Abstract
Background
Evidence on the efficacy of glycemic control for diabetic peripheral neuropathy (DPN) is limited in patients with type 2 diabetes mellitus. Despite the known relationship between hemoglobin A1c (HbA1c) and DPN, the parameters (e.g., mean values or variability) that play an important role have not been elucidated.
Objective
The objective of this study was to explore factors associated with DPN, including long-term HbA1c parameters, among patients with type 2 diabetes, in a large-scale longitudinal study.
Methods
We conducted a case-control study using a medical claims database. We extracted data of patients with type 2 diabetes and disease records of DPN (indicating that they received treatment for DPN) and those without DPN records (controls), and matched for age, sex, index year, and duration since the first type 2 diabetes record. A logistic regression analysis was performed to explore factors associated with DPN, and a receiver-operating characteristic analysis to estimate the optimal mean HbA1c target.
Results
Of 1,792,037 patients with type 2 diabetes, data from 1632 patients (816 per group) were analyzed. The mean HbA1c levels in the 3-year observation period were 7.2 ± 1.0% in the DPN group and 6.9 ± 1.1% in the control group. Elevated 3-year mean HbA1c levels were significantly associated with DPN records (adjusted odds ratio: 1.23, 95% confidence interval 1.06–1.42), while HbA1c variability was not significantly associated. The mean HbA1c levels that discriminated between patients with and without DPN records were 6.5% (unadjusted) and 7.1% (adjusted).
Conclusions
The development or progression of DPN in patients with type 2 diabetes was associated with the 3-year mean HbA1c level in real-world data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This longitudinal real-world data study explored various factors associated with diabetic peripheral neuropathy in patients with type 2 diabetes mellitus, using a large sample size of more than 800 patients per group. |
Higher 3-year mean hemoglobin A1c levels were significantly associated with diabetic peripheral neuropathy records (indicating the receipt of diabetic peripheral neuropathy treatment). |
A mean hemoglobin A1c level of approximately 6.5–7.0% was the optimal cut-off discriminating between with and without the development or progression of diabetic peripheral neuropathy in patients with type 2 diabetes. |
1 Introduction
Diabetic peripheral neuropathy (DPN) is a common microvascular complication of diabetes and is present in 27.6‒35.8% of diabetic patients in Japan [1]. Diabetic peripheral neuropathy is a contributing factor in the development of foot ulceration, which can result in foot amputation [2], and is associated with increased mortality in diabetic patients [3]. Although approximately half of the patients may be asymptomatic [1], some can present symptoms and signs of DPN, including allodynia, hyperalgesia, and pain described as “burning, electric, and stabbing sensations with or without numbness” [4], and patients with painful DPN experience an impaired quality of life and depressive state [5, 6]. Although the pathogenesis of DPN is complex, several factors have been reported to contribute to its pathogenesis by causing cellular stress/damage, including hyperglycemia, dyslipidemia, and impaired insulin signaling, ultimately leading to nerve dysfunction [4].
Glycemic control is considered an important strategy for the prevention of DPN; the current guidelines for the management of diabetes in Japan recommend strict glycemic control to suppress the onset or progression of DPN [7]. However, a previous systematic review reported that while intensive glycemic control significantly reduced the development of DPN in patients with type 1 diabetes mellitus (T1DM), the effect was not significant in patients with type 2 diabetes mellitus (T2DM) [8]. Rather than focusing only on achieving normal hemoglobin A1c (HbA1c) levels, different approaches, such as control of blood pressure and lipid levels or reduction of glucose fluctuations, may be required for the prevention of DPN in patients with T2DM [9].
A previous study suggested that glycemic variability was a significant risk factor for DPN in patients with T2DM, even among those with well-controlled HbA1c [10]. This may be partly explained by the fact that glucose fluctuations contribute more to DNA damage and oxidative stress, which would lead to endothelial cell damage, compared with sustained hyperglycemia [11, 12]. More recent studies have suggested that long-term glycemic variability was also associated with DPN in patients with T2DM [13, 14]. Long-term glycemic variability, or HbA1c variability, may be a better predictor of some macro-vascular and micro-vascular diabetic complications than mean HbA1c levels [15], although its relationship with the development of DPN has yet to be established. To date, the specific glycemic parameters such as mean HbA1c value, area under the curve (AUC) of mean HbA1c, and HbA1c variability, which play important roles in the development of DPN, have not been elucidated.
Given that the pathophysiological mechanism of DPN has not been fully elucidated, it is important to understand the characteristics of patients with DPN and identify the risk factors. Although a number of studies to date have explored the factors associated with an increased risk of DPN among patients with T2DM, these were mostly cross-sectional studies with small sample sizes [16]. Thus, there is lack of evidence that is based on high-quality longitudinal studies with sufficient sample size. Given the long-term hyperglycemic state in diabetes, it is indispensable to evaluate the impact of long-term glycemic exposure longitudinally rather than cross-sectionally. Therefore, to address this need, we explored various factors potentially associated with the disease records of DPN in patients with T2DM, with a particular focus on, but not limited to, long-term HbA1c parameters, in this longitudinal real-world study using a large-scale claims database.
2 Methods
2.1 Study Design and Data Source
This was a case-control study of DPN in patients with T2DM, using de-identified administrative and claims data from Japanese hospitals, compiled by Medical Data Vision Co., Ltd. (MDV, Tokyo, Japan). The MDV database is a large-scale hospital-based database, which contained data of more than 27 million patients in hospitals using the Diagnosis Procedure Combination/Per-Diem Payment System (DPC hospitals), as of March 2019. The database contains both inpatient and outpatient data, including patient demographics (age and sex), diagnosis, procedures, prescriptions, inpatient/outpatient status, and laboratory data (for a limited number of hospitals). This healthcare claims database has been used in many studies on T2DM in Japan [17]. The data of patients with T2DM included in this database from April 2008 (database launch) to March 2019 were analyzed in the present study.
The institutional review board’s ethical approval and patients’ informed consent were not obtained because the study used secondary data that were fully anonymized and collected from a commercial database provided by MDV. Thus, the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects do not apply to this study. Nonetheless, the Ethics Committee at Aichi Medical University was consulted, and the committee decided evaluation by the committee was unnecessary. This study was conducted in accordance with guidelines including Good Pharmacoepidemiology Practices issued by the International Society for Pharmacoepidemiology.
2.2 Study Population
Patients with T2DM were identified using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) codes of E11. Diabetic peripheral neuropathy was defined using the Japanese Disease Name Code 8845100 (type 2 diabetic peripheral neuropathy), 2505018 (diabetic peripheral neuropathy), or 8848768 (diabetic neuropathic pain).
Among patients with T2DM, those with a record of DPN and with HbA1c data at intervals of ≤6 months for at least 3 years before the first DPN diagnosis record were assigned as the DPN group in this study. Patients without a DPN record and with HbA1c data at intervals of ≤6 months for at least 3 years after the T2DM diagnosis were assigned as controls. Patients were excluded if they had (1) T1DM diagnosis (ICD-10 code: E10) prior to the T2DM diagnosis or first DPN diagnosis, whichever occurred first; (2) cancer diagnosis (ICD-10 codes: C00‒C97 and D00–D09) prior to the T2DM diagnosis or first DPN diagnosis, whichever occurred first; or (3) suspicious DPN identified by the uncertain disease flag.
Patients in the DPN group were matched to the patients in the control group for age, sex, calendar year of index date (defined below), and duration since the first T2DM record (i.e., duration from the earliest diagnosis record of T2DM in the patient’s record at the institution to index date), using the coarsened exact matching method [18]. In this method, each variable is first coarsened into several strata, and then an exact match is performed on the data in these strata (1:1 matching); after the data are matched, the original (uncoarsened) values for the variable are passed on for analysis.
Index date was defined as the date of the first DPN diagnosis record for the DPN group and the date of the latest HbA1c record for the control group (Fig. 1). Baseline was defined as the earliest timepoint (month) within the 3-year HbA1c observation period.
Definitions of data period. Index date was defined as the date of the first diabetic peripheral neuropathy (DPN) diagnosis record for the DPN group and the date of the latest hemoglobin A1c (HbA1c) record for the control group. Baseline was defined as the earliest timepoint (month) within the 3-year HbA1c observation period. T2DM type 2 diabetes mellitus
2.3 HbA1c and Other Variables
The HbA1c data during the 3-year observation period were extracted from the database. For glycemic exposure, in addition to the mean HbA1c level during the 3-year observation period, the AUC of mean HbA1c levels, measured at ≤6-month intervals over the 3-year observation period, was also assessed using Tai’s formula [19].
For the HbA1c variability, the following metrics were used: standard deviation (SD) of HbA1c, adjusted SD of HbA1c, and adjusted coefficient of variation (CV) of HbA1c. The CV was defined as the SD of HbA1c divided by the mean HbA1c. The adjusted SD (or CV) of HbA1c was calculated by multiplying the SD (or CV) by the correction coefficient (i.e., \(\sqrt{(n-1)/n}\), where n is the number of HbA1c measurements of each patient).
For other patient characteristics, data at index date (month) for age, sex, test for DPN (e.g., nerve conduction study), and duration since the first T2DM record, and the following data at baseline were extracted from the database: smoking experience, body mass index (BMI), diabetic medicines, diabetes guidance management, and comorbidities/complications. Laboratory data (i.e., blood glucose level, triglyceride level, and low-density lipoprotein and high-density lipoprotein cholesterol levels) within 3 months before and after baseline and hospitalization during the 3-year observation period were also assessed.
2.4 Statistical Analyses
To examine whether glycemic exposure (e.g., elevated HbA1c levels) and other patient characteristics differed between the DPN and control groups in this study, the demographic and clinical characteristics of patients were descriptively summarized for each group. To explore the factors associated with DPN, a univariate conditional logistic regression analysis was performed in the case-control pairs, using DPN diagnosis as the dependent variable and HbA1c metrics or other patient variables as independent variables. Multivariate logistic regression analysis was also conducted using DPN diagnosis as the dependent variable and various patient variables, including HbA1c, as independent variables. For each variable, the odds ratio (OR) for DPN and its corresponding 95% confidence interval (CI) were calculated.
Furthermore, to identify a target mean HbA1c goal for the prevention of DPN, the potential of the mean HbA1c level to discriminate patients with and without a DPN record was evaluated by plotting a receiver operating characteristic (ROC) curve and calculating the AUC. Then, the optimal mean HbA1c cut-off level for discriminating for DPN, which can be considered as the optimal target HbA1c goal, was calculated based on the Youden Index [20]. For the sensitivity analysis, the adjusted ROC curve was also plotted using the multivariate logistic regression model adjusted for the background characteristics of patients (i.e., diabetic medicines, guidance on diabetes, comorbidities/complications, and hospitalization). Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Background Characteristics of Patients
Of the 27,567,797 patient records in the MDV database, 1,792,037 patients with T2DM were identified. Of these, 79,584 patients had confirmed diagnostic records of DPN (indicating that they received treatment for DPN), whereas 1,712,453 patients did not. Of each, 1126 patients with a DPN record and 18,592 patients without a DPN record met the inclusion and exclusion criteria and were eligible for matching. Ultimately, we obtained data of 816 patients each in the DPN and control groups, matched for age, sex, index year, and duration since the first T2DM record (Fig. 2).
The mean age ± SD of the overall study population was 67.0 ± 11.6 years, and 61.8% were male. The mean duration since the first T2DM record was 4.1 ± 1.2 years. The mean HbA1c levels in the 3-year observation period were 7.2 ± 1.0% in the DPN group and 6.9 ± 1.1% in the control group; 48.0% and 62.5% of patients had a mean HbA1c level of <7.0% (Table 1). For HbA1c variability, all metrics were similar between groups.
The majority of the patients used diabetic medicines at baseline, but 25.1% of the DPN group and 37.0% of the control group did not use these medications. The types of medications used were basically similar between the two groups, but the proportion of patients receiving biguanides was higher in the DPN group (39.5% vs 22.9%). Insulin was prescribed to 29.2% of the patients in the DPN group and 22.7% of those in the control group. At baseline, the proportion of patients with cardiovascular diseases was marginally lower in the DPN group than in the control group: coronary heart disease (17.2% vs 24.5%) and angina (16.2% vs 22.5%), with the exception of peripheral arterial disease [PAD] (17.4% vs 15.9%). For microvascular complications, retinopathy was present in 13.5% of the patients in the DPN group and 11.4% of those in the control group, and nephropathy was present in 8.8% and 12.7%, respectively.
3.2 Factors Positively Associated with DPN Records
Among all patient characteristics in Table 1 except for smoking experience, the univariate analysis revealed that patients with elevated 3-year mean HbA1c level (OR: 1.36, 95% CI 1.22–1.51), 3-year mean HbA1c level of ≥7.0% [vs <7.0%] (OR: 1.38, 95% CI 1.24–1.54), higher AUC of mean HbA1c level (OR: 1.05, 95% CI 1.03–1.06), use of any diabetic medicines (OR: 1.75, 95% CI 1.41–2.17), use of glinides (OR: 1.55, 95% CI 1.05–2.28), use of biguanides (OR: 2.23, 95% CI 1.78–2.79), and use of insulin (OR: 1.40, 95% CI 1.12–1.74) were significantly more likely to have DPN records (Table S1 of the Electronic Supplementary Material [ESM]). However, in this analysis, none of the parameters used to measure the HbA1c variability was significantly associated with DPN records.
Among the HbA1c parameters, we chose to include the mean HbA1c level (continuous value) in the multivariate logistic regression model, in addition to all the remaining variables except for matching factors (age, sex, and duration since the first T2DM record), test for DPN, and BMI. Body mass index was excluded because data were available only for a limited number of hospitalized patients. The results of the multivariate analysis showed that patients with elevated 3-year mean HbA1c level (OR: 1.23, 95% CI 1.06–1.42), use of biguanides (OR: 1.67, 95% CI 1.25–2.22), and PAD (OR: 1.45, 95% CI 1.01–2.07) were significantly more likely to have DPN records than those without these factors (Table 2).
3.3 HbA1c Cut-Off Level for the Discrimination for DPN
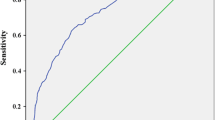
In the ROC curve in Fig. 3, the true-positive rate (sensitivity) and the false-positive rate (1 − specificity) of the mean HbA1c level to detect the presence of DPN records were plotted at each possible HbA1c cut-off level. The AUC was 0.602. The optimal HbA1c cut-off levels as indices for preventing DPN were 6.54% and 6.55%, respectively, with the former having a sensitivity and a specificity of 73.8% and 43.3%, and the latter having a sensitivity and a specificity of 73.4% and 43.6%, respectively.
After adjusting for the background characteristics of patients in our sensitivity analysis, the AUC was 0.680 (Fig. S1 of the ESM). In the adjusted ROC analysis, the optimal HbA1c cut-off level was 7.12%, with a sensitivity of 68.4% and a specificity of 60.4%.
4 Discussion
Targeting normal HbA1c levels has long been considered the main strategy for preventing the development of DPN in patients with diabetes. However, relevant recommendations depend largely on the evidence on the efficacy of intensive insulin therapy in patients with T1DM [21,22,23,24], and evidence for T2DM is relatively scarce [25, 26]. Moreover, the currently available data on factors associated with DPN in patients with T2DM are mostly based on cross-sectional studies conducted at a single center or with a small sample size. Thus, to obtain additional evidence from a higher quality study, these factors were explored in the present longitudinal study using a large sample size, with each study group comprising >800 patients. Among various background factors, the long-term elevated mean HbA1c level was an important factor in the present analysis, whereas HbA1c variability was not a significant factor.
The baseline characteristics of our overall study population were similar to those reported in previous cohort studies in patients with T2DM at hospitals or clinics specializing in diabetes in Japan, in terms of age, sex, BMI, and HbA1c levels [27, 28]. The proportion of patients with hypertension was also similar between this study and previous reports (62.3% vs 59.7–63.0%) [28, 29]. Our DPN group had a median duration since the first T2DM record at the DPC hospital of 3.8 years. Although it is the duration after matching, it seems short given the median duration of diabetes in patients with DPN reported in a recent Japanese study (15.9 years) [27]. However, the definition of this variable does not necessarily indicate the actual diabetes duration, and many patients in our study population were probably referred from clinics or non-diabetes-specialized hospitals; thus, the actual duration of T2DM was probably much longer.
As in any diabetes study, the accurate diabetes duration was unknown. However, the time from onset to diagnosis would not vary vastly among individuals in Japan because of mandatory regular health check-ups and high medical care access owing to the universal health insurance system. Of course, their first T2DM diagnostic records may not be their first diagnosis if they were patients referred from clinics; however, given that the DPN group did not have a DPN diagnosis within 3 years from the first T2DM diagnosis record, such hospital transfers would have occurred for reasons not directly related to DPN (i.e., the conditions were the same in both groups). Altogether, there would have been no large differences between the groups in the time from onset to the first T2DM diagnosis record at the DPC hospital. Therefore, matching by “duration since the first T2DM record” was considered valid, even if it may not be an accurate diabetes duration.
As described above, the characteristics of the overall T2DM population in this study were similar to those in previous studies [27,28,29]. However, the present study used claims data from DPC hospitals, and disease identification was performed based on disease records documented for reimbursement purposes. In Japan, most of the standard treatments, in principle, are covered by health insurance; thus, a disease code is recorded in the patient’s claims data when physicians administer treatments for the disease. Therefore, patients identified as having DPN in this study (n = 79,584) were practically those who received treatment for DPN, and not all patients with T2DM who had DPN. By contrast, the control group may have contained patients with DPN, but without the need for treatment. Our results were comparisons between these two groups of patients, rather than between patients with and without DPN. Our definition of the DPN group may seem strict, but it enabled us to assess factors associated with DPN requiring treatment. The examination of these factors is clinically important because, in clinical practice, it is crucial to prevent progression so that patients with T2DM do not have to undergo treatment for DPN. As for the data size, of 79,584 patients identified as having DPN (i.e., those who received DPN treatment), 816 were finally analyzed. This sample size was obtained by extracting the maximum data from the utilized database according to pre-specified criteria and methods, such as the availability of long-term HbA1c data and matching by baseline variables. Given the purpose of this study, longitudinal data acquisition was more important than increasing the sample size. In addition, matching by baseline factors was essential to minimize bias in observational studies using real-world data.
Between patients in the DPN and control groups, matched for age, sex, duration since the first T2DM record, and index year, no remarkable differences were observed in various baseline variables, including BMI, comorbidities/complications, and lipid profiles. A Japanese study previously reported a higher prevalence of retinopathy in patients with DPN than in those without DPN (43.1% vs 19.2%) [27], but such a trend was not observed in the present study, probably because of the above-mentioned definition of groups and differences in the timing of assessment (whether a cross-sectional study or not). In the present study, the only notable difference between the two groups was the level of glycemic exposure for 3 years; the proportion of patients with a mean HbA1c level of ≥7.0% was 14.5% higher in the DPN group (52.0% vs 37.5%). Patients subsequently requiring treatment for DPN might have needed more intensive glucose lowering at baseline, which probably explained the slightly greater use of insulin or biguanides at baseline among this group than among the control group.
In the multivariate analysis, we found that patients with an elevated 3-year mean HbA1c level, use of biguanides, and PAD at baseline were significantly more likely to have DPN records (indicating that they received treatment for DPN). The association between biguanides and DPN may be the result of peripheral neuropathy due to vitamin B12 deficiency induced by metformin treatment [30]. However, this result probably reflects the severity of the patients at baseline. Unlike in Europe and the USA where biguanides are the first-line therapy [31, 32], no first-line glucose-lowering agent is specified in the Japanese guidelines [7]; therefore, biguanides are less frequently used in primary care in Japan [33] because of their difficulty in use due to the risk of lactic acidosis [34]. By contrast, experienced specialists at DPC hospitals would more aggressively use biguanides at higher doses in patients whose glycemic control is difficult to achieve. Therefore, patients treated with biguanides at baseline were considered to have poor glycemic control. That is, the observed association in this analysis probably reflected the association between poor glycemic control and DPN. Contrastingly, alpha-glucosidase inhibitors were significantly negatively associated with DPN (OR: 0.65, 95% CI 0.44–0.98). Given that alpha-glucosidase inhibitors are relatively safe considering their mild adverse effects and that they are commonly used in primary care, patients who received this medication at baseline presumably had better glycemic control status. This result may indicate that patients with mild T2DM who do not require aggressive glycemic control have a lower chance of progressing to treatment-requiring DPN. Taken together, these results suggest the importance of adequate glycemic control in delaying the onset or worsening of DPN.
Given the significant association between DPN and PAD (including arteriosclerosis obliterans) [35, 36], it is possible that these two have frequently coexisted. However, the observed association between PAD and DPN in this study probably reflected the treatment for progressive diabetic conditions at baseline in the DPN group. For the treatment of diabetic ulcers or gangrene, vasodilators such as prostaglandin E1, lipo-prostaglandin E1, and prostaglandin E2 are used in clinical practice [37], but only some (e.g., alprostadil) are indicated as treatment for PAD-based or diabetic ulcers in order to be covered by insurance. Thus, there might have been cases where physicians made a disease record of PAD in order to prescribe these medications to patients with progressive neurological damage or blood flow disturbance due to diabetes at baseline. This may be one reason why patients with a disease record of PAD at baseline were more likely to have DPN records in this study.
Furthermore, although the association of HbA1c with DPN in patients with T2DM has not necessarily been unequivocal in the existing literature [38], the elevated 3-year mean HbA1c level was significantly associated in the present analysis. This finding was compatible with those of previous studies suggesting that HbA1c is an important modifiable risk factor for DPN in patients with T2DM [16, 39, 40]. In addition, this finding supports the current preventive approaches focusing on strict glycemic control, with additional emphasis on the importance of long-term adequate glycemic control.
In this study, however, the HbA1c variability over the 3-year observation period, which was assessed using various metrics, namely, the SD, adjusted SD, and adjusted CV of HbA1c, was not significantly associated with DPN in patients with T2DM. This result was in contrast to the results of recent studies showing the association between long-term glycemic variability and DPN in patients with T2DM in Taiwan and China, although causality was not determined [13, 14]. However, the observation period for glycemic exposure and the measures of glycemic variability in these previous studies differed from those in our study. In this study, HbA1c data at intervals of ≤6 months for ≥3 years were used to evaluate the long-term glycemic variability, but a possible long-term interval, such as 6 months, may have been inappropriate to adequately capture the HbA1c variability. In addition, the differences in the definition of DPN among studies might also be responsible for this discrepancy. Although the accumulated evidence suggests that long-term glycemic variability may be a predictor of renal diseases and retinopathy [15, 41,42,43], there is still a paucity of relevant data on DPN in patients with T2DM. Hence, further research is warranted to determine its relationship with the development of DPN in patients with T2DM.
Based on our findings that the mean HbA1c level may be an important factor associated with DPN in patients with T2DM, we further explored the mean HbA1c cut-off level that can best discriminate for the presence of DPN records in order to identify a target HbA1c goal for its prevention. Consequently, we obtained the optimal mean HbA1c cut-off level of 6.5%, which was in accordance with the target HbA1c level previously proposed for the prevention of microangiopathy development or progression [25]. The HbA1c level of 6.5% also coincided with the threshold above which the risk of microvascular complications (nephropathy and retinopathy) is considered to increase [44]. An AUC of 0.602 indicated that the ability of this mean HbA1c to discriminate for the presence of DPN records was not sufficiently high; however, its performance slightly increased after adjusting for various patient characteristics (AUC of 0.680), with an optimal mean HbA1c cut-off level of 7.12%. Taken together, although the mean HbA1c may not be the best predictor, at least at this moment where the pathophysiological mechanism of DPN has yet to be fully elucidated, glycemic control with a target mean HbA1c level of around 6.5–7.0% would be the best strategy for the prevention of DPN progression in patients with T2DM. This target level is in line with the recommended glycemic target for the prevention of diabetic complications in the current guidelines in Japan [7]. However, this recommended glycemic target is based on evidence of retinopathy and nephropathy, not DPN [25]. The guidelines even state that the effectiveness of strict glycemic control for the suppression of DPN is apparent in T1DM but has not been thoroughly tested in T2DM. Therefore, our findings would serve as complementary data.
With some reports that patients often predominantly have small-fiber neuropathy in prediabetes [45], DPN during the prediabetic stage has been an important topic in this field. Unfortunately, the present study did not consider DPN at this stage because our focus was on patients already diagnosed with T2DM. In addition, this topic could not be investigated with this database because data on patients with prediabetes were unavailable as there is no insurance-covered treatment indicated for prediabetes. However, it is clinically important to be cautious regarding DPN in this stage. Therefore, DPN in prediabetes, including its relationship with HbA1c values and other factors such as smoking, alcohol abuse, and metabolic syndrome during this stage, should be investigated in future studies.
The present study has several limitations. First, the generalizability of the results may be limited because this study only included patients with longitudinal data, that is, patients who regularly visited hospitals for diabetes treatment. For example, patients with regular outpatient visits to DPC hospitals may have different characteristics than those treated at clinics. Second, because the database was hospital based and not linked to a patient’s medical chart, a complete medical/treatment history could not be obtained. We may not have captured all comorbidities/complications because they are input in claims data only when some medical care is provided for them during the observation period. This characteristic of claims data probably explains the low prevalence of some complications such as the diabetic foot. In this study, no patient had a record of diabetic foot, which may be because most of the cases with diabetic foot were not treated as diabetic foot on claims data, and hence, the disease was not recorded. Although we might have missed some true cases, the impact of such misclassification on our results was not considered significant because medical conditions demanding treatment were captured in claims data, and therefore considered in our analysis. Third, in this database, data on smoking status and BMI were available for only a limited number of hospitalized patients, which made it impractical to evaluate the association of these factors with DPN in this study. As these may be modifiable risk factors, further research is needed to evaluate the relationship between these factors and DPN. Despite these limitations, the present study, which had a longitudinal study design and a large sample size, corroborated and confirmed the findings of previous cross-sectional studies, and provided important evidence on the factors associated with DPN in patients with T2DM.
5 Conclusions
The results of this real-world study demonstrated that the development or progression of DPN in patients with T2DM was associated with the 3-year mean HbA1c level, suggesting the important role of long-term mean HbA1c levels, among the various HbA1c parameters, in DPN in patients with T2DM. Although further research is needed to confirm our findings, targeting HbA1c levels of 6.5–7.0% may be beneficial for patients with T2DM to prevent the development or progression of DPN.
References
Satoh J, Baba M, Yagihashi S, et al. Frequency of diabetic polyneuropathy (DPN) and clinical significance of Achilles tendon reflex in diagnosis of DPN–Survey of 15,000 patients in Tohoku, Japan (in Japanese). J Japan Diabet Soc. 2007;50:799–806.
Amin N, Doupis J. Diabetic foot disease: from the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World J Diabetes. 2016;7:153–64.
Coppini DV, Bowtell PA, Weng C, Young PJ, Sönksen PH. Showing neuropathy is related to increased mortality in diabetic patients: a survival analysis using an accelerated failure time model. J Clin Epidemiol. 2000;53:519–23.
Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–34.
Tsuji M, Yasuda T, Kaneto H, et al. Painful diabetic neuropathy in Japanese diabetic patients is common but underrecognized. Pain Res Treat. 2013;2013: 318352. https://doi.org/10.1155/2013/318352.
Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91:733–7.
Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020;11:1020–76.
Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. https://doi.org/10.1002/14651858.CD007543.pub2.
Callaghan BC, Hur J, Feldman EL. Diabetic neuropathy: one disease or two? Curr Opin Neurol. 2012;25:536–41.
Xu F, Zhao LH, Su JB, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6:139. https://doi.org/10.1186/1758-5996-6-139.
Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–7.
Schisano B, Tripathi G, McGee K, McTernan PG, Ceriello A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetologia. 2011;54:1219–26.
Pai YW, Lin CH, Lin SY, Lee IT, Chang MH. Reconfirmation of newly discovered risk factors of diabetic peripheral neuropathy in patients with type 2 diabetes: a case-control study. PLoS ONE. 2019;14: e0220175. https://doi.org/10.1371/journal.pone.0220175.
Su JB, Zhao LH, Zhang XL, et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol. 2018;17:47. https://doi.org/10.1186/s12933-018-0693-0.
Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38:2354–69.
Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS ONE. 2019;14: e0212574. https://doi.org/10.1371/journal.pone.0212574.
Medical Data Vision Co., Ltd. Publication: academic article. https://en.mdv.co.jp/publication. Accessed 8 Jan 2021.
Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal. 2012;20:1–24.
Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–4.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5.
Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122:561–8.
Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–6.
Reichard P, Berglund B, Britz A, Cars I, Nilsson BY, Rosenqvist U. Intensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 years. J Intern Med. 1991;230:101–8.
Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17.
Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–30.
Yokoyama H, Tsuji T, Hayashi S, Kabata D, Shintani A. Factors associated with diabetic polyneuropathy-related sensory symptoms and signs in patients with polyneuropathy: a cross-sectional Japanese study (JDDM 52) using a non-linear model. J Diabetes Investig. 2020;11:450–7.
Kuroda N, Kusunoki Y, Osugi K, et al. Relationships between time in range, glycemic variability including hypoglycemia and types of diabetes therapy in Japanese patients with type 2 diabetes mellitus: Hyogo Diabetes Hypoglycemia Cognition Complications Study. J Diabetes Investig. 2021;12:244–53.
Yokoyama H, Oishi M, Takamura H, et al. Large-scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40). BMJ Open Diabetes Res Care. 2016;4: e000294. https://doi.org/10.1136/bmjdrc-2016-000294.
Roy RP, Ghosh K, Ghosh M, et al. Study of vitamin B12 deficiency and peripheral neuropathy in metformin-treated early type 2 diabetes mellitus. Indian J Endocrinol Metab. 2016;20:631–7.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;2018(61):2461–98 (Erratum in: Diabetologia. 2019;62:873).
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl. 1):S111–24.
Fukuda M, Doi K, Sugawara M, Naka Y, Mochizuki K. Survey of hypoglycemia in elderly people with type 2 diabetes mellitus in Japan. J Clin Med Res. 2015;7:967–78.
Metformin hydrochloride [package insert]. Tokyo: Daiichi Sankyo Espha Co., Ltd.; 2019.
Zander E, Heinke P, Reindel J, et al. Peripheral arterial disease in diabetes mellitus type 1 and type 2: are there different risk factors? Vasa. 2002;31:249–54.
Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31:464–9.
Japanese Dermatological Association. Wound, pressure ulcer and burn guidelines: 3. Guidelines for diabetic ulcer/gangrene (in Japanese). Japan J Dermatol. 2017;127:1989–2031.
Pan Q, Li Q, Deng W, et al. Prevalence of and risk factors for peripheral neuropathy in Chinese patients with diabetes: a multicenter cross-sectional study. Front Endocrinol (Lausanne). 2018. https://doi.org/10.3389/fendo.2018.00617.
Nisar MU, Asad A, Waqas A, et al. Association of diabetic neuropathy with duration of type 2 diabetes and glycemic control. Cureus. 2015;7: e302. https://doi.org/10.7759/cureus.302.
Hoque S, Muttalib M, Islam MI, Happy TA. Evaluation of different HbA1c levels to assess the risk of peripheral neuropathy among type 2 diabetic patients along with other conventional risk factors. Bangladesh Med Res Council Bull. 2016;42:95–103.
Takao T, Suka M, Yanagisawa H, Matsuyama Y, Iwamoto Y. Predictive ability of visit-to-visit variability in HbA1c and systolic blood pressure for the development of microalbuminuria and retinopathy in people with type 2 diabetes. Diabetes Res Clin Pract. 2017;128:15–23.
Lee CL, Chen CH, Wu MJ, Tsai SF. The variability of glycated hemoglobin is associated with renal function decline in patients with type 2 diabetes. Ther Adv Chronic Dis. 2020. https://doi.org/10.1177/2040622319898370.
Hu J, Hsu H, Yuan X, et al. HbA1c variability as an independent predictor of diabetes retinopathy in patients with type 2 diabetes. J Endocrinol Invest. 2020;44:1229–36.
Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55:636–43.
Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:42.
Acknowledgements
Statistical support and medical writing support were provided by Clinical Study Support, Inc. (Nagoya, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Viatris Pharmaceuticals Japan, Inc.
Conflicts of interest/competing interests
KN is an employee of Viatris Pharmaceuticals Japan, Inc. MI and SK declare that they have no competing interests.
Ethics approval
This observational study solely used secondary data, which were processed to be fully anonymized and contained no personal information. An observational study exclusively using anonymized data is outside the scope of the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Japanese Government. Therefore, ethical committee approval and individual informed consent were not required for this study in accordance with consultation of the ethics committee at Aichi Medical University.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The dataset analyzed in this study are not publicly available because they were obtained from a commercial company, Medical Data Vision Co., Ltd., and used under license. However, data may be available from the corresponding author on reasonable request and with permission from Medical Data Vision Co., Ltd.
Code availability
Not applicable.
Author contributions
All authors made substantial contributions to (1) the conception and design of the study, (2) analysis or interpretation of data, and (3) drafting or revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript to be submitted.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Table S1.
Univariate conditional logistic regression analysis of factors positively associated with DPN records. Supplementary Figure S1. Adjusted ROC curve for mean HbA1c as an indicator of DPN (PDF 215 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nozawa, K., Ikeda, M. & Kikuchi, S. Association Between HbA1c Levels and Diabetic Peripheral Neuropathy: A Case–Control Study of Patients with Type 2 Diabetes Using Claims Data. Drugs - Real World Outcomes 9, 403–414 (2022). https://doi.org/10.1007/s40801-022-00309-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00309-3