in the CHA2DS2-VASc score and the HASBLED score over time despite the changes in the prevalence of comorbidities. This is consistent with the findings of a rapid review on risk profiles of DOAC and VKA users which also suggested that these scores are suboptimal to detect relevant differences in the risk profiles between users of different oral anticoagulants [23]. Different reasons for this have been discussed. The HAS-BLED score, for example, does not take into account, diabetes mellitus or heart failure, so changes in these comorbidities are not reflected by this score. The CHA2DS2-VASc score includes information on heart failure, hypertension, diabetes mellitus, prior stroke or transient ischemic attack, prior vascular diseases as well as sex and age. Being aged > 74 years adds two points to the score, while having diabetes, for example, adds only one point to the score. Changes in the age distribution over the years may thus hide changes in the prevalence of comorbidities. Also an increasing share of older women (> 65 years) without comorbidity could hide a decrease in the prevalence of comorbidities among men given that solely being female adds one point to the score. These aspects point to the importance of reporting the prevalence of relevant comorbidities for different patient groups in addition to reporting scores.
This study was based on two well-established healthcare databases commonly used for pharmacoepidemiological research which cover about one fifth of the respective population. The dispensing of medication in these databases has been shown to be representative of the respective country [24, 25]. Given the absence of non-responder and recall bias the results of this analysis are likely to reflect routine clinical practice in Germany and the Netherlands. There are also limitations to our study. Although the design and conduct of the studies was harmonized as much as possible, some differences remained. Furthermore, variation in the prevalence of certain diagnoses (e.g., hypertension) between countries may not reflect true differences but result from differences between databases as well as healthcare systems. In German health claims data, defining comorbidity by diagnostic codes recorded in outpatient setting without further criteria tends to lack specificity whereas electronic medical record data (e.g., PHARMO database) do not suffer from this problem. This may also explain the observed differences in the values of HAS-BLED and CHA2DS2-VASc scores between countries. Even though this has to be kept in mind, it is less critical for our study as it focused on comparing changes in the prevalence of comorbidities over time within each country rather than comparing prevalences between countries. Even though the sample from the Netherlands also covered a relevant proportion of the Dutch population, the absolute sample size per year was still limited, i.e., statistical variation may also explain some differences between years. Interpretation should therefore focus on overall patterns rather than on changes in prevalences between single years. While our study showed interesting time trends, it was merely descriptive, so it did not allow us to identify the reasons behind these trends. Finally, due to the design of the post-authorization safety study in which our analysis was embedded, a person could not be included both as first-time user of RVX and as first-time user of VKA, i.e., if a person initiated VKA treatment during the study period, it was no longer eligible as potential first-time user of RVX. However, we do not think that the number excluded due to this criterion was very high as it only affected new users of VKA rather than the large group of prevalent VKA users.
As our study was conducted in the context of a post authorization safety study on RVX it focused on only one DOAC and on the first years after market approval of RVX. In future studies it would be interesting to investigate whether the changes in risk profiles we observed in our study continued or whether the distribution of risk profiles tended to be more stable in recent years. The fact that the increase in the proportion of new RVX users in our study mainly increased until 2015 but less afterwards could indicate a trend towards a more stable patient population but this requires empirical confirmation. Furthermore, it will be interesting to also investigate time trends in risk profiles for other DOACs. The number of available DOACs has increased over time (apixaban approved in 2012 and edoxaban in 2015), and with the approval of edoxaban, RVX was no longer the only DOAC with a once-daily dose regimen. Also marketing strategies and reimbursement issues may have changed over time and may have varied between DOACs. Only empirical data will help to shed light on the question how this complex interplay affected the risk profiles of the users of different DOACs over time. In a more general sense, the methodological implications of changes in risk profiles among users of a new drug after market approval may also be relevant to consider when investigating the effectiveness or safety of other new drugs with observational data.
5 Conclusion
Patient characteristics of new rivaroxaban users with nvAF changed between 2011 and 2016/17, but changes differed between countries. While there was a general trend towards a lower prevalence of comorbidities in both countries this pattern tended to be more pronounced in Germany. These patterns have methodological implications. They have to be considered in the interpretation of existing and planning of new observational studies comparing oral anticoagulants, especially regarding potential bias due to unmeasured confounding.
References
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62. https://doi.org/10.1016/S0140-6736(13)62343-0.
Friberg L, Oldgren J. Efficacy and safety of non-vitamin K antagonist oral anticoagulants compared with warfarin in patients with atrial fibrillation. Open Heart. 2017. https://doi.org/10.1136/openhrt-2017-000682.
Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, et al. Estimated effectiveness and safety of nonvitamin K antagonist oral anticoagulants compared with optimally acenocoumarol anticoagulated “real-world” in patients with atrial fibrillation. JACC. 2018;122(5):785–92. https://doi.org/10.1016/j.amjcard.2018.05.012.
Rodríguez-Bernal CL, Santa-Ana-Téllez Y, García-Sempere A, et al. Clinical outcomes of nonvitamin K oral anticoagulants and acenocoumarol for stroke prevention in contemporary practice: a population-based propensity-weighted cohort study. Br J Clin Pharmacol. 2021;87(2):632–43. https://doi.org/10.1111/bcp.14430.
Hohnloser SH, Basic E, Hohmann C, et al. Effectiveness and safety of non–vitamin K oral anticoagulants in comparison to phenprocoumon: data from 61,000 patients with atrial fibrillation. Thromb Haemost. 2018;118(03):526–38. https://doi.org/10.1160/TH17-10-0733.
Loo SY, Dell’Aniello S, Huiart L, et al. Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharmacol. 2017;83(9):2096–106. https://doi.org/10.1111/bcp.13299.
Paschke LM, Klimke K, Altiner A, et al. Comparing stroke prevention therapy of direct oral anticoagulants and vitamin K antagonists in patients with atrial fibrillation: a nationwide retrospective observational study. BMC med. 2020;18(1):1–13. https://doi.org/10.1186/s12916-020-01695-7.
Schwabe U, Paffrath D, Ludwig W-D, et al. Arzneiverordnungs-report 2019. Berlin: Springer; 2019.
Stichting Farmaceutische Kengetallen. 2017.
Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612.
García-Rodríguez LA, Wallander M-A, Friberg L, et al. Rationale and design of a European epidemiological post-authorization safety study (PASS) program: rivaroxaban use in routine clinical practice. Expert Opin Drug Saf. 2020;19(11):1513–20. https://doi.org/10.1080/14740338.2020.1798928.
Chao T-F, Liu C-J, Liao J-N, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133(16):1540–7. https://doi.org/10.1161/CIRCULATIONAHA.115.019794.
Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta-analysis. BMJ Open. 2014;4(5):e004587. https://doi.org/10.1136/bmjopen-2013-004587.
Camm AJ, Accetta G, Ambrosio G, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017;103(4):307–14. https://doi.org/10.1136/heartjnl-2016-309832.
Huiart L, Ferdynus C, Renoux C, et al. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open. 2018;8(3):e018180. https://doi.org/10.1136/bmjopen-2017-018180.
Vora P, Morgan Stewart H, Russell B, et al. Time trends and treatment pathways in prescribing individual oral anticoagulants in patients with nonvalvular atrial fibrillation: an observational study of more than three million patients from Europe and the United States. Int J Clin Pract. 2022. https://doi.org/10.1155/2022/6707985.
Ibáñez L, Sabaté M, Vidal X, et al. Incidence of direct oral anticoagulant use in patients with nonvalvular atrial fibrillation and characteristics of users in 6 European countries (2008–2015): a cross-national drug utilization study. Br J Clin Pharmacol. 2019;85(11):2524–39. https://doi.org/10.1111/bcp.14071.
Goodman SG, Wojdyla DM, Piccini JP, et al. Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). JACC. 2014;63(9):891–900. https://doi.org/10.1016/j.jacc.2013.11.013.
Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47. https://doi.org/10.1093/eurheartj/ehs253.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. https://doi.org/10.1093/eurheartj/ehw210.
Voss A, Ruigomez A, Smits E, et al. Time trends in patient characteristics of new rivaroxaban (RVX) users with atrial fibrillation (AF) across four countries. In: Pharmacoepidemiol drug saf. Hoboken: Wiley; 2021.
The Wise List 2015. 2015.
Krueger K, Jobski K, Voss A, et al. Different risk profiles of European patients using direct oral anticoagulants or vitamin K antagonists: a rapid review. Curr Epidemiol Rep. 2020. https://doi.org/10.1007/s40471-020-00257-y.
Fassmer A, Schink T. Repräsentativität von ambulanten Arzneiverordnungen in der German Pharmacoepidemiological Research Database (GePaRD). Jahrestagung der Deutschen Gesellschaft für Epidemiologie (DGEpi). 2014;17(20):2014.
Kuiper JG, Bakker M, Penning-van Beest FJ, et al. Existing data sources for clinical epidemiology: the PHARMO database network. Clin Epidemiol. 2020;12:415. https://doi.org/10.2147/CLEP.S247575.
Pharmacoepidemiology ISf. Guidelines for good pharmacoepidemiology practices (GPP). 1996 June 2015.
Acknowledgements
The BIPS team would like to thank all statutory health insurance providers which provided data for this study, namely AOK Bremen/Bremerhaven, DAK-Gesundheit, Die Techniker (TK), and hkk Krankenkasse. The authors would also like to thank Inga Schaffer and Philipp Alexander Volkmar for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
These studies were funded by Bayer AG, Berlin, Germany.
Conflict of interest
AV, TS and UH work for the Leibniz Institute for Prevention Research and Epidemiology—BIPS, an independent, non-profit research institute, which performs amongst others financially supported studies for government and related healthcare authorities and pharmaceutical companies; RH, LS and KS-P work for the PHARMO Institute for Drug Outcomes Research, an independent research institute which performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies; YB and KS-W are employees of Bayer AG; GB was an employee of Bayer AB at the time the study programme was conducted. This post-authorization safety study (PASS) was requested by the EMA, financed by Bayer AG and performed in line with the ENCePP Code of Conduct.
Ethics and approval
Approval of individual study protocols has been granted by the appropriate research ethics committees and regulatory authorities. All studies are registered on the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) electronic register and/or the ClinicalTrials.gov website. All of the studies were conducted in collaboration with ENCePP centres and in accordance with Good Pharmacoepidemiology Practices [26].
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
As we are not the owners of the data we are not legally entitled to grant access to the data of the German Pharmacoepidemiological Research Database. In accordance with German data protection regulations, access to the data is granted only to employees of the Leibniz Institute for Prevention Research and Epidemiology—BIPS on the BIPS premises and in the context of approved research projects. Third parties may only access the data in cooperation with BIPS and after signing an agreement for guest researchers at BIPS.
Author contributions
All authors contributed to the study conception and design or the data acquisition. The first draft of the manuscript was written by AV and UH. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Voss, A., Smits, E., Swart, K.M.A. et al. Time Trends in Patient Characteristics of New Rivaroxaban Users with Atrial Fibrillation in Germany and the Netherlands. Drugs - Real World Outcomes 10, 215–224 (2023). https://doi.org/10.1007/s40801-022-00350-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00350-2