Abstract
Background
Despite the dynamic treatment landscape for EGFR mutant-positive metastatic non-small cell lung cancer (EGFRm+ mNSCLC), most of the earlier studies have focused on US or Western populations.
Objective
The objective of this study was to explore real-world treatment patterns and outcomes of South Korean patients with EGFRm+ mNSCLC.
Methods
Retrospective chart review of adult patients with EGFRm+ mNSCLC who received systemic treatment between January-2019 and June-2019.
Results
A total of 162 patients were included from 21 hospitals, with a median follow-up of 15.6 months. Median age was 65.0 years, 22% had central nervous system metastasis, and 57% and 38% had exon 19 deletion and exon 21 L858R, respectively. Among 144 patients (89%) who received first-line EGFR-tyrosine kinase inhibitor, afatinib was most the common (44%), followed by gefitinib (28%) and erlotinib (13%). First-line chemotherapy was more common when an EGFR-mutation was detected after versus before first-line treatment initiation (31% vs 5%). Discontinuation of first-line treatment was mostly due to disease-progression (81%) and toxicity (7%). Among 58 (78%) patients who received second-line treatment, osimertinib was the most common (40%). Most (60%) patients reported ≥1 Grade ≥3 adverse event during first-line treatment. Following initiation of first-line treatment, physician visits and chest X-rays were the most frequent healthcare utilisation events. Rates of emergency-room visits and hospitalization were 12% and 16%, respectively, with a mean length-of-stay of 10.4 days. At 12 months, overall survival rate was 95%, and numerically worse for patients with exon 21 versus 19 mutations.
Conclusions
Characteristics and clinical outcomes of Korean patients with EGFRm+ mNSCLC in real-world practice were comparable to those observed in clinical trials. As osimertinib was not reimbursed for first-line treatment before study completion, further investigation is warranted to explore evolving treatment practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Among first-line EGFR-tyrosine kinase inhibitors, afatinib was the most common (44%), followed by gefitinib (28%) and erlotinib (13%). In the second-line treatment, osimertinib was most common (40%). |
Discontinuation of first-line treatment was mostly due to disease-progression (81%) and toxicity (7%). |
The clinical outcomes of Korean patients with EGFR-mutated metastatic non-small cell lung cancer were comparable with those observed in clinical trials. |
1 Introduction
Cancer is the leading cause of death in South Korea, accounting for one in four deaths. Lung cancer was the leading cause of cancer-related death in both sexes in 2018. In recent decades, survival rates of lung cancer have increased [1]. However, the financial burden of lung cancer treatment in South Korea is substantial, with reported expenditure in 2017 for 1 year after diagnosis reaching approximately 30% of national GDP per capita [2].
The treatment landscape for metastatic non-small-cell lung cancer (mNSCLC) has evolved dramatically with the advance of targeted therapies for patients with oncogenic driver mutations. Mutations in the EGFR gene represent an important driver in mNSCLC, occurring in approximately 40% of cases in Asian patients [3]. EGFR mutations occur at a higher rate in women than in men, in non-smokers than ever smokers, and in adenocarcinomas than other NSCLC histologies [3]. The Asia–Pacific geographic region has the highest (47%) reported EGFR-mutation frequency in patients with NSCLC [4]. Targeted agents against EGFR mutations have become the standard first-line (1L) therapy for EGFRm+ mNSCLC, including first-generation (erlotinib and gefitinib), second-generation (afatinib and dacomitinib) and third-generation (osimertinib) EGFR-tyrosine kinase inhibitors (EGFR-TKIs) [5,6,7].
Earlier studies have well described the real-world practice patterns for EGFRm+ mNSCLC, however most have focused on US or Western populations. Given the dynamic landscape of treatment options for EGFRm+ mNSCLC, exploration of recent real-world treatment patterns and outcomes in these patients would provide practical insight of the treatment strategies for patients with EGFRm+ mNSCLC, amidst the rapidly evolving therapeutic landscape. In this context, this study presents real-world data regarding the patterns of diagnosis, treatment and associated clinical outcomes of EGFRm+ mNSCLC in South Korea. These data may enhance the implementation of results from clinical trials to our daily practice.
2 Patients and Methods
2.1 Study Design
A retrospective, observational physician-based chart review was conducted to gauge the data regarding treatment patterns, clinical outcomes, and associated healthcare resource utilisation (HRU) for patients with EGFRm+ mNSCLC in South Korea. The study was implemented online using an electronic data abstraction form (eDAF). Data collection information is outlined in the supplemental material. Physicians, who treat patients with mNSCLC in advanced general hospitals in South Korea, were asked to report anonymised patient-level information on treatment and outcomes for 2–5 randomly selected patients. Physicians were asked to select eligible patients based on the inclusion and exclusion criteria, applying system-generated random months of birth to minimise selection bias. Data abstraction took place from August 11, 2020, to September 2, 2020, over a year after the cut-off date for the 1L treatment initiation period (January 2019–June 2019).
The primary objective was to describe the treatment patterns of patients with EGFRm+ mNSCLC. The secondary objectives were to describe for treated patients with EGFRm+ mNSCLC the demographic, clinical and disease characteristics, EGFR testing patterns, clinical outcomes including tumour response, progression-free survival (PFS) and overall survival (OS), selected relevant Grade ≥ 3 adverse events (AEs) and economic outcomes including HRU and costs. HRU included outpatient visits, hospitalisations, emergency room visits, palliative and supportive care treatments and procedures. Exploratory objectives were to explore associations between patient characteristics and treatment patterns, clinical outcomes and economic outcomes. Using the eDAF, physicians were asked to report on selected relevant Grade ≥ 3 AEs reported by patients from a list of common Grade ≥ 3 AEs of EGFR-TKIs and chemotherapy based on published evidence [8,9,10,11,12,13,14,15] and expert opinion.
Eligible physicians must have medical specialties in medical oncology or pulmonology, have at least three years of experience treating patients with EGFRm+ mNSCLC and treat at least three patients with EGFRm+ mNSCLC per month.
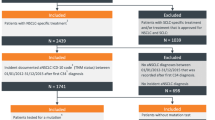
Eligible patients, aged ≥ 18 years, had initiated 1L systemic treatment of mNSCLC between January 2019 and June 2019, histologically confirmed metastatic (stage IV) NSCLC and documented EGFR activating mutations based on any molecular diagnostics prior to or following initiation of 1L systemic treatment of mNSCLC. Patients who participated in a clinical trial for NSCLC after diagnosis of EGFRm+ mNSCLC were excluded. The sample size for this single cohort study was based on precision of the primary and secondary outcomes. Using the proportional precision based on standard sampling methodology without finite population correction [16] it was estimated that a sample of 120–160 patients would provide reasonable precision for the outcomes of interest, e.g., proportion of patients using a specific systemic therapy, and HRU incidence rates.
Institutional Review Board (IRB) approval of the study protocol and questionnaire was obtained with waiver of informed consent (Advarra IRB) as this was a noninterventional study using deidentified, routinely collected data.
2.2 Statistical Analysis
Descriptive statistics were used to characterise the primary, secondary and exploratory objectives. These consist of frequencies and percentages for the categorical variables and means, standard deviations, medians and ranges for the continuous variables. Range details minimum to maximum values while interquartile range (IQR) details the first to third quartile.
PFS and OS from 1L treatment initiation date were assessed using Kaplan-Meier methods. 1L progression (PFS) was defined as discontinuation of 1L treatment due to disease progression, death, or commencement of the next line of therapy, whichever occurred earlier. Data for patients not known to have progressed, died, or started subsequent therapy were censored at their last contact date (e.g., last visit to the clinic). PFS from initiation of the 2L of treatment was defined similarly.
For sensitivity analysis, the percentage and the associated 95% confidence intervals (CIs) for each treatment were adjusted for clustering using hospital as the primary sampling unit. Rates per person-year and 95% CIs for HRU were evaluated using a negative binomial model, including a random effect for hospital to adjust for clustering, and an offset for patient observation time. Rates relate to all study patients, which include patients with no tests or office visits. All results presented are annualised rates and relate to the study observation period, from commencement date of 1L systemic anti-cancer therapy post-mNSCLC diagnosis until the last date of chart data available or death. Bootstrapping was used to estimate CIs for the mean annual health care unit costs.
Given that high-prescribing physicians may have greater influence on practice patterns and that our data suggest variation in treatment patterns by the prescribing volume of physicians, a sensitivity analysis was conducted to assess the impact of weighting each study patient by the total number of patients their physician treated each month; thereby giving physicians who treat a high number of patients more influence on the 1L monotherapy treatment patterns.
Statistical analyses were performed using SAS Software, Version 9.4 (SAS Institute, Cary, North Carolina).
3 Results
3.1 Physician and Patient Characteristics
A total of 39 physicians from 21 hospitals, consisting of 20 (51.3%) pulmonologists and 19 (48.7%) medical oncologists, provided data for 162 patients. Physicians had mean (standard deviation [SD]) experience of 12.5 (5.2) years of treating patients with EGFRm+ mNSCLC and treated a mean (SD) of 30 (20.7) patients with EGFRm+ mNSCLC per month. Physicians were based across regions of Korea including Seoul (n = 17 [43.6%]), Gyeonggi (n = 11 [28.2%]), Gyeongsang (n = 5 [12.8%], Incheon (n = 4 [10.3%]) and Chungcheong/Jeolla (n = 1 [2.6%]; Table S1).
The median (range) age of patients was 65.0 (38-90) years. The majority of patients were female (n = 100 [61.7%]). Most patients (n = 92 [56.8%]) were never smokers, 65 (40.1%) were ever smokers and 5 (3.1%) had unknown smoking status. Exon 19 deletions were detected in 92 (56.8%) patients and exon 21 L858R substitutions were detected in 61 (37.7%) patients. Exon 20 T790M substitutions were observed in 2 (1.2%) patients and 7 (4.3%) patients showed other genetic mutations. At the time of mNSCLC diagnosis, 35 (21.6%) patients had central nervous system (CNS) involvement (Table 1).
3.2 NSCLC Systemic Treatment Patterns
The median (IQR) time from NSCLC diagnosis to initiation of 1L treatment was 2 (1–3) weeks, and the median (IQR) duration of 1L treatment was not reached (35.0–NA) (Table 2). Most patient 1L treatments were ongoing (n = 88 [54.3%]) at the time of data abstraction. Median (IQR) study follow-up time for patients was 15.6 (13.7–17.3) months in line with the 1L treatment initiation and data abstraction dates (Tables 2 and 3).
Overall, 144 (88.9%) patients received an EGFR-TKI monotherapy as 1L systemic treatment, with afatinib (n = 71 [43.8%]) being the most commonly chosen drug. Gefitinib (n = 46 [28.4%]) and erlotinib (n = 21 [13.0%]) were the second and third most common 1L treatment options, respectively. Thirteen patients (8.0%) received chemotherapy (Table 2). EGFR testing was performed before 1L treatment initiation for 139 (85.8%) patients, and after 1L treatment initiation for 13 (8.0%) patients. Testing date is not recorded for 10 (6.2%) patients. Chemotherapy was more commonly chosen than EGFR-TKI monotherapy when an EGFR-mutation status was tested after 1L systemic treatment initiation versus before (30.8% vs 5.0%; Table S3). Among the 7 patients who received 1L chemotherapy after testing, 3 (43%) had exon 19 deletions, 3 (43%) had exon 21 L858R substitutions, and 1 (14%) had other mutations. Of the 4 patients who received 1L chemotherapy before testing, 2 (50%) had exon 19 deletions, 1 (25%) had exon 21 L858R substitutions, and 1 (25%) had other mutations. The above findings are derived without adjustment for the difference in volume of patients that different physicians tend to treat and gave equal weight to physicians regardless of the volume of patients treated in practice. However, based on the post-hoc sensitivity analysis with adjustment for the difference in physician volume, physicians in the high-prescribing group, in terms of the number of patients treated per month, tend to prescribe first-generation EGFR-TKIs more than physicians from other groups (n = 24 [53.3%] vs n = 20 [37.0%]) who tended to prescribe more second-generation EGFR-TKIs (Fig. S1). Compared with unweighted results, weighted results showed an increase in the use of first-generation TKIs (gefitinib and erlotinib) from 41.4 to 45.1%, respectively, and a decrease in the use of the second-generation TKI, afatinib, from 43.8 to 41.7%, respectively (Table S4).
Efficacy was the most important factor (n = 137 [84.6%]), as noted by physicians, in selecting 1L treatment, followed by tolerability (n = 20 [12.3%]). Just 3.1% (n = 5) physicians selected 1L treatment for financial reasons (Table 2). Among the patients who discontinued 1L treatment (n = 74 [45.7%]), the most frequent reason was disease progression (n = 60 [81.1%]; Table 2). Of those 74 patients, 58 (78.4%) had second-line (2L) therapy. EGFR-TKI was the most common 2L treatment, of which osimertinib monotherapy (n = 23 [39.7%]; Table 2) was most commonly prescribed. The treatment sequence for 2L therapy are detailed in Table S2.
3.3 Clinical Efficacy Outcomes
Most patients (n = 146 [90.1%]) were alive at the time of data abstraction. Also, less than half of patients (n = 70 [43.2%]), progressed during 1L treatment within the follow-up time (Fig. 1a and b). Therefore, median OS and PFS are not reached in the study, the median observation period was 15.8 months (IQR 13.9–17.6). Four (3.1%) patients developed CNS metastases post-commencement of 1L treatment (Table 3).
The PFS rates (95% CI) of patients who received 1L therapy at 12 and 18 months were 63.8% (55.8–70.8) and 53.1% (44.2–61.3), respectively. The OS rates (95% CI) at 12 months and 18 was 94.8% (89.9–97.4%) and 92% (84.8–95.8%), respectively (Table 3). OS at 12 and 18 months were numerically lower for patients with an exon 21 L858R vs exon 19 deletion (Table S5).
3.4 Adverse Events
A total of 98 (60.5%) patients reported at least one Grade ≥ 3 AE during 1L therapy. Of the AEs, the most common were thrombocytopenia (n = 35 [21.6%]), diarrhoea (n = 32 [19.8%]), and decreased appetite (n = 30 [18.5%]; Table 4). For the 58 patients receiving second-line treatment during the observation period, 31 (53.4%) reported at least one Grade ≥ 3 AEs. The most common AEs reported during second-line therapy were fatigue (n = 12 [20.7%]), decreased appetite (n = 11 [19.0%]) and diarrhoea (n = 7 [12.1%]).
3.5 Supportive Care and Healthcare Resource Utilisation
Approximately 70% of patients (n = 110 [67.9%]) received some level of palliative and supportive care; the most common types were pain medications (29.6%), antidiarrheal agents (21.6%) and antiemetic drugs (21.0%; Table S6).
Events with a relative higher rate of resource utilisation included physician (oncologist or pulmonologist) visits occurring at an average rate (95% CI) of 11.8 (9.3–15.0) per person-year. Chest radiographs and computerised tomography (CT) scans were also frequent at a rate of 12.9 (10.6–15.7) and 5.1 (4.7–5.4) per person-year, respectively. Following initiation of 1L treatment, 19 patients (11.7%) had a total of 33 emergency room visits due to EGFRm+ mNSCLC or related complications and 26 (16%) patients had at least one hospitalisation with a mean (SD) length-of-stay of 10.4 (7.51) days. Hospitalisations occurred at a rate of 0.2 (0.1, 0.4) events per person-year. The most common reason for hospitalisation was NSCLC treatment (n = 11 [26.2%]) followed by diarrhoea (n = 5 [11.9%]) and neoplastic pleural effusion (n = 5 [11.9%]) (Table 5).
4 Discussion
In this real-world chart review, we present an overview of current treatment patterns and HRU in patients with EGFRm+ NSCLC in South Korea. The findings presented here show that treatment patterns in real-world practice are substantially in line with the recent 2019 Pan Asian guidelines [17]. EGFR-tyrosine kinase inhibitors were the most commonly selected treatment of both 1L and 2L treatment and second-generation afatinib was the most frequently prescribed EGFR-TKI in the 1L setting, more commonly prescribed than first-generation gefitinib or erlotinib.
Baseline characteristics were comparable to previous Phase 3 clinical trials in patients with untreated EGFRm+ mNSCLC. These include the open-label, randomised, Phase 3 LUX-Lung 6 study, which compared second-generation EGFR-TKI afatinib with the gemcitabine plus cisplatin chemotherapy regimen in Asian patients [8] and the worldwide, double-blind, Phase 3 RELAY and FLAURA studies that compared erlotinib plus ramucirumab with erlotinib plus placebo and osimertinib with first-generation EGFR-TKIs (gefitinib or erlotinib), respectively [10, 11]. Median age was 65 years in this study, 58 years in LUX-Lung 6 and 64–65 years in RELAY and FLAURA. Here, 62% of patients were women compared with 62–68% in the treatment groups of LUX-Lung 6, RELAY and FLAURA. 57% never smoked compared with 60–65% in the Phase 3 trials and an EGFR exon 19 deletion was detected in 57% in this study compared with 50–63% in LUX-Lung 6, RELAY and FLAURA.
In this study, afatinib was the most frequently prescribed EGFR-TKI in the 1L setting. A similar observation was made in the real-world study by Lee et al. 2021 in a cohort of 235 patients from South Korea treated between January 2015 and December 2017 [18]. However, these findings contrast from real-world observations in the USA of 782 patients who initiated 1L treatment between January 2016 and July 2019, for whom erlotinib was the most common 1L treatment [19]. This may be explained in part by findings from Kim et al. 2019, examining 467 South Korean patients treated between 2014 and 2016, who showed a significantly better median PFS of patients treated with 1L afatinib than that of erlotinib or gefitinib (19.1 months vs. 14.0 and 13.7 months, respectively; p =0.001) [13]. In that study, PFS improvements in patients treated with afatinib were more pronounced in those with exon 19 deletion and uncommon mutations than exon 21 L858R mutations [13]. In addition, patients with EGFRm+ NSCLC treated with afatinib had improved clinical outcomes and had a more favourable safety profile than chemotherapy in the Phase 3 LUX-lung 3 and 6 studies [8, 20]. Afatinib improved outcomes in treatment-naïve patients compared with gefitinib in the Phase 2B LUX-Lung 7 trial [21]. However, a recent meta-analysis found that afatinib did not have a greater efficacy than gefitinib or erlotinib as a 1L therapy. This study included mostly East Asian studies consisting of 8 randomised trials and 82 cohort studies with a total of 17,621 patients. While afatinib appeared to be associated with longer PFS compared to the first-generation EGFR-TKIs, the benefit differed considerably between studies and more importantly no benefit in terms of OS was observed [22]. Post-hoc weighted sensitivity analysis conducted in this study found that physicians who treat more than 40 patients with EGFRm+ mNSCLC per month (high prescribers) tend to prescribe first-generation TKIs (gefitinib or erlotinib) more often than physicians who treat either 20–40 (medium-prescribers) or less than 20 (low-prescribers) patients with EGFRm+ NSCLC per month (Fig. S1). This post-hoc analysis suggests that treatment patterns observed in the study are sensitive to the volume of patients per physician, a finding which warrants further investigation.
Regulatory approval of osimertinib as a 1L treatment option in South Korea was received in December 2018; however, reimbursement status of osimertinib as a 1L therapy is not yet approved in Korea [23]. Therefore, only 3.7% of patients in the 1L setting received osimertinib in this study. Osimertinib is a third-generation EGFR-TKI that is currently recommended as a preferred 1L treatment option in patients with EGFRm+ mNSCLC in the National Comprehensive Cancer Network (NCCN) guidelines [24]. This recommendation is based on the clinically meaningful improvement in efficacy and tolerability observed with osimertinib treatment, in patients with previously untreated EGFRm+ advanced NSCLC, compared to first-generation EGFR-TKIs in the Phase 3 FLAURA trial [10]. Improved efficacy was also observed in a subset analysis of Asian patients in the FLAURA trial. Asian patients who received osimertinib treatment had a significantly longer mPFS of 16.5 months, an overall response rate (ORR) of 80%, a not calculable median CNS PFS and fewer Grade ≥ 3 AEs (40%) compared to the mPFS of 11.0 months, 75% ORR, 13.8 months median CNS PFS and 48% Grade ≥ 3 AEs reported for patients in the first-generation EGFR-TKI groups. However, final OS analysis showed the greatest numerical between-group differences in the hazard ratios (HRs) for OS were between Asian and non-Asian patients, with HR 1.00 (95% CI 0.75–1.32) in the Asian subset [25]. The FLAURA China trial, a double-blind, randomised, Phase 3 trial which assessed first-line osimertinib in Chinese patients with EGFRm+ advanced NSCLC, also reported that the HR for OS was 0.85 (95% CI 0.56–1.29; nominal p = 0.442) compared with EGFR-TKI group (gefitinib or erlotinib; all sites selected gefitinib) [26].
Synchronous and metachronous brain metastasis rates were numerically lower in this study than have been observed in other retrospective Korean studies of EGFRm+ NSCLC. In one such analysis of patients who received EGFR-TKIs, 35.4% of 559 patients had synchronous brain metastasis compared to 21.6% in this study [27]. In a study by Baek et al., 27.4% of 73 Korean patients with EGFRm+ NSCLC had synchronous CNS metastases. Of those with EGFRm+ NSCLC, 9.6% developed brain metastases, with a median time to brain metastases of 13.4 months, compared to 3.1% of the patients without initial CNS involvement in this study [28]. A large retrospective nationwide study of Korean patients with advanced NSCLC reported that the overall cumulative incidence of brain metastasis was significantly higher in patients with targeted therapy than in patients with chemotherapy. After failure of the 1L treatment, the incidence of brain metastasis increased [29]. The numerically lower observed rates of initial and developed brain metastasis in this study may be associated with the shorter median follow-up time of 15.6 (IQR 13.7–17.3) months.
The 1-year OS (95% CI) of 94.8% (89.9–97.4%) in our study is consistent with that reported in previous Phase 3 clinical trials. The 1-year OS (95% CI) in the RELAY study was 93% (89–96%) and 94% (90–96%) for the ramucirumab plus erlotinib group and placebo plus erlotinib groups, respectively, and in the FLAURA study, was 89% (85–92%) in the osimertinib group and 82% (77–86%) for those treated with first-generation EGFR-TKI. Moreover, in a subset analysis of East Asian patients in the RELAY study, 1-year OS was 94.4% (89.4–97.0%) and 95.2% (90.7–97.6%) for the ramucirumab plus erlotinib and placebo plus erlotinib arms, respectively [12].
This study was designed to allow sufficient follow-up time of at least 1 year after initiation (January 2019–June 2019) of 1L treatment, with 50% of included patients followed between 13.7 and 17.3 months. On one hand, this helped to capture relatively recent treatment patterns but on the other hand restricted the observation period for more recently introduced treatments to be included. As such, treatment patterns observed in this study may not fully reflect rapidly evolving clinical practice in the treatment of EGFRm+ mNSCLC. Examples of the changing mNSCLC treatment landscape include regulatory approval of osimertinib for 1L treatment in 2018, followed by regulatory approval of ramucirumab plus erlotinib as well as dacomitinib for 1L treatment in 2020. In November 2020, dacomitinib received reimbursement approval in South Korea with a lower price than the weighted average price of gefitinib, erlotinib, and afatinib, meaning that dacomitinib is now cheaper than afatinib which may change the EGFR-TKI market [23]. Moreover, lazertinib received regulatory approval for the second-line treatment of patients with EGFR T790M mutation positive mNSCLC in January 2021 and obtained reimbursement approval in the 2L setting with a lower price than osimertinib in July 2021 [30]. Future studies should investigate how these recent approvals and reimbursements will affect real-world treatment of EGFRm+ NSCLC in Korea.
The severity of EGFRm+ mNSCLC may be reflected in the level of HRU including physician visits and chest X-rays [31]. Consistent with this, levels of palliative and supportive care were also substantially high, especially for the management of pain. Although the mean length of hospital stay was somewhat longer for patients in our study compared with South Korean patients (n = 150) analysed in another real-world multinational study (10.4 days vs 7.1 days) the hospitalisation rate and the rate of emergency room visits during the follow-up duration were lower in our study (16% vs 55% and 11.7% vs 42.7%, respectively) [31]. However, direct comparisons between studies should be limited due to the differences between patient characteristics, treatment patterns, study timelines and the shorter abstraction and follow-up periods.
There were some additional limitations of this study which need to be highlighted. First, as physician agreement was required for patient participation this provides potential for selection bias. As such, treatment pattern information may not be representative of all physicians or the treatments for all patients with EGFRm+ NSCLC in South Korea. Data extracted for the study is based on patient charts, therefore only information captured there could be used for the analysis, some missing data is unavoidable. Second, the majority of patient data came from the Seoul, Gyeonggi and Incheon region reflective of the majority of cancer treatment being centred in this region in Korea. Third, as more than half of patients were on 1L treatment at the time of data abstraction due to study follow-up time, the percentage of patients receiving the 2L therapy may be underestimated. In addition, this likely impacted estimates of OS and PFS in the 1L, where progression was not observed in the majority of patients, and most were alive at the time of data extraction. Consequently, PFS for patients on 2L therapy were not fully captured, and the treatment sequencing from 1L to 2L should be interpreted cautiously. Lastly, the post-1L treatment initiation follow-up period overlapped with the COVID-19 pandemic and subsequently during a national strike for physicians. It is unclear if any treatments or HRU were provided or reported at a reduced rate than there would have been before the pandemic. However, the accessibility of hospitals and the severity of cancer diseases are thought to have limited any reduction in HRU due to COVID-19 in clinical practice in South Korea [32, 33].
5 Conclusions
Our study illustrates the landscape of real-world treatment patterns and resource utilisation amongst patients with EGFRm+ mNSCLC in Korea. Patient characteristics, clinical outcomes and HRU in real-world practice in Korean patients were comparable to those observed in clinical trials. These observations provide useful information for tailoring upcoming treatment strategy in patients with EGFRm+ mNSCLC and estimating their health economic impact. Further timely investigation is warranted integrating more recent treatment options for these patients.
References
Hong S, Won YJ, Lee JJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53(2):301–15.
Jeon SM, Kwon J-W, Choi SH, et al. Economic burden of lung cancer: a retrospective cohort study in South Korea, 2002–2015. PLoS ONE. 2019;14(2): e0212878.
Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–93.
Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892.
Planchard D, Popat S, Kerr K, et al. Correction to: “Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.” Ann Oncol. 2019;30(5):863–70.
Stock-Martineau S, Shepherd FA. EGFR tyrosine kinase inhibitor monotherapy should remain the standard first-line treatment in advanced EGFR-Mutant NSCLC. J Thorac Oncol. 2021;16(11):1793–7.
Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113–22.
Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22.
Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–66.
Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25.
Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655–69.
Nishio M, Seto T, Reck M, et al. Ramucirumab or placebo plus erlotinib in EGFR-mutated, metastatic non-small-cell lung cancer: East Asian subset of RELAY. Cancer Sci. 2020;111(12):4510–25.
Kim Y, Lee SH, Ahn JS, et al. Efficacy and safety of afatinib for EGFR-mutant non-small cell lung cancer, compared with gefitinib or erlotinib. Cancer Res Treat. 2019;51(2):502–9.
Cho BC, Kim DW, Park K, et al. Real-world use of osimertinib in non-small cell lung cancer: ASTRIS study Korean subgroup analysis. Curr Med Res Opin. 2020;36(3):477–82.
Choi MK, Ahn JS, Kim YC, et al. Afatinib in heavily pretreated advanced NSCLC patients who progressed following prior gefitinib or erlotinib: compassionate use program in Korea. Lung Cancer. 2018;119:36–41.
Scheaffer RL, Mendenhall W, Ott RL. Elementary survey sampling. 5th ed. Belmont: Wadsworth Publishing Company; 1996. p. 96–100.
Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS SSO and TOS. Ann Oncol. 2019;30(2):171–210.
Lee JC, Hung J-Y, Kim Y-C, et al. Real-world treatment patterns in patients with EGFR mutation-positive NSCLC receiving a first-line, first-or second-generation EGFR tyrosine kinase inhibitor in South Korea and Taiwan. Asian Pac J Cancer Biol. 2021;6(2):123–32.
Winfree KB, Sheffield KM, Cui ZL, et al. Study of patient characteristics, treatment patterns, EGFR testing patterns and outcomes in real-world patients with EGFRm(+) non-small cell lung cancer. Curr Med Res Opin. 2022;38(1):91–9.
Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3342–50.
Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–89.
Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805–19.
HIRA. Drug reimbursement evaluation committee (DREC) report of afatinib. Wonju. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030014040000&brdScnBltNo=4&brdBltNo=46872&pageIndex=2#none. Accessed on: 27 Jul 2021.
NCCN. Non-Small Cell Lung Cancer. National Comprehensive Cancer Network: National Clinical Practice Guidelines in Oncology 2021; Version 7 (2021): Available from: www.nccn.org.
Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
Cheng Y, He Y, Li W, et al. Osimertinib versus comparator EGFR TKI as first-line treatment for EGFR-mutated advanced NSCLC: FLAURA China. A randomized study. Target Oncol. 2021;16(2):165–76.
Jung HA, Woo SY, Lee SH, et al. The different central nervous system efficacy among gefitinib, erlotinib and afatinib in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer. Transl Lung Cancer Res. 2020;9(5):1749–58.
Baek MY, Ahn HK, Park KR, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med. 2018;33(1):168–75.
Lee JS, Hong JH, Sun S, et al. The impact of systemic treatment on brain metastasis in patients with non-small-cell lung cancer: a retrospective nationwide population-based cohort study. Sci Rep. 2019;9(1):18689.
Dhillon S. Lazertinib: first approval. Drugs. 2021;81(9):1107–13.
Lee DH, Isobe H, Wirtz H, et al. Health care resource use among patients with advanced non-small cell lung cancer: the PIvOTAL retrospective observational study. BMC Health Serv Res. 2018;18(1):147.
Kang Y-J, Oh SJ, Baek JM, et al. Impact of COVID-19 pandemic in 2020 on the diagnosis and management of breast cancer in Korea: a multi-institutional study. J Clin Oncol. 2021;39(15 suppl):10566–10566.
HIRA. Medical Expenditure Statistics. Wonju.2020. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020045030000&brdScnBltNo=4&brdBltNo=2413&pageIndex=1. Accessed on: 11 Nov 2021.
Acknowledgements
We thank Mark Steven Leusch from Eli Lilly and Company for medical support and Engels Chou from TechData Service for statistical support on this work. Dwayne Byrne, PhD, provided scientific communication expertise and project management support and Eglantine Julle-Daniere, PhD, provided scientific communication expertise, both are employees of Eli Lilly and Company.
National Comprehensive Cancer Network (NCCN) and NCCN are registered trademarks of NCCN.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
JL, SC and AS, employees of Labcorp Drug Development, conducted contract research for Eli Lilly and Company. JMC, MJK, YD and SK are employees of Eli Lilly and Company. CM and KLT are employees and shareholders of Eli Lilly and Company. CM manufactures ramucirumab indicated in combination with erlotinib for treatment of EGFRm+ NSCLC. HKA reports consulting fees and honoraria from Eisai Korea, Yuhan, Boryung, Everest medicine, Hanmi, Daiichi Sankyo, BMS, MSD, Eli Lilly and Company, Boehringer Ingelheim, Roche and Pfizer. JHP, MHH and HRK have nothing to disclose.
Author Contributions
CM, JMC, JL, YD, SK, SC, AS conceived and designed the analysis. JL, SC and AS collected the data. JL, YD, SK, SC and AS contributed data or analysis tools. JL and SC performed the analysis. CM, JC and YD interpreted the study data, wrote and provided intellectual contribution to the study manuscript. JL, MJK, SK, SC, SK, AS, JHP, HKA, MHH, KLT, HRK interpreted study data and provided intellectual contribution and critical revision of the study manuscript. All authors read and approved the final version.
Funding
Funding for this study was provided to Covance Inc. (now LabCorp Drug Development Inc.) by Eli Lilly and Company.
Ethical Approval and Consent
Institutional Review Board (IRB) approval of the study protocol and questionnaire was obtained with waiver of informed consent (Advarra IRB) as this was a noninterventional study using deidentified, routinely collected data.
Data Sharing Statement
Lilly provides access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Molife, C., Cho, J.M., Lapthorn, J. et al. Treatment Patterns, Clinical Outcomes and Health Care Resource Utilisation in Patients with EGFR-mutated Metastatic Non-Small Cell Lung Cancer: A Real-World Study in South Korea. Drugs - Real World Outcomes 10, 131–143 (2023). https://doi.org/10.1007/s40801-022-00344-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00344-0