Abstract
Background
The multikinase inhibitors (MKIs) sorafenib, lenvatinib, and vandetanib are approved for advanced thyroid cancer (TC) in Japan. How sequential treatment with MKIs is conducted in Japanese clinical practice is unknown.
Methods
This retrospective observational cohort study used a Japanese administrative claims database (April 2008–September 2021). Patients with a confirmed TC subtype diagnosis of papillary (PTC), follicular (FTC), medullary (MTC), or anaplastic (ATC), who received MKI treatment after TC diagnosis within the index period (June 2014–August 2021), were included. Overall MKI treatment duration was estimated by Kaplan–Meier analysis.
Results
The analysis population included 795 patients (PTC, N = 447; FTC, N = 86; MTC, N = 32; ATC, N = 230). Median age was ≥ 64 years; most patients (> 60%) were female except for the MTC subgroup (43.8%). First-line (1L) MKI treatment was mainly lenvatinib for PTC (81.7%), FTC (83.7%), and ATC (97.8%), and vandetanib for MTC (62.5%). Among patients discontinuing 1L MKI treatment and evaluable for subsequent therapy [PTC: 57.9% (259/447); FTC: 48.8% (42/86); MTC: 62.5% (20/32); ATC: 70.4% (162/230)], 26.3% (68/259), 21.4% (9/42), 50.0% (10/20), and 4.9% (8/162) of PTC, FTC, MTC, and ATC patients, respectively, received second-line (2L) treatment. Median (95% CI) overall MKI treatment duration was 21.2 (17.9–27.5), 43.9 (30.9–not assessable), 39.0 (17.7–not assessable), and 4.0 (3.0–4.8) months for PTC, FTC, MTC, and ATC, respectively.
Conclusion
Advanced TC treatment options are limited. In this study, most patients received only 1L MKI treatment; of those who discontinued 1L, ≤ 50% progressed to 2L.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The real-world treatment patterns with multikinase inhibitors (MKIs) approved in Japan for patients with advanced thyroid cancer were assessed. |
Lenvatinib was the main MKI prescribed for first-line treatment for patients with differentiated thyroid cancer or anaplastic thyroid cancer, and vandetanib was the main MKI prescribed for patients with medullary thyroid cancer. Many patients received only one line of MKI treatment. |
Treatment options for patients with advanced thyroid cancer are limited. Newer treatments with high efficacy and safety, potentially in combination with companion diagnostic tests and/or complemented by cancer genomic profiling, are needed to improve the outcome of these patients. |
1 Introduction
In 2018, 18,636 cases of thyroid cancer (TC) were reported in Japan with an incidence rate of 21.3 per 100,000 in women and 7.8 per 100,000 in men [1]. These data align with global figures in which the incidence rate of TC is threefold higher in women than in men [2]. The main histologic types of TC are differentiated thyroid cancer (DTC), medullary thyroid cancer (MTC), and anaplastic thyroid cancer (ATC) [3]. DTC accounts for > 90% of TC cases and can be further classified into papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and Hürthle cell carcinoma [4]. PTC is the most common type of TC and has the best overall prognosis. In Japan, cause-specific survival rates were 78.8% and 76.3% at 5 and 10 years in patients with M1 (distant metastasis at presentation) PTC, compared with 41.0% and 30.8%, respectively, in patients with M1 FTC [5, 6]. MTC accounts for approximately < 2% of TC cases [7]. The majority of MTCs are sporadic, with approximately 25% of cases having a familial predisposition, including mutations in the RET gene [8]. ATC is an undifferentiated TC, and although rare, it is the most aggressive form of TC with a median overall survival of 3–4 months [9].
Treatment for TC consists of surgery, radioactive iodine (RAI) treatment, radiotherapy, chemotherapy, and molecular targeted therapy [9, 10]. In Japan, three multikinase inhibitors (MKIs), sorafenib, lenvatinib, and vandetanib, are available for the treatment of TC. Sorafenib was approved for unresectable DTC based on the randomized, double-blind, phase 3 DECISION study [11, 12] and for MTC based on a phase 2 trial in Japanese patients [13]. Lenvatinib was approved [14] based on the results of the randomized, double-blind, phase 3 SELECT study in patients with RAI-refractory DTC [14, 15], and can be used for MTC and ATC in Japan based on the results of a phase 2 study [16]. Vandetanib was approved for the treatment of unresectable MTC based on the results of the randomized, double-blind, phase 3 ZETA trial and a phase 1/2 open-label study in Japan [17, 18].
The Japan Association of Endocrine Surgeons (JAES) clinical practice guidelines for the treatment of patients with TC in Japan provide guidance on the preferred MKI treatment for advanced TC. The current JAES guidelines recommend lenvatinib and sorafenib as the only available treatments for advanced/recurrent DTC, strongly recommend vandetanib and weakly recommend sorafenib and lenvatinib for advanced/recurrent MTC, and recommend lenvatinib for unresectable advanced/recurrent ATC [9]. However, the treatment pattern of how these MKIs are used nationwide in real-world clinical practice in Japan is poorly understood. The primary objective of this study was to describe the real-world treatment pattern with MKIs for patients with advanced TC in Japan using data from a nationwide administrative claims database. Factors associated with MKI treatment continuation were also explored.
2 Materials and Methods
2.1 Study Design
This was a retrospective observational cohort study analyzing data from an anonymized hospital-based claims database [Medical Data Vision Co., Ltd. (MDV)] in Japan. The MDV database comprises de-identified inpatient and outpatient administrative claims, discharge summaries, and Diagnosis Procedure Combination (DPC) data from approximately 26% of acute care hospitals in Japan, with the starting month of data capture varying from hospital to hospital [19]. As of 1 December 2021, the database covered approximately 38.2 million patients [20]. The data used in this study comprised the whole database for the period of 1 April 2008 to 30 September 2021. The study was conducted in accordance with the Declaration of Helsinki and was consistent with Good Pharmacoepidemiology Practices. Ethical review and informed consent were not required because all data in this study were de-identified and collected retrospectively.
2.2 Study Population
For this analysis, patients were eligible for inclusion in the study if they: (1) had a confirmed diagnosis of TC (International Classification of Diseases, Tenth Revision (ICD-10) code C73) in the database at any time during the study period (1 April 2008 to 30 September 2021); (2) had received a prescription of an MKI [lenvatinib (claims codes 622416101 and 622416001), sorafenib (claims code 620006778), or vandetanib (claims code 622441001)] in or after the first month of TC diagnosis, and the first prescription date (defined as the MKI index date) was within the MKI index period (1 June 2014 to 31 August 2021); and (3) had a confirmed diagnosis of PTC, FTC, MTC, or ATC based on the specific Japanese disease name code for each TC subtype (claims codes: PTC, 1930010; FTC, 1939010; MTC, 1939006; ATC, 1939009) in the 180 days before the MKI index date (inclusive) (Fig. 1). Patients who had a diagnosis of multiple TC subtypes were excluded, except for those who had a diagnosis of ATC. In these cases, the patient was included in the ATC subgroup because treatment for ATC would have been prioritized. Patients were also excluded if they had any MKI drugs prior to the MKI index date (not inclusive) or had records of hospital admission as part of a clinical trial after the MKI index date (inclusive). Patients were followed from their MKI index date to the end of available data (September 2021) for their antitumor treatment with guideline-recommended systemic antitumor drugs for advanced TC [an MKI or taxane (paclitaxel, nab-paclitaxel, or docetaxel)] [9], clinical outcomes (time to death and time to discontinuation), and supportive care (Fig. 1).
2.3 Variables/Measures
Hospital information, patient characteristics, and clinical information at baseline (defined as the period of 180 days before the MKI index date) were extracted from the database. Some clinical information, including weight, body mass index (BMI), and 10-item Barthel activities of daily living (ADL) index [21] at hospital admission, was available only from inpatient discharge summaries and therefore was limited to patients with hospitalizations within the baseline period. ADL was defined as “independent” if all 10 items were recorded as independent and “dependent” if any items were recorded as not independent. ADL was defined as “missing” for patients with any ADL items missing.
Treatment was defined as the drug that was prescribed on the first date of each line of therapy. Start of first-line (1L) MKI treatment was the MKI index date, and the start for each subsequent line was the date when the new MKI drug (or taxane) was prescribed. The end of each treatment line was when all drugs were terminated, or when a new MKI (or taxane) was started. The end date of each treatment line was defined as the date of the last prescription plus the number of days’ supply minus 1 day. Patients without additional hospital visits after the end date of the line were censored on that date because such patients could be on treatment at the end date of the line. The proportion of patients who received subsequent lines of therapy after 1L MKI treatment was reported only for the patients who discontinued 1L MKI treatment. Overall MKI treatment duration was defined as the time between the index date (first MKI dose) and the expected final dose of any MKI drugs in the database (date of the last prescription of any MKI drugs plus number of days’ supply minus 1 day). Patients without additional hospital visits after the expected final dose of any MKI drug were censored at that dose.
The initial MKI dose (mg/day) at the start of 1L was the intended dose prescribed on the first day of 1L MKI treatment. Mean dose per day within 1L MKI treatment was the averaged prescribed amount of the MKI per day in all periods. This was calculated by dividing the total amount of the MKI prescribed by the number of days of 1L MKI treatment (i.e., dates between the start and end of 1L). Dose reduction was defined as any reduction in 1-day dose (prescribed amount as the intended dose per day) in milligrams compared with the initial dose per day. The initial MKI dose was calculated by the MKI index year (index year ≤ 2017; index year ≥ 2018; i.e., before or after the inclusion of MKI treatment in the Japanese clinical practice guidelines for advanced TC [9]).
In the MDV database, death information is available only from hospitalization records (i.e., death during hospitalization) in the same treating hospital; therefore, time from the first MKI dose to death during hospitalization was evaluated, where a patient was censored at the last hospital visit if the patient did not have a record of death.
Records of supportive care and comprehensive genomic profiling were evaluated during the baseline period and during 1L MKI treatment. Variables included general patient management (emergent hospital admission), supportive drugs (antihypertensive drugs, drugs related to skin conditions, antidiarrheal drugs, painkillers, bone-modifying agents (BMAs), systemic hemostatic agents, and Kampo drugs), and comprehensive genomic profiling.
2.4 Statistical Analysis
All available and eligible patients in the MDV database were included in the study. The objectives of this study are descriptive and no statistical comparison between patient groups or treatment groups was planned; therefore, no statistical adjustments for bias or confounding factors were conducted. Mean, SD, median, and range are used for continuous variables. Frequency counts and percentages are used for categorical variables. Given the descriptive nature of the study objectives, there is no comparative delta to be achieved in hypothesis testing; 95% confidence intervals (CIs; alpha = 0.05) were determined by the sample size to enable interpretation of point estimates.
Overall MKI treatment duration and time to death during hospitalization were estimated by the Kaplan–Meier method. Multivariable Cox proportional hazards regression analysis was conducted to investigate factors associated with MKI treatment discontinuation in patients with DTC (PTC and FTC subgroups). Covariates were those with potential clinical relevance for treatment continuation and which were available in the MDV database: patient characteristics, clinical information, supportive care drugs, and Charlson comorbidities (excluding cancer) occurring in approximately ≥ 10% of patients (data not shown). Missing data were not imputed and are reported in the descriptive summary tables for their frequency count and percentage. Extraction of the cohort and creation of analytic variables were performed using the Instant Health Data platform (Panalgo, Boston, MA, USA), and statistical analyses were undertaken with R, Version 3.2.1 or higher (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Demographic and Baseline Clinical Characteristics
A total of 1717 patients with TC started 1L MKI treatment (June 2014 and August 2021) and met the criteria to be included in the study before TC subgrouping (Fig. 2). These patients were divided into TC subgroups according to the subtype disease codes in the baseline period (PTC, N = 447; FTC, N = 86; MTC, N = 32; ATC, N = 230) and were included in this analysis. Patients without any specific TC subtype code or with more than one subtype code (except for ATC) were excluded.
Median age at first MKI prescription was ≥ 64 years in all subgroups (Table 1). In each subgroup, most patients (> 60%) were female except for the MTC subgroup, in which there were more men than women. Almost all patients (> 88%) in each subgroup were prescribed MKI treatment in a designated cancer hospital. MKIs were prescribed mainly in the internal medicine department for patients in the PTC (40.5%), FTC (53.5%), and MTC (40.6%) subgroups, and in the otorhinolaryngology department for patients in the ATC (39.5%) subgroup. Approximately one-third of patients were prescribed MKI treatment within a surgical department. At baseline the most common metastasis site was the lung for the PTC and ATC subgroups, and bone for the FTC subgroup; in the MTC subgroup, lung metastasis, bone metastasis, and liver metastasis were reported in ten patients each (Table 1). The proportion of patients with a poorer ADL (i.e., dependent ADL) status was numerically greater in the FTC and ATC subgroups compared with the PTC and MTC subgroups (Table 1).
3.2 Treatment Patterns with Multikinase Inhibitors (MKIs) and Taxanes
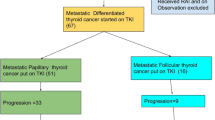
In the PTC, FTC, and ATC subgroups, > 80% of patients were prescribed lenvatinib as 1L MKI treatment (81.7% (365/447); 83.7% (72/86); 97.8% (225/230), respectively), and in the MTC subgroup, 62.5% (20/32) of patients were prescribed vandetanib (Fig. 3). Among the patients who were evaluable for subsequent therapy [PTC: 57.9% (259/447); FTC: 48.8% (42/86); MTC: 62.5% (20/32); ATC: 70.4% (162/230)], the transition rate to second-line (2L) was 26.3% (68/259) for PTC, 21.4% (9/42) for FTC, 50.0% (10/20) for MTC, and 4.9% (8/162) for ATC. Of those who did transition to 2L treatment, most received a subsequent MKI (i.e., lenvatinib, sorafenib, or vandetanib), except for patients with ATC where a taxane was prescribed more often (Fig. 3). Subsequent 3L treatment was received by 14 patients with PTC, one with FTC, three with MTC, and four with ATC. and comprised mainly an MKI (Fig. 3).
Treatment patterns with MKI and taxane. aThe number of patients who were evaluable for 2L treatment analyses were those who had finished or terminated 1L treatment. The end of 1L treatment was when the patient terminated all the drugs in the regimen or started a new MKI drug or taxane that was not included in the regimen, whichever occurred first: PTC, N = 259; FTC, N = 42; MTC, N = 20; ATC, N = 162. 1L/2L/3L first-/second-/third-line, ATC anaplastic thyroid cancer, FTC follicular thyroid cancer, LEN lenvatinib, MKI multikinase inhibitor, MTC medullary thyroid cancer, PTC papillary thyroid cancer, SOR sorafenib, TAX taxane, VAN vandetanib
3.3 Dose and Dose Reduction of MKIs in First-Line (1L) Treatment
The initial dose and the average dose of lenvatinib were numerically higher in the ATC subgroup compared with the other subgroups (Table 2). The initial sorafenib dose was numerically higher but the average dose was numerically lower in the ATC subgroup compared with the other subgroups (Table 2). More than 60% of the patients in each subgroup required a dose reduction of lenvatinib during 1L treatment, and approximately 40–60% of patients required a dose reduction of sorafenib (Table 2); 50.0% of patients in the MTC subgroup receiving vandetanib required a dose reduction during 1L treatment (Table 2).
We evaluated the initial dose of MKI at the start of 1L by the MKI index year (started 1L in 2017 or earlier; started 1L in 2018 or later). The mean initial dose of lenvatinib was numerically lower in the group with an index year of 2018 or later compared with an index year of 2017 or earlier (18.8 vs. 20.5 mg/day), both of which were lower than the approved dose of 24 mg/day (Table 3). Overall, of those who received lenvatinib in 2017 or earlier and in 2018 or later, 36.9% and 47.9%, respectively, received lenvatinib at an initial dose less than the approved 24 mg/day. Analysis of the initial dose of lenvatinib in 1L MKI treatment (< 24 mg/day; 24 mg/day) by baseline characteristics was performed. Patients who received a lower initial dose of lenvatinib were those who were older, those who had PTC, and those who had an ADL status of “dependent” at baseline (Table 4).
3.4 Overall MKI Treatment Duration and Time to Death During Hospitalization
Overall MKI treatment duration and time to death during hospitalization were both numerically shorter for patients in the ATC subgroup compared with patients in the FTC, PTC, and MTC subgroups (Fig. 4). Median (95% CI) treatment duration was 4.0 (3.0–4.8) months for the ATC subgroup compared with 21.2 (17.9–27.5), 43.9 [30.9–not assessable (NA)], and 39.0 (17.7–NA) months for the PTC, FTC, and MTC subgroups, respectively (Fig. 4a). Median (95% CI) time to death during hospitalization was 7.4 (6.1–10.9) months for the ATC subgroup compared with 65.3 (52.7–NA) and 67.5 (67.5–NA) months for the FTC and PTC subgroups, respectively; median time to death during hospitalization for the MTC group was not reached (Fig. 4b).
Factors significantly associated with MKI treatment discontinuation in patients with DTC (i.e., PTC and FTC subgroups only, N = 533) were age ≥ 75 years (hazard ratio (HR) 1.86; 95% CI 1.45–2.39), PTC at baseline (HR 1.76; 95% CI 1.16–2.66), and use of opioids at baseline (HR 2.69; 95% CI 1.71–4.24). Findings were similar for the subgroup analysis of patients whose ADL independence and BMI were available (N = 268) (Table 5). The factor significantly associated with continuation of treatment was total ADL independence (HR 0.50; 95% CI 0.32–0.76).
3.5 Supportive Care and Comprehensive Genomic Profiling
In general, the use of supportive care increased after the initiation of MKI treatment for all subgroups (Table 6). At baseline, the proportion of patients with an emergent hospital admission was numerically highest in the ATC subgroup (12.6%) compared with the other subgroups (FTC: 2.3%; PTC: 4.0%; MTC: 0.0%). The proportion of patients who received opioids at baseline was numerically higher in the ATC (16.5%) and FTC (15.1%) subgroups compared with the PTC (6.7%) and MTC (12.5%) subgroups, and BMAs were numerically more commonly prescribed to patients in the FTC (25.6%) subgroup than to those in the ATC (2.6%), PTC (4.0%), and MTC (9.4%) subgroups. A small proportion of patients in the ATC and PTC subgroups only received comprehensive genomic profiling at baseline and during 1L treatment after starting MKI treatment (Table 6).
4 Discussion
This is the first study in Japan to describe the real-world treatment patterns with MKIs approved in Japan (lenvatinib, sorafenib, and vandetanib) for patients with TC using a nationwide, large-scale administrative database. In this analysis of TC subtypes, lenvatinib was the main MKI prescribed for 1L treatment for patients with DTC (PTC and FTC) or ATC. The higher proportion of prescriptions for lenvatinib compared with sorafenib may be related to its greater apparent efficacy, as mentioned in the JAES guidelines [9], although direct comparisons between clinical trials cannot be made. Vandetanib was the main MKI prescribed for patients with MTC, consistent with the JAES guidelines, which strongly recommend vandetanib for MTC based on phase 3 efficacy data [9, 17]. For patients who discontinued 1L MKI treatment and were evaluable for subsequent therapy (MKI or taxane), the transition rate to 2L was low (4.9–26.3%) for ATC, FTC, and PTC, where only two MKIs are indicated (lenvatinib and sorafenib) and was higher (50%) for patients with MTC where three MKIs are available (lenvatinib, sorafenib, and vandetanib). For FTC, PTC, and MTC, the treatment pattern consisted of 1L MKI followed by a subsequent MKI treatment; for ATC, 1L MKI treatment was more often followed by a taxane. Subsequent 3L treatment, although rare, was in most cases the MKI received during 1L MKI treatment, except for patients with MTC who received an MKI that was not received during 1L or 2L. The results of this study indicate that the concept of “sequential treatment” has not yet been established for MKI treatment for patients with TC in Japan, and this may be due to the lack of multiple effective treatment options. Although sequential treatment is evident elsewhere [22], further investigation to establish its benefit to survival is warranted.
The approved starting dose of lenvatinib for the treatment of unresectable TC is 24 mg/day. Due to concerns of adverse events associated with the drug, some physicians will prescribe lenvatinib at a lower starting dose [23,24,25]. However, a phase 2 study compared lenvatinib at 18 mg/day with the approved starting dose of 24 mg/day in patients with RAI-refractory DTC. Safety was similar between the two dose levels, but lenvatinib at 18 mg/day did not demonstrate noninferiority compared with a starting dose of 24 mg/day [26]. In a post-marketing observational study of lenvatinib treatment in Japan, approximately 75% of patients with unresectable TC did receive the approved standard starting dose of 24 mg/day, but the mean dose throughout the study period was lower (range: 12.06–15.71 mg/day across the TC subgroups) [25]. In the current study, 44% of patients prescribed lenvatinib had a starting dose lower than 24 mg/day. The mean prescribed starting dose was lower than the approved starting dose (24 mg/day) for all TC subgroups (range: 17.3–20.8 mg/day), and dose reductions of lenvatinib during 1L treatment were required for approximately 60–70% of the patients. Mean dose per day averaged through 1L treatment was consistent with that observed in the Japanese post-marketing study of lenvatinib [25]. Interestingly, when analyzed by 1L MKI index year, numerically more patients were prescribed 1L lenvatinib treatment at a starting dose < 24 mg/day in 2018 and after, compared with those prescribed treatment in 2017 or earlier (47.9% vs. 36.9%, respectively); as reported by Brose et al. [26], a starting dose of 24 mg/day is important for the optimization of lenvatinib treatment. In this study, patients who were prescribed lenvatinib at a dose lower than the approved starting dose tended to be older and have a poorer ADL status (i.e., dependent) than those who were prescribed lenvatinib at a starting dose of 24 mg/day; this may have been due to safety concerns related to a higher dose in the older, more frail patients.
ATC is known to be a more aggressive TC tumor type with a poorer prognosis than PTC, FTC, and MTC [3], and this is what we observed in this study. Patients with ATC had a shorter duration of MKI treatment and time to death during hospitalization than patients with PTC, FTC, and MTC. The trends were similar to that observed in the Japanese post-marketing observational study of lenvatinib, in that patients with ATC had a shorter overall survival and shorter time-to-treatment failure than patients with DTC or MTC [25]. Furthermore, in this database study, patients with ATC tended to be elderly and required supportive care (e.g., emergent hospitalization, opioids). Evaluation of their lenvatinib dosing regimen indicated that their antitumor treatment was intense, which may have been due to the aggressive disease progression characteristic of ATC and thus the shorter post-dose reduction period. Overall, this study indicates that there is an unmet need for better treatment for these vulnerable patients.
In this analysis of TC subtypes, most patients (88–97%) were prescribed MKI treatment in a designated cancer hospital. In a post hoc analysis of all patients with a confirmed diagnosis of TC in the whole database (N = 89,953; Fig. 2), regardless of stage or treatment received, 78% of patients were treated in a designated cancer hospital, indicating that a high proportion of patients with TC in Japan, and in particular those receiving MKI treatment, are treated in a designated cancer hospital. Furthermore, approximately 30% of patients in the TC subtype analysis were prescribed MKI treatment in a surgical department. The involvement of the surgical department in prescribing MKI treatment appears to be unique to Japan, with studies in the USA indicating that the surgical department/surgeons were rarely involved with such prescriptions [22, 27]. Trends of age and sex for each TC subgroup were consistent, but the percentage of patients with metastasis was smaller in this study than in the post-marketing observational study for lenvatinib in Japan (e.g., lung metastases: 31% vs. 44% for MTC patients and 26% vs. 77% for ATC patients, respectively) [25]. This may be because this is a claims database study and treatment for metastases may not have been recorded as a specific claim for reimbursement; therefore, the metastases would not have been captured in this observational study. However, the trends for each TC subtype for common metastatic sites in this study were consistent with the literature [3] and the post-marketing observational study of lenvatinib in Japan [25]: PTC had a high percentage of lung metastases; FTC had mainly bone metastases; MTC had lung, bone, and liver metastases; and ATC had a high percentage of lung metastases. In this study, patients with FTC or ATC had a poorer ADL status than those with PTC or MTC, likely reflective of the presence of bone metastases in patients with FTC, which leads to pain and possibly a poorer ADL status, and the aggressive nature of ATC leading to a poor prognosis [3].
To improve the health-related quality of life for patients with advanced TC, supportive care is recommended, particularly for patients with ATC [9, 28]. In this study, patients with ATC had a numerically higher percentage of emergent hospitalization in baseline compared with the other TC subgroups, and opioid use was common especially in the ATC and FTC subgroups. BMAs were common for patients with FTC, likely associated with the frequent bone metastasis observed in this subgroup. Furthermore, old age, PTC (vs. FTC), use of opioids in baseline (implying existing pain symptoms), and dependent ADL status were negative factors for continuation of MKI treatment in DTC patients. As shown by Brose et al. [26], a lower dose of lenvatinib (18 mg/day) did not demonstrate noninferiority compared with a starting dose of 24 mg/day in objective response rate, and the lower dose showed a tendency for shorter progression-free survival (PFS). In this database study, the dose reduction rate for lenvatinib was higher, and the average dose of lenvatinib in 1L was lower for PTC than FTC, suggesting that patients with PTC were not managing well with lenvatinib, which may have resulted in earlier treatment discontinuation. Additionally, the PTC subgroup was older than the FTC subgroup, which may have contributed to the shorter treatment duration. Other contributing factors to the observed difference in treatment duration between the FTC and PTC subgroups may exist and require further investigation in future studies.
This study also elucidated that some patients received comprehensive genomic profiling during baseline and during 1L MKI treatment. We hypothesize that comprehensive genomic profiling was conducted on those patients who were seeking experimental treatment options targeting gene alterations before completing the currently available treatments [29]. In particular, patients with ATC have a poor prognosis and limited treatment options; therefore, they may be exploring clinical trials. Clinically relevant gene alterations for TC include the BRAFV600E mutation, RET mutations, RET/PTC rearrangements, and NTRK gene fusions [4]. The most common driver mutation is the BRAFV600E mutation, which occurs in approximately 45% of PTCs [30]. RET/PTC rearrangements are found in 10–20% of PTC and RET mutations in > 95% of hereditary and up to 50% of sporadic MTC [4]. NTRK gene fusions, although rare in adult TC, have been reported in predominantly PTC at a prevalence of 5.7% [31]. Molecular targeted therapies for TC that require molecular testing include the RET inhibitors selpercatinib [32] and pralsetinib [33], the BRAF/MEK inhibitor combination of dabrafenib plus trametinib [34], and the tyrosine receptor kinase inhibitors larotrectinib [35] and entrectinib [36] for NTRK fusion-positive tumors. At the time of this study, molecular targeted therapies for the treatment of TC that require companion diagnostics, except for larotrectinib and entrectinib, were not yet approved in Japan, and therefore companion diagnostics were not captured in this study; selpercatinib was approved in Japan in February 2022 and approved RET companion diagnostics are now available. Although the proportion of patients who received comprehensive genomic profiling was very low, it is expected to increase in the future with the availability of newly developed molecular targeting agents.
In Japan, unlike other countries, all medical costs are covered by the public insurance system and paid to hospitals as reimbursements (i.e., claims). The use of administrative claims in a nationwide database enabled the longitudinal collection of real-world data, especially the collection of the real-world pattern of MKI drug prescriptions and concomitant therapies. The use of the large-scale database also meant that a relatively large number of patients with TC treated with MKIs could be included. This study was further strengthened by the disease name nomenclature used in Japanese claims, enabling the examination of TC subtype within the ICD-10 code for thyroid cancer (C73), which is not possible with the standard ICD-10 coding nomenclature. Furthermore, the availability of ADL data, although limited as described below, which can be used to capture the general health status of the patients and analyze its association with prognosis, is a strength of the MDV database compared with other claims databases that do not include clinical information.
This database study was limited in that the MDV database only includes a portion of DPC hospitals, which can potentially introduce bias in the hospital characteristic observed. In addition, the database cannot track a patient across multiple hospitals, and therefore patients may be lost or counted multiple times if they received treatment at more than one hospital. Furthermore, some important clinical information (e.g., cancer stage, performance status) and common effectiveness endpoints (e.g., PFS, tumor response) were not available or were largely missing from the database. Other clinical information including ADL, BMI, and death records can only be obtained from hospitalization records. As a result, a death event can only be captured when it occurs during hospitalization in the same treating hospital. If patients are transferred to other facilities for terminal care or any other purposes, death events are not captured, and the patients are censored for the time-to-death analysis. Because of the lack of clinical information that is essential for treatment choice decisions in clinical practice within the database, results were not adjusted for patient background, and statistical comparison for treatment outcome between the treatment groups was not conducted. Furthermore, the database does not contain information on treatment lines, and so treatment lines were defined using a prespecified definition of treatment sequence. Despite the availability of disease name codes specific for the TC subtypes, as mentioned above, not all patients with TC (ICD-10 code C73) in the database have a record of these specific TC subtype codes in their claims. As a result, a large proportion of the potential study population was lost by TC subgrouping (Fig. 2). Lastly, Cox proportional hazards regression analysis was conducted to investigate factors associated with MKI treatment discontinuation in patients with DTC (PTC+FTC) only. This was because the overall MKI treatment duration for patients with ATC was too short to examine, and the MTC sample size was too small.
5 Conclusions
This study clarified the real-world MKI treatment patterns for patients with advanced TC in Japan, where many patients received only one line of MKI treatment. Treatment options for patients with advanced TC are still very limited, and newer treatments with high efficacy and safety, potentially in combination with companion diagnostic tests and/or complemented by cancer genomic profiling, are needed to improve the outcome of these patients.
References
Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare). Section 2.2. Affected. 1. National Cancer Registration. 2016–2018. https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#anchor2. Accessed 21 Jun 2022.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–95. https://doi.org/10.1016/s0140-6736(16)30172-6.
Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):569–80. https://doi.org/10.1038/nrendo.2011.142.
Ito Y, Masuoka H, Fukushima M, Inoue H, Kihara M, Tomoda C, et al. Prognosis and prognostic factors of patients with papillary carcinoma showing distant metastasis at surgery (M1 patients) in Japan. Endocr J. 2010;57(6):523–31. https://doi.org/10.1507/endocrj.k10e-019.
Sugino K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, et al. Prognosis and prognostic factors for distant metastases and tumor mortality in follicular thyroid carcinoma. Thyroid. 2011;21(7):751–7. https://doi.org/10.1089/thy.2010.0353.
Ezaki H, Ebihara S, Fujimoto Y, Iida F, Ito K, Kuma K, et al. Analysis of thyroid carcinoma based on material registered in Japan during 1977–1986 with special reference to predominance of papillary type. Cancer. 1992;70(4):808–14. https://doi.org/10.1002/1097-0142(19920815)70:4%3c808::aid-cncr2820700415%3e3.0.co;2-l.
Moo-Young TA, Traugott AL, Moley JF. Sporadic and familial medullary thyroid carcinoma: state of the art. Surg Clin North Am. 2009;89(5):1193–204. https://doi.org/10.1016/j.suc.2009.06.021.
Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020;67(7):669–717. https://doi.org/10.1507/endocrj.EJ20-0025.
Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1856–83. https://doi.org/10.1093/annonc/mdz400.
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–28. https://doi.org/10.1016/s0140-6736(14)60421-9.
Bayer receives approval for Nexavar® (sorafenib) in Japan for treatment of differentiated thyroid cancer [press release]. Bayer AG; 20 Jun 2014.
Ito Y, Onoda N, Ito KI, Sugitani I, Takahashi S, Yamaguchi I, et al. Sorafenib in Japanese patients with locally advanced or metastatic medullary thyroid carcinoma and anaplastic thyroid carcinoma. Thyroid. 2017;27(9):1142–8. https://doi.org/10.1089/thy.2016.0621.
Lenvatinib approved for certain thyroid cancers. Cancer Discov. 2015;5(4):338. https://doi.org/10.1158/2159-8290.Cd-nb2015-029.
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–30. https://doi.org/10.1056/NEJMoa1406470.
Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, et al. A phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019;15(7):717–26. https://doi.org/10.2217/fon-2018-0557.
Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–41. https://doi.org/10.1200/jco.2011.35.5040.
Uchino K, Komoda M, Tomomatsu J, Okamoto T, Horiuchi K, Tsuji A, et al. Safety and tolerability of vandetanib in Japanese patients with medullary thyroid cancer: a phase I/II open-label study. Endocr Pract. 2017;23(2):149–56. https://doi.org/10.4158/ep161259.Or.
Japanese Society for Pharmacoepidemiology. Survey of Japanese databases in Japan available for clinical/pharmacoepidemiology research. October 8, 2021. https://www.jspe.jp/mt-static/FileUpload/files/JSPE_DB_TF_E.pdf. Accessed 14 Jun 2022.
Medical Data Vision Co., Ltd. Introducing the Medical Database Service. December 2021.
Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988;10(2):64–7. https://doi.org/10.3109/09638288809164105.
Dacosta Byfield SA, Adejoro O, Copher R, Chatterjee D, Joshi PR, Worden FP. Real-world treatment patterns among patients initiating small molecule kinase inhibitor therapies for thyroid cancer in the United States. Adv Ther. 2019;36(4):896–915. https://doi.org/10.1007/s12325-019-0890-6.
Locati LD, Piovesan A, Durante C, Bregni M, Castagna MG, Zovato S, et al. Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur J Cancer. 2019;118:35–40. https://doi.org/10.1016/j.ejca.2019.05.031.
Kim SY, Kim SM, Chang H, Kim BW, Lee YS, Chang HS, et al. Safety of tyrosine kinase inhibitors in patients with differentiated thyroid cancer: real-world use of lenvatinib and sorafenib in Korea. Front Endocrinol (Lausanne). 2019;10:384. https://doi.org/10.3389/fendo.2019.00384.
Takahashi S, Tahara M, Ito K, Tori M, Kiyota N, Yoshida K, et al. Safety and effectiveness of lenvatinib in 594 patients with unresectable thyroid cancer in an all-case post-marketing observational study in Japan. Adv Ther. 2020;37(9):3850–62. https://doi.org/10.1007/s12325-020-01433-8.
Brose MS, Panaseykin Y, Konda B, de la Fouchardiere C, Hughes BGM, Gianoukakis AG, et al. A randomized study of lenvatinib 18 mg vs 24 mg in patients with radioiodine-refractory differentiated thyroid cancer. J Clin Endocrinol Metab. 2022;107(3):776–87. https://doi.org/10.1210/clinem/dgab731.
Parikh R, Hess LM, Esterberg E, Bhandari NR, Kaye JA. Diagnostic characteristics, treatment patterns, and clinical outcomes for patients with advanced/metastatic medullary thyroid cancer. Thyroid Res. 2022;15(1):2. https://doi.org/10.1186/s13044-021-00119-9.
Husson O, Haak HR, Buffart LM, Nieuwlaat WA, Oranje WA, Mols F, et al. Health-related quality of life and disease specific symptoms in long-term thyroid cancer survivors: a study from the population-based PROFILES registry. Acta Oncol. 2013;52(2):249–58. https://doi.org/10.3109/0284186x.2012.741326.
Bun S, Yonemori K, Sunadoi H, Nishigaki R, Noguchi E, Okusaka T, et al. Safety and evidence of off-label use of approved drugs at the National Cancer Center Hospital in Japan. JCO Oncol Pract. 2021;17(3):e416–25. https://doi.org/10.1200/op.20.00131.
Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–62. https://doi.org/10.1677/erc.1.0978.
Pekova B, Sykorova V, Mastnikova K, Vaclavikova E, Moravcova J, Vlcek P, et al. NTRK fusion genes in thyroid carcinomas: clinicopathological characteristics and their impacts on prognosis. Cancers (Basel). 2021;13(8):1932. https://doi.org/10.3390/cancers13081932.
Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825–35. https://doi.org/10.1056/NEJMoa2005651.
Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9(8):491–501. https://doi.org/10.1016/s2213-8587(21)00120-0.
Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7–13. https://doi.org/10.1200/jco.2017.73.6785.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9. https://doi.org/10.1056/NEJMoa1714448.
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–82. https://doi.org/10.1016/s1470-2045(19)30691-6.
Acknowledgements
Medical writing assistance was provided by Prudence Stanford, PhD, CMPP, and Rebecca Lew, PhD, CMPP, of ProScribe—Envision Pharma Group, and was funded by Eli Lilly Japan K.K. ProScribe’s services complied with international guidelines for Good Publication Practice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly Japan K.K., manufacturer/licensee of selpercatinib. Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Conflict of interest
CM and TO have no conflicts of interest to declare. KS received honorarium for lectures from Bayer, Eisai Co., Ltd. and Eli Lilly and Company. YT, KN, YO, and ZC are employees of Eli Lilly Japan K.K. and own stocks of Eli Lilly and Company.
Ethics approval
Ethical review and informed consent were not required because all data in this study were de-identified and collected retrospectively.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Code availability
Not applicable.
Author contributions
All authors were involved in the study design and participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. YT and ZC conducted the statistical analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Masaki, C., Sugino, K., Tanizawa, Y. et al. Multikinase Inhibitor Treatment Patterns for Advanced Thyroid Cancer in Japan: An Administrative Claims Database Study. Drugs - Real World Outcomes 10, 145–158 (2023). https://doi.org/10.1007/s40801-022-00346-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00346-y