Abstract
Background
Elderly patients are at high risk of unintentional medication discrepancies during transition of care as they are more likely to have multiple comorbidities and chronic diseases that require multiple medications.
Objective
The aim of the study was to assess the frequency of unintentional medication discrepancies and identify the associated risk factors and potential clinical impact of them in elderly inpatients during hospital admission.
Patients and Methods
A prospective observational study was conducted from July to December 2018 in an 800-bed geriatric hospital in Hanoi, North Vietnam. Patients over 60 years of age, admitted to one of selected internal medicine wards, taking at least one chronic medication before admission, and staying at least 48 h were eligible for enrollment. Medication discrepancies of chronic medications before and after admission of each participant were identified by a pharmacist using a step-by-step protocol for the medication reconciliation process. The identified discrepancies were then classified as intentional or unintentional by an assessment group comprising a pharmacist and a physician. A logistic regression model was used to identify risk factors of medication discrepancies.
Results
Among 192 enrolled patients, 328 medication discrepancies were identified, with 87 (26.5%) identified as unintentional. Nearly a third of enrolled patients (32.3%) had at least one unintentional medication discrepancy. The most common unintentional medication discrepancy was omission of drugs (75.9% of 87 medication discrepancies). The logistic regression analysis revealed a positive association between the number of discrepancies at admission and the type of treatment wards.
Conclusions
Medication discrepancies are common at admission among Vietnamese elderly inpatients. This study highlights the importance of obtaining a comprehensive medication history at hospital admission and supports implementing a medication reconciliation program to reduce the negative impact of medication discrepancy, especially for the elderly population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first study that assessed the frequency of unintentional medication discrepancies and the associated risk factors in elderly patients at hospital admission in Vietnam. |
Unintentional medication discrepancy was common in elderly inpatients at admission and persisted throughout the patients’ hospital stay until discharge. |
The study highlights the importance of implementing standard operating procedures to attain a complete preadmission medication history for patients as well as implementing a medication reconciliation program in Vietnam. |
1 Introduction
Medication discrepancies are defined as inconsistencies between two or more medication lists of patients and can occur during the transition between healthcare facilities, including on admission, transfer, and discharge [1]. The discrepancies (e.g., medication omission, addition of a new medication, change in medication dose, or change in the route of administration) can be either intentional or unintentional, but not documented in any of the patients’ medical records [1]. These discrepancies, especially those that are unintentional, can often lead to preventable medication errors and potentially be harmful to patients [2, 3]. In practice, medication errors due to unintended discrepancies have been reported to occur in up to 50–70% of patients during transitions in care [3].
The majority of these medication discrepancies can be intercepted through medication reconciliation at all transitions in care [1]. Many organizations have demonstrated that implementing medication reconciliation at every interface of care is an effective and necessary strategy for identifying medication discrepancies and thus ensuring patient safety [1, 4, 5]. According to the World Health Organization (WHO), “medication reconciliation is the formal process in which health care professionals partner with patients to ensure accurate and complete medication information transfer at interfaces of care” [1]. The Institute for Healthcare Improvement (IHI) defines medication reconciliation as the process of creating the most accurate list possible of all medications a patient is taking and comparing that list against the physician’s order at all transition of care [5]. The medication reconciliation service has shown to be successful in identifying most discrepancies and preventing harm to patients [3, 6], thus resulting in significant financial saving [7, 8]. Currently, medication reconciliation has become a standard healthcare practice recommended by the WHO [9] and many countries [5, 10, 11].
Often with multiple morbidities requiring multiple medications, elderly patients theoretically have a high risk of many medication issues, including inappropriate prescribing [12], drug–drug interactions, drug–disease interactions, adverse drug events (ADEs) [13], and medication errors, especially medication discrepancies [14]. Actually, regarding medication discrepancies, prevalences of 49.5–81.9% during transitions in care had been reported in this population [15,16,17,18]. Furthermore, elderly patients can also have psychological (e.g., anxiety, depression, and dementia) and physiological factors (e.g., impaired hearing and vision function) that may impair their ability to communicate effectively with medical and healthcare staff, thus further contributing to potential medication discrepancies in this population. Elderly patients are, therefore, more vulnerable to medication discrepancies and should be a priority target population for medication reconciliation.
In Vietnam, obtaining the medication history of patients is the responsibility of doctors, nurses, and clinical pharmacists during ward rounds. However, the concept of medication reconciliation is still very new and has not been mandated in any government regulations or standard professional practice guidelines. As such, there is no standard operating procedure for medication reconciliation in Vietnam. This is further attested by a literature search performed by our research team that found no studies on this topic performed in Vietnam to date. Hence, the frequency and clinical impact of medication discrepancies remain unknown as a potential clinical problem in Vietnam. Without this information, it is difficult to request allocation of appropriate resources to rectify this clinically important but amendable problem.
Therefore, the main objective of the present study was to assess the frequency of medication discrepancies and identify the associated risk factors and potential clinical impact of them in elderly patients at hospital admission in Vietnam. The results were expected to support the importance of obtaining a comprehensive medication history at hospital admission and implementing a medication reconciliation program to reduce the negative impact of medication discrepancy, especially for the elderly population. This would also provide evidence to persuade the healthcare administrators in Vietnam to allocate additional resources to rectify this problem.
2 Methods
2.1 Study Setting and Patient Recruitment
This prospective observational study was conducted at Friendship Hospital, an 800-bed public geriatric hospital in Hanoi, which has 23 clinical units in total with 22,700 admissions in 2018. Patients over 60 years of age, admitted to seven selected internal medicine units of the hospital, taking at least one chronic medication before admission, and staying at least 48 h were eligible for enrollment. The selected internal medicine units were endocrine and metabolism, orthopedics, cardiovascular, respiratory, gastroenterology, psychiatry and neurology, and general internal medicine (coded from 01 to 07, respectively, in the present study). These selected units practically covered all the internal medical specialties at the hospital. Patients were excluded if they were unable to give consent due to their clinical conditions or refused to participate in the study. The patient recruitment process took place over 14 non-consecutive weeks from July 2018 to December 2018, with 2 weeks of recruitment for each unit. During this period, all patients admitted to the units who met the selection criteria were eligible to be included in the study.
2.2 Data Collection
For each enrolled participant, the following information was collected: age, gender, comorbidity, current admission diagnosis, treatment unit, outpatient management status (i.e., whether the patient was managed as an outpatient by the study hospital), patient’s existing chronic medical conditions, and the available sources for patients’ medication information (e.g., electronic medical records, paper-based outpatient medical records, and paper-based inpatient medical records). The patients’ medication history and current treatment during the hospital admission were collected as part of the medication reconciliation process described below.
2.3 Process of Identifying Medication Discrepancies
At the time of the study, there was no standard operating procedure (SOP) available for healthcare staff to obtain the medication history from patients and to reconcile the information with the admission medication prescriptions. The physician or nurse would normally collect the information regarding patients’ preadmission medications during the medical examination and record this in the patients’ medical record (paper-based medical record) without a SOP to perform any reconciliation for discrepancy. To identify any medication discrepancies at admission, the research group conducted a process of medication reconciliation that was independent of the normal practice of other healthcare professional staff (i.e., physicians and nurses). The activities of the study researchers did not interfere with the healthcare process for the patients.
Using the information from the WHO High 5s program, a step-by-step protocol for the medication reconciliation process was developed and training was provided for a group of study data collectors. Overall, the process of medication reconciliation for each participant consisted of the following key steps:
-
Step 1: Obtain the Best Possible Medication History (BPMH) list for patients. The BPMH form suggested by the WHO High 5s program was employed to obtain preadmission medication information of patients [1]. The BPMH was obtained from multiple available sources, including patient interviews, computer-based medical record systems, and paper-based medical records. Patient interviews were conducted at the patients’ bedside, using a structured form to guide the interview and record the data (Supplementary file 1: Interview guide; see electronic supplementary material [ESM]).
-
Step 2: Identify medication discrepancies in chronic medications. The list of admission medication prescriptions (i.e., prescribed during the first 24 h after a patient’s admission to the hospital) was collected from paper-based medical records for each patient. The list was then compared with the BPMH obtained by a study researcher as described above. Any differences between the chronic medications on the BPMH and admission medication prescription list was considered a potential medication discrepancy. Herbal products, traditional herbal medicine, dietary supplements, and other nonprescription medications were excluded from assessment as these products were usually stopped by the physicians at patient’s admission.
To examine the extent of the medication discrepancy resolution by physicians during the patients’ hospital stay, the medication discrepancy was also assessed at 48 h after admission and at the time of discharge using the same approach described above. After this time, each potential medication discrepancy was discussed with the physician to determine if it was intentional or unintentional. To ensure the accuracy of the process for determining the reason of each medication discrepancy, several potential reasons were considered such as diagnosis of a new clinical condition, occurrence of adverse drug events, or a specific medication was unavailable in the Department of Pharmacy at the hospital (Supplementary file 2: Process of Medication Discrepancies Classification, see ESM). Medication discrepancies that were accepted by the physician as being unreasonable were classified as unintentional medication discrepancies. Each unintentional medication discrepancy was then classified by drug class (according to the Anatomical Therapeutic Chemical Classification System—ATC) [19] and type of unintentional medication discrepancy (e.g., omission of medication, change of medication, extra medication, or difference in dose or dosing frequency).
The potential clinical impact of unintentional medication discrepancies was assessed and rated jointly by a panel of clinical experts (TXP Dong, TT Nguyen and TTV Pham) using both an explicit tool and clinical judgment. A consensus was reached by the expert panel for potential clinical impact of all discrepancies after group discussion. In particular, for omission discrepancies, the panel used the “Reducing Harm from omitted and delayed medicines in hospital” tool developed by the Specialist Pharmacy Service, United Kingdom, which is a list of drug groups evaluated according to the degree of impact on the clinical condition if delayed in treatment [20]. Finally, each discrepancy was classified into three categories based on the following classifications used by several studies [2, 21,22,23]: Risk 1 discrepancies with the potential to cause mild discomfort or clinical deterioration; Risk 2 discrepancies with the potential to cause moderate discomfort or clinical deterioration; and Risk 3 discrepancies with the potential to result in severe discomfort or clinical deterioration.
2.4 Ethics Approval
This study was granted ethics approvals by The Hospital Science and Technology Committee at Friendship Hospital (Vietnam) and the Human Research Ethics Committee (HREC) at the University of Newcastle (Australia).
2.5 Data Analysis
The collected data were analyzed using the Statistical Package for Social Sciences (SPSS), version 20.0 (IBM statistics, Armonk, NY, USA). Percent and frequency were used to describe medication discrepancy.
Multivariate logistics regression was used to identify risk factors associated with unintentional medication discrepancies in our study population. The Backward Stepwise (Wald) method was employed to identify appropriate multivariate logistic regression, with p values at 0.10 as the threshold for entering or removing variables. Based on previous research and our experience, we selected the independent variables that could have a significant impact on the likelihood of unintentional medication discrepancies. The independent variables then were examined to include in the logistic regression model by the univariate analysis. The regression analysis results were expressed as odds ratios with 95% confidence intervals. The influence of factors was considered to be statistically significant with p < 0.05.
3 Results
3.1 Demographics and Baseline Characteristics of the Participants
During the study period, a total of 395 patients were admitted to the study units. Of these, 203 patients were excluded from the study—14 were admitted for <48 hours, 127 were not taking any chronic medications or had no chronic disease, 30 refused to participate, and 32 were unable to give consent. There was a total of 192 eligible patients included in the study (Fig. 1).
The demographics and baseline characteristics of the 192 patients included in the study are shown in Table 1. The average age of the study participants was 75.6 (± 7.0) years and 77.1% were males. Polypharmacy (at least 5 medications) before admission was seen in almost half of the patients (44.8%). The most common chronic diseases in the study participants were hypertension (86.5%), hyperlipidemia (61.5%), type 2 diabetes mellitus (45.3%), chronic coronary syndrome (37.0%), and osteoarthritis (25.5%). The average number of co-morbidities was 5.1 ± 1.8.
3.2 Frequency and Type of Medication Discrepancy
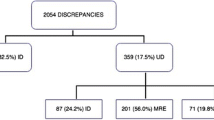
Among the 192 patients recruited, there were 328 chronic medication discrepancies identified between the BPMH list and the 24-h medication prescription (intentional and unintentional), with a mean ± SD of 1.7 ± 1.4 discrepancies per patient. All of the identified discrepancies had no documented reason in either the paper-based medical records or electronic medical records of the patients. After discussion with the physicians in charge, 87 discrepancies were classified as unintentional in 32.3% of patients (n = 62). The frequency of medication discrepancies among the study population is presented in Table 2. Among the types of unintentional medication discrepancies, medication omission accounted for the highest proportion (75.9%), followed by medication change (21.8%). After the first 48 hours of admission, the number of unintentional medication discrepancies remained high (90.8%) and persisted until the time of discharge (77.0%).
Cardiovascular agents were the most common drug therapies involved in medication discrepancies among the study participants. This included lipid-modifying drugs (39 cases, 44.8%), antihypertension drugs (18 cases, 20.7%), and antithrombotic drugs (11 cases, 12.7%) (Table 3).
3.3 Risk Factors Associated with Unintentional Medication Discrepancies
The study used multivariate logistics regression with the Backward Stepwise (Wald) method to eliminate variables and selected suitable multivariate models to identify factors associated with the likelihood of unintentional medication discrepancies. Accordingly, the frequency of unintentional medication discrepancies was significantly higher among patients admitted to the orthopedics, respiratory, and gastroenterology units in comparison to those admitted to the endocrine and metabolism unit (odds ratio 10.03, 5.44 and 6.98, respectively; p < 0.05). In addition, the risk of medication discrepancy significantly increased among patients using at least five chronic medications (polypharmacy) before admission compared with patients who were taking only one or two chronic medications at preadmission (odds ratio 4.65, p < 0.05) (Table 4).
3.4 Clinical Importance of Unintentional Medication Discrepancies
Most of the unintentional medication discrepancies (n = 69, 79.3%) were classified into the risk 1 group (i.e., associated with mild potential harm or deterioration in patients). There were three discrepancies belonging to the risk 3 group, including the omission of dabigatran in a patient with atrial fibrillation and the omission of levodopa + benserazide in a patient with Parkinsonism (Table 5).
4 Discussion
To the best of our knowledge, this is the first study to examine the frequency of medication discrepancies among hospital inpatients in Vietnam. The study was focused on elderly patients, as they are a particularly vulnerable population to medication discrepancies and other drug-related problems (e.g., inappropriate indication, dose, or adverse effects). While the discrepancies can come from all kinds of patients’ preadmission medications, including for both chronic and non-chronic medical conditions, we focused only on chronic medications because of their importance in managing long-term conditions of the elderly. The results showed an average of 1.7 (SD 1.4) medication discrepancies per patient at the time of admission and 32.3% of the study participants had at least one unintentional medication discrepancy regarding their chronic medications.
To interpret the results meaningfully, we compared our findings with similar studies conducted in other countries, which also focused on identifying unintentional medication discrepancies in elderly patients during admission from 2010 onwards. As shown in Table 6, the prevalence of unintentional medication discrepancies varied widely between the published studies from other countries. The studies that showed a much higher rate include those conducted by Belda-Rustarazo et al. in 2015 (64.5%) [24], Vargas et al. in 2016 (49.5%) [15], and Magalhães et al. in 2014 (48.0%) [25]. Similar and lower prevalence rates were reported in the study by Cornu et al. in 2012 (40.9%) [16], Quélennec et al. in 2013 (33.2%) [21], and Climente-Martí et al. in 2010 (9.1%) [22]. Reasons for the marked variation in results include differences in the study population, the definition of unintentional medication discrepancy used, and the protocol applied to conduct medication reconciliation. For example, the studies by Belda-Rustarazo et al. [24], Vargas et al. [15], and Magalhães et al. [25] selected patients with at least three or five preadmission medications, whereas our study only required at least one preadmission medication. This may explain why the frequency of unintentional medication discrepancy is lower in our current study when compared with some other published studies. Furthermore, we only classified medication discrepancies as being ‘unintentional’ after clarification and approval from the managing physicians, which could have reduced the proportion of unintentional medication discrepancies identified. Despite this, our study still indicates a relatively high frequency of unintentional medication discrepancies, and the current practice of obtaining the medication history of patients and reconciliating this with the medications prescribed at hospital admission is not adequate in Vietnam.
This study also demonstrates that the number of unintentional medication discrepancies remained very high at 48 h after admission (90.8%) and even persisted until the patient was discharged (77.0%). Discrepancies in medication records can occur during transition between various healthcare facilities. If they are not identified and effectively communicated to the patient or the patient’s general practitioner (GP) following hospital discharge, the unresolved medication discrepancies may continue indefinitely and can lead to adverse consequences for the patient (e.g., omission of a vital medication).
The most frequent type of unintentional medication discrepancy was medication omission (75.9%), followed by medication change (21.8%). This result is in line with previous studies that have reported medication omission as the most common type of discrepancy [15, 22, 24, 26]. Potential reasons for the unintentional omission of medications when patients are admitted to hospital or leave hospital include incomplete information regarding patients’ preadmission medication lists, issues surrounding amnesia of patients during interviews, and the complexity of patients’ medication regimens. These findings suggest the need for strategies to identify and improve barriers in the transition of care pathways to ensure continuity and integration of care for the patient.
In term of medication class, unintentional medication discrepancy was identified mostly for cardiovascular drugs (e.g., lipid-modifying agents, antihypertensive agents, and antithrombotic agents), followed by blood glucose-lowering drugs. Other medication reconciliation studies had also identified cardiovascular drugs as being one of the most frequent drug classes associated with medication discrepancies [15, 24, 25]. Other frequently reported medication classes include drugs affecting the blood and hematopoietic system [22, 24], the nervous system [15, 24], and the gastrointestinal system [15, 22]. This may suggest that some medication classes require special attention when implementing medication reconciliation procedures.
Assessment of the potential clinical impact of the unintentional discrepancies detected in the current study showed that 20.7% of unintentional medication discrepancies were judged to be from risk 2 and risk 3 groups, indicating that they had the potential to cause moderate discomfort or clinical deterioration (17.2%) or severe discomfort or clinical deterioration (3.5%). In comparison, several previous studies showed a wide variation of proportions of unintentional medication discrepancies (from 1.5 to 65.0%) at hospital admission that were able to cause moderate to severe discomfort or clinical deterioration [2, 21,22,23, 27, 28]. The lack of an appropriate explicit assessment tool can be the main reason leading to these differences. Nevertheless, our findings highlighted the necessity to detect and resolve these discrepancies in a timely manner.
Associations between the number of unintentional medication discrepancies and the type of internal medicine unit as well as the number of medications at admission were found in the present study. The unintentional medication discrepancies were 10.03, 5.44 and 6.98 times more likely to occur among patients admitted to the orthopedics, respiratory, and gastroenterology units, respectively, in comparison to patients admitted to the endocrine and metabolism unit. Similar variations in the prevalence of unintentional medication discrepancy among hospital wards were also reported by other studies [22, 29]. For example, Tamiru et al. [22, 29] found that the frequency of medication discrepancy was significantly reduced among patients admitted to the surgery ward compared with patients admitted to the medical ward (adjusted odds ratio 0.27 [0.10–0.74]). These variations may be due to not having a standard operating procedure for medication reconciliation in the different units of the study hospital. The different characteristics of patients admitted to these units and the different specialties of the physicians in these units may also be contributing factors. In resource-limited settings such as Vietnamese hospitals, this information could help the hospital administrators to strategically assign resources.
Furthermore, the likelihood of medication discrepancy was also significantly increased among patients taking a least five chronic medications prior to hospital admission compared with patients who had one or two preadmission chronic medications. This finding was consistent with other studies regarding the risk factors of unintentional medication discrepancies [15, 29]. For example, Vargas et al. reported that the risk of unintentional medication discrepancies increased by 20% for each additional drug [15]. In addition, this study also found that patients with unintentional medication discrepancies took significantly more medications than those without unintentional medication discrepancies (9.2 vs 7.6; p < 0.01). Similarly, Cornu et al. showed that for every additional drug in the medication history, the likelihood of experiencing one or more drug discrepancies increased by 47% (adjusted OR 1.47; 95% CI 1.24–1.74; p < 0.001) [16]. These findings suggest that medication reconciliation by clinical pharmacists can be prioritized to elderly inpatients with polypharmacy at hospital admission if resources are limited. It should be noted that, in contrast to several previous studies [15, 22, 24], multivariate logistic regression analysis did not show any associations between number of unintentional medication discrepancies and age, gender, or number of comorbidities in our present study. The absence of these associations may be due to the small sample size of our study, the different patient population, or the different study setting. In addition, the lack of association between the number of comorbidities and the number of unintentional medication discrepancies (while the number of medications was a risk factor in the study) might be explained by the commonly observed phenomenon of under-treatment for elderly patients in Vietnamese hospitals.
There are a few limitations that should be considered when interpreting the findings of the present study. First, the study period was over 14 non-consecutive weeks during 6 months, which may potentially affect the discrepancy rate due to variation in types of patients being admitted during the study period. Nevertheless, the study only identified discrepancies related to medications in patients with chronic diseases, where their admissions were much less affected by season. In addition, the study took place within the same year with no change in the hospital formulary nor any SOP affecting our study. Therefore, we considered the effect of prolonged sampling time in the study to be minimal. Second, the results may not represent the current practice of the whole country, as the study was only conducted at a single hospital in Vietnam. However, as mentioned above, the concept of ‘medication reconciliation’ is still very new in Vietnam and has not been mentioned in any official documents or professional practice standards. Hence, there is a lack of SOPs in Vietnamese hospitals for this practice. In addition, the study hospital is one of the biggest geriatric hospitals in Vietnam with a large number of elderly patients admitted each year. Therefore, the current results are likely to be applicable to other Vietnamese hospitals. The third limitation is that the review of the medications prescribed was limited to chronic medical conditions, which may have led to an underestimation of the frequency of unintentional medication discrepancies. We only focused on this group of medications due to their importance in managing the conditions of the elderly population. Lastly, the potential clinical impact of some of the unintentional medication discrepancies identified was assessed by an expert panel due to the lack of an appropriate assessment instrument.
5 Conclusion
This study highlights that the frequency of medication discrepancies among elderly patients admitted to hospital in Vietnam is similar to the study results reported in other jurisdictions. The most frequent type of unintentional medication discrepancy was medication omission, which commonly occurred for drugs of the cardiovascular system. Another important observation from our study was that unintentional medication discrepancies persisted throughout the patients’ hospital stays until discharge. Overall, our results support the importance of implementing SOPs to obtain a complete preadmission medication history of patients as well as implementing a medication reconciliation program in Vietnam to facilitate better healthcare management for patients. Besides filling the information gap of unintentional medication discrepancies among Vietnamese patients with chronic disease at hospital admission, our results may provide some reference values for countries in a similar position to Vietnam for healthcare planning or conducting similar studies.
References
World Health Organization. Standard operating protocol assuring medication accuracy at transitions in care: medication reconciliation—The High 5s Project Implementation Guide. 2014. https://www.who.int/patientsafety/implementation/solutions/high5s/h5s-sop.pdf. Accessed 9 May 2021
Cornish PL, Knowles SR, Romina M, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165:424–9. https://doi.org/10.1001/archinte.165.4.424.
Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15:122–6. https://doi.org/10.1136/qshc.2005.01534.
The Joint Commission. Hospital: 2021 National Patient Safety Goals. https://www.jointcommission.org/-/media/tjc/documents/standards/national-patient-safety-goals/2021/npsg_chapter_hap_jan2021.pdf. Accessed 9 May 2021
Institute for Healthcare Improvement. Medication reconciliation to prevent adverse drug events. http://www.ihi.org/Topics/ADEsMedicationReconciliation/Pages/default.aspx. Accessed 9 May 2021
Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2): e010003. https://doi.org/10.1136/bmjopen-2015-010003.
Kilcup M, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53(1):78–84. https://doi.org/10.1331/japha.2013.11250.
Sebaaly J, et al. Clinical and financial impact of pharmacist involvement in discharge medication reconciliation at an academic medical center: a prospective pilot study. Hosp Pharm. 2015;50(6):505–13. https://doi.org/10.1310/hpj5006-505.
World Health Organization, Assuring medication accurracy at transitions in care: medication reconciliation—The High 5s Project Implementation Guide. 2014. https://www.who.int/patientsafety/implementation/solutions/high5s/h5s-fact-sheet.pdf?ua=1. Accessed 9 May 2021
The Society of Hospital Pharmacists of Australia. Standards of Practice for Clinical Pharmacy Services. 2016. http://www.cec.health.nsw.gov.au/patient-safety-programs/medication-safety/continuity-of-medication-management. Accessed 9 May 2021
American Society of Hospital Pharmacist. ASHP statement on the pharmacist’s role in medication reconciliation. Am J Health Syst Pharm. 2013;70(5):453–6. https://doi.org/10.2146/sp120009.
Steinman MA, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54(10):1516–23. https://doi.org/10.1111/j.1532-5415.2006.00889.
Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. https://doi.org/10.1517/14740338.2013.827660.
Stawicki SP, Gerlac AT. Polypharmacy and medication errors: stop, listen, look, and analyze. Opus Sci. 2009;3(1):6–10.
Vargas BR, et al. Prevalence and risk factors for medication reconciliation errors during hospital admission in elderly patients. Int J Clin Pharm. 2016;38(5):1164–71. https://doi.org/10.1007/s11096-016-0348-8.
Cornu P, et al. Effect of medication reconciliation at hospital admission on medication discrepancies during hospitalization and at discharge for geriatric patients. Ann Pharmacother. 2012;46(4):484–94. https://doi.org/10.1345/aph.1q594.
Steurbaut S, et al. Medication history reconciliation by clinical pharmacists in elderly inpatients admitted from home or a nursing home. Ann Pharmacother. 2010;44:1596–603. https://doi.org/10.1345/aph.1p192.
Villanyi D, Fok M, Wong R. Medication reconciliation: identifying medication discrepancies in acutely ill hospitalized older adults. Am J Geriatr Pharmacother. 2011;9:339–44. https://doi.org/10.1016/j.amjopharm.2011.07.005.
World Health Organization. Anatomical therapeutic chemical (ATC) classification. https://www.who.int/medicines/regulation/medicines-safety/toolkit_atc/en/#:~:text=In%20the%20Anatomical%20Therapeutic%20Chemical,groups%20at%20five%20different%20levels. Accessed 9 May 2021
Specialist Pharmacy Service. Tool to reduce harm from omitted and delayed medicines. 2017. https://www.sps.nhs.uk/articles/npsa-alert-reducing-harm-from-omitted-and-delayed-medicines-in-hospital-2010/. Accessed 9 May 2021
Quelennec B, et al. Potential clinical impact of medication discrepancies at hospital admission. Eur J Intern Med. 2013;24(6):530–5. https://doi.org/10.1016/j.ejim.2013.02.007.
Climente-Martí M, et al. Potential risk of medication discrepancies and reconciliation errors at admission and discharge from an inpatient medical service. Ann Pharmacother. 2010;44(11):1747–54. https://doi.org/10.1345/aph.1p184.
Abdulghani KH, et al. The impact of pharmacist-led medication reconciliation during admission at tertiary care hospital. Int J Clin Pharm. 2018;40(1):196–201. https://doi.org/10.1007/s11096-017-0568-6.
Belda-Rustarazo S, et al. Medication reconciliation at admission and discharge: an analysis of prevalence and associated risk factors. Int J Clin Pract. 2015;69(11):1268–74. https://doi.org/10.1111/ijcp.12701.
Magalhães GF, et al. Medication reconciliation in patients hospitalized in a cardiology unit. PLoS ONE. 2014;9(12): e115491. https://doi.org/10.1371/journal.pone.0115491.
Marinović I, et al. Clinical pharmacist-led program on medication reconciliation implementation at hospital admission: experience of a single university hospital in Croatia. Croat Med J. 2016;57(6):572–81. https://doi.org/10.3325/cmj.2016.57.572.
Giannini O, et al. Prevalence, clinical relevance and predictive factors of medication discrepancies revealed by medication reconciliation at hospital admission: prospective study in a Swiss internal medicine ward. BMJ Open. 2019;2019(9): e026259. https://doi.org/10.1136/bmjopen-2018-026259.
Andreoli L, et al. Medication reconciliation: a prospective study in an internal medicine unit. Drugs Aging. 2014;31(5):387–93. https://doi.org/10.1007/s40266-014-0167-3.
Tamiru A, et al. Magnitude and factors associated with medication discrepancies identified through medication reconciliation at care transitions of a tertiary hospital in eastern Ethiopia. BMC Res Notes. 2018;11(1):554. https://doi.org/10.1186/s13104-018-3668-z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
All data generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors’ contributions
Phuong Thi Xuan Dong: conceptualization, methodology, data collection, formal analysis, writing—original draft, writing—review and editing. Van Thi Thuy Pham: conceptualization, methodology, writing—review and editing. Thao Thi Nguyen: data collection, writing—review and editing. Huong Thi Lien Nguyen: conceptualization, methodology, writing—review and editing. Susan Hua: conceptualization, methodology, writing—review and editing, supervision. Shu Chuen Li: conceptualization, methodology, writing—review and editing, supervision, project administration. All authors read and approved the final manuscript.
Ethics approval
This study was granted ethics approvals by The Hospital Science and Technology Committee at Friendship Hospital, Vietnam (approved on 28 March, 2018) and the Human Research Ethics Committee (HREC) at the University of Newcastle, Australia (Approval Number H-2018-0130). The study was performed in accordance with the Declaration of Helsinki. The participants were informed of the objectives of the study and the risks and benefits of the explorations to be carried out (Informed Consent).
Consent to participate
All participants have provided written consent to participate.
Consent to publication
Not applicable.
Acknowledgments
The authors wish to thank Dr Van Anh Le and the staff at the Department of Pharmacy as well as the physicians, nurses, and patients at the Friendship Hospital for their collaboration on this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dong, P.T.X., Pham, V.T.T., Nguyen, T.T. et al. Unintentional Medication Discrepancies at Admission Among Elderly Inpatients with Chronic Medical Conditions in Vietnam: A Single-Centre Observational Study. Drugs - Real World Outcomes 9, 141–151 (2022). https://doi.org/10.1007/s40801-021-00274-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-021-00274-3