Abstract
Background
An internal quality assurance review at AtlantiCare Regional Medical Center (ARMC) revealed that patients admitted with skin and soft tissue infections (SSTIs) remained in hospital post-resolution of acute symptoms and demonstrated a delayed transition to step-down oral antibiotic therapy. A non-mandatory institutional pathway was developed and implemented in 2016 to expedite hospital discharge in hemodynamically stable SSTI patients by utilizing oritavancin.
Objective
To describe the outcomes associated with use of single-dose oritavancin therapy to expedite hospital discharge in hemodynamically stable inpatients with SSTIs.
Methods
A retrospective, descriptive cohort was evaluated for outcomes of patients with SSTIs who received either oritavancin to expedite discharge or were discharged on oral step-down antibiotics. Patients were included in this analysis if they were: ≥ 18 years old; hospitalized; received empiric vancomycin; not pregnant or nursing; hemodynamically stable at the time of assessment; and received either oritavancin or oral step-down antibiotics to facilitate discharge. The primary outcomes were index hospital length of stay (LOS), 30-day SSTI-related readmissions, and 30-day SSTI progression.
Results
Overall, 199 patients met the study criteria (oritavancin = 99 and oral step-down antibiotics = 100). Groups were well matched at baseline. Patients who received oritavancin had a shorter mean index hospital LOS than those in the oral step-down antibiotic group (3.5 days vs. 5.6 days). Patients receiving oritavancin also had lower SSTI 30-day readmission and SSTI-progression rates.
Conclusions
An institutional pathway that used oritavancin to expedite hospital discharge of hemodynamically stable SSTI patients resulted in shorter hospital LOS, less 30-day SSTI-related hospital readmissions, and decreased SSTI progression relative to those discharged on conventional oral step-down therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Single-dose IV oritavancin was successfully used to expedite hospital discharge in hospitalized patients with skin and soft tissue infections (SSTIs) who were hemodynamically stable. |
Patients who received single-dose IV oritavancin to expedite hospital discharge had shorter hospital length of stay, lower 30-day SSTI-related hospital readmission rates, and less SSTI progression relative to patients discharged on oral antibiotics. |
1 Introduction
With rising healthcare costs, there is a growing emphasis for hospitals to improve the quality and efficiency of healthcare delivery [1, 2]. Management of hospitalized patients with skin and soft tissue infections (SSTIs) is a potential quality improvement initiative for hospitals given its prevalence and associated costs. Each year, there are approximately 850,000 SSTI-related hospital admissions, accounting for 2% of all hospital admissions in the USA [3,4,5]. The cost of each SSTI-related hospital admission is estimated to be between US$6000 and US$20,000, resulting in an economic burden to the US healthcare system that exceeds US$15 billion annually [3, 6, 7]. Data indicate many patients admitted with SSTIs have minimal co-morbid conditions and mild or no systemic signs of infection, and often remain hospitalized after their acute infection resolves solely for continued intravenous (IV) antibiotics [3, 8, 9]. The consequences of this practice on the healthcare system are substantial and decrease hospital quality and efficiency.
Due to the significant hospitalization costs associated with SSTIs and potential for improvement in healthcare delivery, a quality assurance project in hospitalized adult patients with SSTIs was conducted at AtlantiCare Regional Medical Center (ARMC). Vancomycin was identified as the most commonly used intravenous agent for patients with SSTIs. Patients treated with vancomycin stayed in the hospital on average for 5.6 days, and the cost of each hospital day was estimated to be US$2700 based on hospital specific data [10]. Consistent with other studies [3, 8], a review of data also indicated that the majority of patients who received vancomycin for SSTIs had zero or minimal uncontrolled co-morbid conditions and were limited to no systemic signs and symptoms. More importantly, most SSTI patients stayed in the hospital post-resolution of acute symptoms of infection and there was a slow transition to oral antibiotic therapy post-resolution of symptoms.
Based on the collected data, a non-mandatory institutional pathway was developed and implemented at ARMC in 2016 that encouraged providers to expedite hospital discharge of hemodynamically stable SSTI patients treated with oritavancin. Oritavancin is a US Food and Drug Administration (FDA)-approved, single-dose IV therapy for the treatment of acute bacterial skin and skin structure infections (ABSSSIs) caused or suspected to be caused by susceptible Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) [11]. Because it is a single-dose therapy, it was anticipated that select inpatients may be discharged earlier in their hospitalization thus decreasing time spent in the hospital and subsequently costs. Additionally, utilizing oritavancin in this population eliminates the need for oral step-down antibiotic therapy and negates the commonly reported negative consequences associated with oral antibiotics secondary to poor patient adherence [12]. This descriptive study evaluated the outcomes of ARMC inpatients with SSTIs who received conventional intravenous antibiotics (i.e., vancomycin-containing regimens) followed by oritavancin to expedite discharge relative to those who received oral antibiotics at discharge. The primary outcome measures assessed were index hospital length of stay (LOS), 30-day SSTI progression rates, and 30-day SSTI-related readmission rates. As this was a descriptive study with no formal hypothesis testing, no inferential statistics were performed.
2 Methods
2.1 Study Design and Population
A retrospective descriptive cohort study was conducted among patients receiving care at ARMC between May 2017 and March 2019. Patients were included in this analysis if they (1) were aged ≥ 18 years, (2) hospitalized, (3) had a diagnosis code for an SSTI, (4) had confirmation of SSTI on electronic medical record review, (5) received vancomycin for initial treatment of the SSTI, (6) were not pregnant or nursing, (7) were hemodynamically stable, and (8) received oritavancin or oral step-down antibiotics to facilitate discharge. Patients were excluded if they received oritavancin or vancomycin for treatment of a condition other than an SSTI (e.g., infective endocarditis, osteomyelitis, etc.), or if they were receiving weight-based heparin.
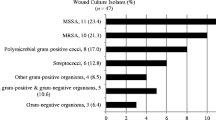
Trained reviewers collected data from patients’ electronic medical records. Data elements that were extracted included demographics, co-morbidities, course of hospitalization, antimicrobial medications, laboratory values, and type of SSTI. Baseline demographic characteristics included age, sex, actual body weight, race, and pre-admission location. Pre-admission location was categorized as community transfer from another hospital/inpatient facility, nursing home, or other/unknown. The following were collected from the patient’s medical record and history of present illness: active malignancy or cancer, decubitus ulcers, concomitant bacteremia, diabetes, intravenous drug use (IVDU), human immunodeficiency virus (HIV), renal dysfunction (creatinine clearance < 30 mL/min [13]), and antimicrobial therapy within 30 days prior to admission. Course of hospitalization included hospital length of stay (LOS) prior to receiving oritavancin, antibiotics received during the hospitalization, oral step-down antibiotics to facilitate discharge, and total LOS. Serum creatinine at admission was used for creatinine clearance (CLCR) calculation (Cockcroft–Gault method [13]). All microbiologic results available during the hospital course were collected. Variables related to types of SSTI included presence of cellulitis, abscess, chronic ulcerative infection, post-operative infection, lymphadenitis, and location of SSTI.
2.2 Outcomes
The following clinical outcomes were assessed: (1) mean (SD) index hospital length of stay (LOS), (2) 30-day SSTI-related readmission rates, and (3) SSTI 30-day readmissions with disease progression. All 30-day readmissions after discharge were screened for the presence of an SSTI diagnosis code. To determine if SSTI disease progression occurred during the SSTI-related 30-day readmission, the trained reviewers assessed if any of the following were present: SSTI, bacteremia, osteomyelitis, or infective endocarditis. As part of their assessment, reviewers determined if the source of each resultant progression infection type was related to their initial SSTI based on medical record notes and microbiologic data (i.e., Gram-negative co-infection). The location of the initial SSTI was considered for patients who presented with osteomyelitis at their subsequent hospital admission.
As this was a descriptive study, no inferential statistics were performed. Only summary statistics were provided by treatment group for outcomes, including number of patients, arithmetic mean, and standard deviation (SD).
3 Results
During the study period, 199 patients met the study criteria. There were 99 patients in the oritavancin group and 100 patients in the oral step-down antibiotic group. Baseline comparison between oritavancin and oral step-down antibiotic group are shown in Table 1. The most frequently administered oral antibiotics upon discharge in the oral step-down antibiotic group included doxycycline, sulfamethoxazole–trimethoprim, and cephalexin. Overall, the groups were well matched at baseline. There were no considerable differences in demographics, pre-clinical co-morbidities, type of skin infection at diagnosis, infection severity at diagnosis, or pre-healthcare resource utilization with the exception of the receipt of antibiotics in the previous 30 days and the incidence of intravenous drug use (IVDU). Both variables were more common in the oritavancin group.
Outcomes of the two treatment groups are shown in Table 2. Patients who received oritavancin had a shorter initial mean (SD) index hospital LOS than those in the oral step-down antibiotic group (3.5 vs. 5.6 days, respectively). Patients receiving oritavancin also had a lower 30-day SSTI-related hospital readmission rate compared to those receiving oral step-down antibiotics (7.1% in oritavancin group vs. 18.0% in oral step-down antibiotic group). Of the seven oritavancin patients with a 30-day SSTI-related readmission, six had a Gram-negative organism identified as the SSTI cause including one patient with Gram-negative bacteremia as a result of a persistent SSTI. In contrast, seven of 18 patients in the oral step-down group with a 30-day SSTI-related readmission had a bloodstream infection, including one case of emerging osteomyelitis, as a result of a persistent SSTI. Six of the seven bacteremia patients in the oral step-down group had Gram-positive organisms identified.
4 Discussion
This descriptive investigation sought to evaluate the outcomes associated with a non-mandatory clinical pathway implemented at ARMC that permitted clinically stable, adult, hospitalized SSTI patients without compromised renal function to receive oritavancin in an effort to facilitate hospital discharge. Consistent with previous studies [3, 8], a review of internal data revealed there was an opportunity to improve the efficiency of care of SSTI patients as the majority of patients who received vancomycin for an SSTI stayed in the hospital post-resolution of acute symptoms of infection and there was a delayed transition to oral step-down antibiotic therapy. When evaluating the outcomes of patients who received oritavancin relative to those who received conventional oral step-down care after implementation of the pathway, there were several noteworthy findings. Patients who received oritavancin had a shorter hospital LOS relative to those who received oral step-down therapy post-receipt of IV vancomycin. The shorter LOS in the oritavancin group was most likely a function of implementing a standardized institutional process to assess SSTI patients and expediting discharge in hemodynamically stable hospitalized patients with SSTIs after administration of a single dose of oritavancin. When reviewing these findings, it should not be assumed that expedited discharge can be accomplished with a single-dose antibiotic like oritavancin alone, as data demonstrate that the implementation of institutional pathways along with healthcare provider education are often required to facilitate early hospital discharge in clinically stable patients with infections [14, 15].
Another notable finding was the observed numerical differences in 30-day SSTI-related readmissions and 30-day SSTI progression rates between patients who received oritavancin relative to oral step-down antibiotic therapy. Among the 99 patients who received oritavancin, seven (7.1%) were re-admitted and only one had a documented progression of their SSTI with a Gram-positive infection. The low 30-day readmission and infection progression rates observed among patients who received oritavancin are consistent with other studies of an identical nature. In a multicenter, retrospective study that evaluated the use of oritavancin upon hospital discharge to complete treatment of an SSTI, infection-related 30-day readmission rates were 2.6% [16]. Similarly, no patients had to be readmitted within 14 days of oritavancin therapy in an evaluation of oritavancin use at a community hospital to facilitate hospital discharge [17].
Nearly 20% of patients in the oral step-down therapy group had a 30-day readmission, and disease progression was reported in seven patients. The exact reasons for the higher 30-day re-admission and disease progression rates in the oral step-down therapy are not entirely clear as most patients received at least 5 days of vancomycin therapy prior to switching to oral antibiotics. Unfortunately, patients’ compliance with oral therapy was not assessed. Studies indicate that poor patient adherence is commonplace with oral antibiotics and failure to take antibiotics post-hospital discharge for SSTIs is associated with suboptimal outcomes. In an assessment of the relationship between adherence to oral antibiotics and post-discharge clinical outcomes among patients hospitalized with S. aureus uncomplicated skin infections, mean electronically measured adherence to antibiotic therapy was only 57% and was considerably lower than self-reported adherence (96%). In the multivariable analysis, lower adherence was an independent risk factor for poor clinical outcome, which was defined as the presence of relapsing or a new SSTI or a need for additional antibiotics or surgical procedures [12]. Another potential reason for the observed findings is the oral step-down antibiotics used. Patients included in this study most commonly received doxycycline, sulfamethoxazole-trimethoprim, ciprofloxacin, and cephalexin as oral step-down antibiotic therapy at the time of discharge. While these are considered appropriate antibiotics for management of most SSTIs, their efficacy for patients with more complicated SSTIs has not been well established in contemporary clinical trials.
4.1 Limitations
This descriptive investigation does have limitations, which should be taken into account when interpreting these findings. First, this was a single-center, retrospective, cohort study, and it is subject to all the limitations and biases associated with this design. Most notably, we cannot fully gauge the impact of prescribing bias on the observed findings. After implementation of the “SSTI hospital discharge” pathway, clinicians were encouraged but not mandated to prescribe oritavancin post-receipt of empiric intravenous antibiotics and were able to choose the step-down therapy they deemed most appropriate. The choice to expedite discharge with oritavancin was frequently initiated by pharmacists or infectious diseases physicians rather than the provider. Furthermore, oritavancin was primarily used in patients perceived as potentially noncompliant, which was internally recognized as IVDUs, homeless, and those with social issues. We were not able to account for the potential effects of this prescribing bias on the observed results. However, the findings suggest that a collaborative, standardized institutional process is needed to expedite discharge with oritavancin. Furthermore, use of oritavancin to facilitate hospital discharge does not appear to lead to an increase in infection-related readmission or SSTI progression.
This was a study of adult, non-neutropenic, clinically stable, non-dialysis SSTI patients, and the observed findings may not be applicable to other populations. Although we included a number of proxy measures for patients’ disease severity, detailed clinical information to calculate acute disease severity measures like the acute physiology and chronic health examination (APACHE-II) or Pitt bacteremia score were not collected. The effect of the inability to include an acute disease severity measure on the observed outcomes is unknown, but it is likely to be minimal as patients had to be hemodynamically stable before switching to either oritavancin or oral step-down therapy. Clinical stability was also a part of the inclusion criteria for this study, further negating the potential influence of acute disease severity on the observed outcomes. Finally, our outcomes were limited to objective measures to minimize any subjective biases that may result from assessing and interpreting observational clinical data, and may not include all outcomes that are relevant to SSTI patients. This study did not take into account the patient perspective and patient-reported outcomes, which are an increasingly important metric for healthcare systems. However, data indicate that patients with SSTIs preferred to be treated at home versus the hospital and with a single IV dose therapy relative to other therapies [18].
5 Conclusion
In an era of healthcare reform, hospitals are mandated to deliver high-quality care at the lowest cost. For patients with SSTIs, there is an opportunity to reduce costs while maintaining quality by shortening hospital LOS and reducing subsequent SSTI-related admissions. At ARMC, improvements in the efficiency of care of adult, hospitalized SSTI patients was achieved by prioritizing use of oritavancin relative to step-down oral antibiotics to expedite hospital discharge in hemodynamically stable patients. Utilizing oritavancin for treatment of SSTIs to expedite hospital discharge resulted in a shorter hospital LOS, less 30-day SSTI-related hospital readmissions, and decreased SSTI progression relative to conventional oral step-down therapy.
As this is a single-center retrospective study, the results should be interpreted with caution. Future randomized, multicenter comparator studies are required to properly ascertain the outcomes and potential benefits associated with oritavancin and other long-acting single-dose lipoglycopeptides relative to other commonly used antibiotics as step-down agents in hospitalized patients with SSTIs.
References
The Patient Protection and Affordable Care Act (PPACA), Pub. L. No. 111-148, 124 Stat. 119; 2010.
Burwell SM. Setting value-based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–9.
Lodise TP, Fan W, Sulham KA. Hospital admission patterns in adult patients with skin and soft tissue infections: Identification of potentially avoidable hospital admissions through a retrospective database analysis. Hosp Pract. 2015;43(3):137–43.
Kaye KS, et al. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS One. 2015;10(11):e0143276.
Edelsberg J, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis. 2009;15(9):1516–8.
Healthcare Costs and Utilization Project [Internet]. HCUPnet; 2019. https://hcupnet.ahrq.gov/-query/ey. Cited 29 Mar 2019.
Suaya JA, et al. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis. 2014;14:296.
Dryden M, et al. Antibiotic stewardship and early discharge from hospital: impact of a structured approach to antimicrobial management. J Antimicrob Chemother. 2012;67(9):2289–96.
Talan DA, et al. Factors associated with decision to hospitalize emergency department patients with skin and soft tissue infection. West J Emerg Med. 2015;16(1):89–97.
Whittaker C, et al. Expediting discharge in acute bacterial skin and skin structure infections: a clinical and economic comparison between Vancomycin and Oritavancin in hospitalized patients. Open Forum Infect Dis. 2018;5(Suppl 1):S118.
Melinta Therapeutics Inc. Orbactiv® (oritavancin) for injection, for intravenous use. (Package insert); 2019. http://www.orbactiv.com/pdfs/orbactiv-prescribing-information.pdf.
Eells SJ, et al. Relationship between adherence to oral antibiotics and postdischarge clinical outcomes among patients hospitalized with Staphylococcus aureus skin infections. Antimicrob Agents Chemother. 2016;60(5):2941–8.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Carratala J, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172(12):922–8.
Jenkins TC, et al. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
Estrada S, Lodise TT, Delaportas D. The real world economic and clinical management of adult patients with skin and soft tissue (SSTI) infections with oritavancin: data from two multi-center observational cohort studies; 2019.
Co D, Roebuck L, VanLandingham J. Evaluation of oritavancin use at a community hospital. Hosp Pharm. 2018;53(4):272–6.
Almarzoky Abuhussain SS, et al. Patient preferences for treatment of acute bacterial skin and skin structure infections in the emergency department. BMC Health Serv Res. 2018;18(1):932.
Author information
Authors and Affiliations
Contributions
All authors had a role in study design, conceiving and writing the manuscript. According to the guidelines of the International Committee of Medical Journal Editors (ICMJE, http://www.icmje.org) all authors met the criteria for authorship and no deserving authors have been omitted.
Corresponding author
Ethics declarations
Funding
No funding was received to complete this study, nor in the writing of the manuscript. This article is part of a supplement wholly funded by Melinta Therapeutics.
Conflict of Interest
JR is a speaker for Melinta Therapeutics. TL is a speaker and consultant for Melinta Therapeutics. CW and EN have no disclosures.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the hospital Institutional Review Board.
Additional information
Digital features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12221690.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Whittaker, C., Lodise, T.P., Nhan, E. et al. Expediting Discharge in Hospitalized, Adult Patients with Skin and Soft Tissue Infections Who Received Empiric Vancomycin Therapy with Oritavancin: Description of Findings from an Institutional Pathway. Drugs - Real World Outcomes 7 (Suppl 1), 30–35 (2020). https://doi.org/10.1007/s40801-020-00196-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-020-00196-6