Abstract
Background
To understand the extent to which a large-scale healthcare claims database (DB) captures the safety profile of eribulin mesylate (Halaven®, Eisai Co., Ltd., Japan), we compared patient characteristics, drug use, and adverse events (AEs) between data for patients treated with eribulin retrieved from a DB and data for metastatic breast cancer patients from a conventional prospective post-marketing surveillance (PMS).
Methods
We descriptively summarized patient characteristics and AEs of 551 and 951 patients retrieved from DB and PMS, respectively, during 2011‒2013. Using 2814 patient data from the DB during 2011‒2016, the drug use and AE incidence over time were assessed.
Results
In both datasets, 99.8% were females, and the mean age was 57.8 ± 10.7 years. The mean number of eribulin administration was 11.1 ± 10.9 and 10.1 ± 7.8 in DB and PMS, respectively. Although, overall, the difference in AE incidence between the two datasets was moderate, gaps were larger for nausea (DB: 73.32% vs. PMS: 15.77%), neutropenia (20.87% vs. 66.67%), stomatitis (37.39% vs. 10.94%), and alopecia (0.36% vs. 12.09%). During 2011‒2016, the observed incidence of anemia or pyrexia significantly decreased (trend test, p = 0.0009 for both).
Conclusion
Generally, patient characteristics, drug use, and AE incidence between the DB and PMS were comparable; however, AEs such as neutropenia may require defining based on the laboratory data to achieve more comparable results in DBs. Besides the usefulness of healthcare claims DBs for long-term assessments, they may also serve as a good complementary to PMS in the pharmacovigilance of eribulin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overall, the data for demographic and clinical characteristics for patients treated with eribulin were comparable between the database (DB) and post-marketing surveillance (PMS). |
Overall, the differences in adverse event (AE) incidence between the data retrieved from the DB and PMS were moderate (< 10%); however, larger gaps were observed for nausea, neutropenia, stomatitis, and alopecia. |
The study suggests that healthcare claims DBs could be used for the pharmacovigilance of eribulin as a complementary study to conventional PMS. |
1 Introduction
In the past couple of decades, pharmacoepidemiology has gained significant momentum, globally, for assessing the long-term safety and efficacy of a new medication in the post-approval setting. The pharmacoepidemiological investigations include two types: prospective post-marketing surveillance (PMS) studies and retrospective database (DB) studies. In clinical practice, the PMS of a novel therapy or drug is mandated by the Ministry of Health, Labor, and Welfare (MHLW) in Japan for distribution by pharmaceutical companies. Notably, conventional PMS studies remain at the center stage of these types of studies. The real-world PMS studies are mostly prospective studies that are conducted in compliance with a ministerial ordinance of good post-marketing study practice (GPSP) in Japan. Conventional PMS studies, however, are more advantageous in monitoring the safety event in a wider patient population within the clinical setting than in the clinical trial phases, because of the strict inclusion and exclusion criteria employed. In addition, prospectively collecting data is also essential in assessing the data from multiple healthcare institutions [1, 2]. Higher cost and resource utilization, limited plausible follow-up times, selection bias in both the study site and patient recruitment, and limited generalizability inherited from PMS studies cannot be avoided [1, 3].
An amendment was made to GPSP by the Pharmaceuticals and Medical Devices Agency (PMDA), which became effective in April of 2018 [4]. This amendment allowed the use of an existing DB to evaluate the safety and efficacy of a new pharmaceutical agent during the post-marketing phase. At present, the DBs available for pharmacoepidemiology in Japan can be broadly classified as administrative claims, hospital-based care information and electronic medical record, and disease-based registration [5]. Hence, DBs should be selected based on the purposes of the original data collection and individual PMS study, as recommended in the guidelines for the DB research [6, 7]. Considering the definitions of population, events [e.g., adverse events (AEs)], and time covered are important when a DB is used as an alternative data source. This is because the data commonly included in DBs are limited (e.g., disease name, drug prescription, and the treatment and test performed). The use of DBs as an alternative source functions as an advantage, since DB enables the analysis of large-scale data, all of which are based on the real world.
Very few studies [8, 9] have been reported that examined the possibility of using a DB as an alternative to the conventional PMS studies in Japan. Hence, the objective of this study was to understand the extent to which the healthcare claims DB captures the safety profile of patients prescribed eribulin mesylate (Halaven®, Eisai Co., Ltd., Japan) through the comparison of patient characteristics, drug use, and adverse event (AE) incidence between the data extracted from a large-scale DB and data extracted from a conventional prospective PMS of metastatic breast cancer patients treated with eribulin. The changes and tendencies of drug use and AE incidence, over time, were also assessed using the DB data.
2 Methods
2.1 Data Source
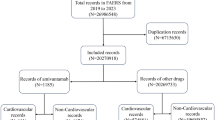
In this comparative study, the data for patients who were treated with eribulin for breast cancer were extracted from the DB and PMS. The DB data were extracted from a hospital-based claims DB, constructed by Medical Data Vision Co., Ltd. (MDV; Tokyo, Japan). As of April 2018, this DB included the data for approximately 1.8 million patients, collected from 294 hospitals with advanced treatment capabilities; these hospitals employed diagnosis procedure combination/per diem payment system (DPC/PDPS). Patient information including age, sex, diagnosis, prescription, and medical practice (e.g., treatment, operation, and test) were included in the data extracted from the DB. The PMS data were extracted from a conventional 1-year prospective study conducted by Eisai Co., Ltd. from 2011 to 2013 [10], where 968 patients with inoperable or recurrent breast cancer receiving treatment with eribulin for the first time were enrolled at 325 centers (ClinicalTrials.gov ID: NCT01463891). Results of this PMS study have been reported elsewhere [10].
To compare the safety profile of the DB with that of the PMS, the data for 551 patients who were treated with eribulin during 2011–2013 were retrieved from the DB, while data for 951 patients included in the safety analysis were retrieved from the PMS. For the assessment of drug use and AE incidence over time, 2814 patient data were retrieved from the DB for treatments that occurred between 2011 and 2016.
2.2 Definition
In DB, the following 14 AEs recorded between the starting and ending months of eribulin administration were identified according to the Japanese disease name codes: alopecia, anemia, decreased appetite, dysgeusia, febrile neutropenia, interstitial pneumonia, leukopenia, lymphopenia, malaise, nausea, neutropenia, peripheral neuropathy, pyrexia, and stomatitis. The Japanese disease name codes along with their corresponding International Classification of Diseases and Related Health Problems, 10th revision (ICD-10) codes for the defined AEs are shown in Table 1.
For the concomitant hormone therapy, data on progestins, luteinizing hormone-releasing hormone (LHRH) analogs, anti-estrogen, aromatase inhibitors, and female and male hormones used at least once during eribulin administration were identified in the DB.
The AEs in PMS were categorized according to the Japanese version of the Medical Dictionary for Regulatory Activities (version 16.1).
2.3 Statistical Analysis
To compare the DB and PMS data, patient characteristics, drug use, and AE incidence were descriptively analyzed upon retrieval from the DB (n = 551) and PMS (n = 951); data retrieved were for patients treated from 2011 to 2013. To assess the trend in drug use and AE incidence during the 2011–2016 period, the number of eribulin administrations and concomitant hormone therapy with eribulin, and the AE incidence in the DB were descriptively summarized (n = 2814 for all). Subsequently, a linear trend contrast, based on the ANOVA model, was performed for the tendency to administer eribulin. The Cochran–Armitage trend test was performed for the trend of occurrence for concomitant hormone therapy and AE incidence. Owing to the limited availability of data, the number of eribulin administrations was assessed from 2011 to 2015. p < 0.05 was considered statistically significant. For the descriptive analysis of the PMS data, SAS release 9.1.3 was used, and SAS release 9.4 used for the rest of the analysis.
3 Results
3.1 Patient Characteristics Collected from the Database and Post-Marketing Surveillance Data
Overall, the patient characteristics were comparable between the DB and PMS data (Table 2). Females accounted for 99.8% of the patients, with a mean ± standard deviation (SD) age of 57.8 ± 10.7 years in both datasets. The mean number of eribulin administrations was 11.1 ± 10.9 and 10.1 ± 7.8 based on the data from the DB and PMS, respectively. However, the proportion of patients with human epidermal growth factor receptor type 2-positive (HER2/neu) and those with triple negative was higher in the data from the DB (24.0% and 29.0%, respectively) compared to data from the PMS (18.4% for both). More patients, based on the data from the DB (16.9%), received concomitant chemotherapy; however, patients in this dataset received less concomitant hormone therapy with eribulin (10.9%) when compared to those from the PMS (7.7% and 16.3% for concomitant chemotherapy and concomitant hormone therapy, respectively).
3.2 Hematologic and Non-Hematologic Adverse Events (AEs)
The most frequently reported hematologic or non-hematologic AE was nausea (73.32%), followed by stomatitis (37.39%), neutropenia (20.87%), and peripheral neuropathy (16.33%) in the DB. However, neutropenia (66.67%), followed by peripheral neuropathy (16.93%), nausea (15.77%), and alopecia (12.09%) were frequently reported in the PMS (Table 3). Overall, the difference in AE incidence between the DB and PMS data was moderate, with a gap < 10%. This gap was, however, smaller in interstitial pneumonia (0.91% and 0.74% in DB and PMS, respectively), peripheral neuropathy (16.33% and 16.93%, respectively), and anemia (8.53% and 7.26%, respectively). Contrarily, the largest gap in AE incidence was observed for nausea (73.32% and 15.77% in the DB and PMS, respectively), followed by neutropenia (20.87% and 66.67%, respectively), stomatitis (37.39% and 10.94%, respectively), and alopecia (0.36% and 12.09%, respectively).
3.3 Changes and Trends of Drug Use and AE Incidence From 2011 to 2016
There was a significant increase in the mean number of eribulin administrations, from 10.14 in 2011 to 12.37 in 2015 (a linear trend contrast, p = 0.0246) (Fig. 1a). Contrarily, the proportion of patients receiving concomitant hormone therapy with eribulin significantly decreased from 13.04% to 6.88% between 2011 and 2016 (Cochran-Armitage trend test, p = 0.0141) (Fig. 1b). With regard to the incidence of AEs, although the incidence of peripheral neuropathy was almost constant throughout the period, the incidences of AEs such as anemia and pyrexia showed a significant decrease (p = 0.0009 for both) (Fig. 1c). A statistically significant trend was not observed for the remaining AEs.
Changes and trends of drug use and adverse event (AE) incidence from 2011 to 2016 (n = 2814). a Number of eribulin administrations from 2011 to 2015. Vertical bars indicate standard errors. Data from 2011 to 2015 were used for these analyses, owing to the limited data available from the database (DB). *A linear trend contrast based on ANOVA model. b Concomitant hormone therapy use. *Cochran–Armitage trend test. c AE incidence. *Cochran–Armitage trend test
4 Discussion
In this comparative study, we examined the extent to which the healthcare DB claims captured the safety profile of eribulin through the comparison of patient characteristics, status of drug use, and AE incidence in patients treated with eribulin with the previously conducted PMS of eribulin in Japan [10]. The results demonstrated that, generally, the patient demographics, clinical characteristics, status of eribulin use, concomitant therapy use, and AE incidence based on the data from the DB and PMS were comparable in the pharmacoepidemiological investigations of eribulin.
For most of the AEs observed during eribulin administration, the occurrence was comparable based on the data from the DB and PMS. This was particularly notable for interstitial pneumonia, peripheral neuropathy, and anemia. The occurrence of AEs such as nausea, neutropenia, stomatitis, and alopecia varied between the two datasets. Overall, the AEs that were consistent between the DB and PMS data were symptomatic and may have required hospital treatment. However, more frequent nausea and stomatitis and less frequent alopecia were reported in the DB than the PMS. Such differences in AE incidence may have been attributable to the differences in the treatment of patients between the two datasets. For example, more patients in the DB received concomitant chemotherapy than in those in the PMS data (16.9% vs. 7.7%) and a slightly higher dose of initial eribulin (1.4 mg/m2 vs. 1.3 mg/m2). The differences in AE incidence also indicate that the diagnosis code for nausea and stomatitis were entered in the DB, for reimbursement purposes, indicating that treatments were required or prophylactic therapy was administered. AEs such as alopecia may have been less frequently entered into the DB when no treatment was required. Patients with moderate-to-severe but asymptomatic neutropenia may not have been diagnosed unless a blood test was performed. In addition, even if a patient was diagnosed with neutropenia, treatment may not have been administered unless required. In contrast, in the PMS data, neutropenia was determined based on the neutrophil count collected for the study; thus, regardless of symptoms, any occurrences of neutropenia were captured. Using the limited laboratory data available in the DB (n = 79), we further defined neutropenia as neutrophils < 1000 to 500/mm3 (Grade 3) or < 500/mm3 (Grade 4) in accordance with the Common Terminology Criteria for Adverse Events v3.0, when such an event had been recorded at least once, between the day after the start of eribulin administration and 14 days after the end of eribulin administration. Subsequently, the result was then compared to Grade 3 or 4 neutropenia from the PMS data, and small discrepancies of 43.04% and 59.83%, respectively, between the DB and PMS data, were observed. Based on these findings, PMS may be more appropriate for the collection of data on symptoms, including those that may not necessarily require treatment. To precisely capture AEs such as neutropenia, the definition of events based on their severity should also be carefully considered when using the MDV DB. As recommended in the guidelines for database research [6, 7], the use of an appropriate DB based on the purpose, with the original objective of the data source in mind, is essential. A health claims database may be particularly suitable for capturing events leading to reimbursements, but not for events affecting a patient’s quality of life. Thus, the decision on whether to conduct a PMS or to use an existing DB, based on the safety events to be assessed, is important.
In this study, use of the DB enabled assessments of the trends in drug use and AE incidence; we observed an increasing trend in the number of eribulin administrations and a decreasing trend in the use of concomitant hormone therapy with eribulin and AEs, such as anemia and pyrexia, over 5 or 6 years. The increased number of eribulin administrations and the decreased incidence of AEs may be because physicians have become more capable in the management adverse drug reactions, including the prevention of AEs. Furthermore, the use of concomitant hormone therapy may have decreased owing to the accumulated experience obtained by physicians of the administration of eribulin as a single agent. There may also have been a shift in the type of patient administered eribulin, from patients with hormone receptor-positive breast cancer to those without (patients with triple-negative status account for 18.4% of patients in the early post-marketing phase of eribulin [10], and patients with triple-negative status account for 25.1% of patients among those with HER2-negative cancer in the subsequent post-marketing phase [11]). Overall, the experience obtained by physicians of the administration of eribulin may be a common reason behind these three trends in our results.
Although moderately comparable results can be obtained from both the MDV DB and PMS data, the PMS is associated with an increased time and cost burden [3, 7] as previously stated. PMS and DB are non-interventional studies, and conducting a PMS, which has a prospective design, requires site contract, study monitoring, inquiries, and data entry by competent persons. There is a high cost and time requirement associated with this, even when the observational period is only a few months. Studies using a DB, which are of a retrospective design, take a shorter time to complete, and are relatively inexpensive. Biases are another issue in PMS. PMS exhibits strength in “all-case surveillance”; however, the data are not necessarily entered on time at the study sites, which results in under-reporting (recall bias) [12]. PMS does not necessarily cover “all cases” when the patients and study sites are selected by physicians and sponsoring pharmaceutical companies, respectively (selection bias). If the drug safety can be evaluated by using the existing secondary data, the study can be conducted in a cost- and time-efficient manner, and the results obtained may be less biased. This would lead to the acquisition of data that are more organic and reflective of the real-world perspective. In addition, a DB-based study enables the assessment of long-term trends, with the retention of a large sample size. The PMS-based study, in contrast, has limits on both the time period and sample size. The DB-based studies have the following inherent limitation that makes it impossible to detect events pertaining to prophylaxis: limited availability of information to define target events. As these studies are, for the most part, retrospective, the time lag that occurs before the collected data are reflected in the database is another potential limitation. However, the biases vary depending on the target disease and drug.
Post-marketing safety assessments using DB and PMS data have both the aforementioned advantages and disadvantages. Our results suggest that MDV DB, instead of conducting PMS, can be used for the safety assessment of eribulin, especially for events that have triggered reimbursement. In addition, it should be noted that some AEs, such as neutropenia, alopecia, nausea, and stomatitis, may be under- or over-reported in the MDV DB, implying that both types of post-marketing studies are complementary in nature.
The coverage of patients in the DB depends on the target disease. We targeted patients with breast cancer treated with eribulin. The number of shipped eribulin vials from Eisai Co. Ltd. depicts a fair consistency with the number of administered eribulin included in the DB. Our findings can therefore be generalized to patients treated with eribulin in Japan during the study period.
Our study is associated with a few limitations. Selection bias was inevitable both in the PMS and the DB used for our study. As previously mentioned, the participating sites and the patients participating in the study for PMS were selected by the pharmaceutical company and the attending physicians, respectively. For DB, all data included in the MDV DB were provided by hospitals with advanced medical capabilities, where DPC/PDPS is employed. The results also required careful interpretation, and data availability was limited in the DB. The AEs were also defined based on the disease name entered as part of the data required for reimbursement purposes, serving as another limitation. In addition, the acquired results may vary depending on the definition employed, and prophylactic treatments could not be differentiated in the DB.
5 Conclusion
The results of this study indicated that, generally, patient characteristics, status of drug use, and the AE incidence in patients treated with eribulin were comparable in retrospective DB-based studies and prospective PMS-based studies, when under- or over-reporting of AEs such as neutropenia, alopecia, nausea, and stomatitis was considered. The results from DB-based studies also showed that the changes and trends in the status of drug use and AE incidence over time can be described by using the data. This suggests that the healthcare claims DBs could be used for the pharmacovigilance of eribulin as a complementary study to conventional PMS. In addition, as DBs cover a longer period than conventional PMS, DBs could be useful for the assessment of long-term safety, as well as the status of drug use, based on the annual trends and changes in AEs and drugs, which may be difficult to assess with PMS.
References
The Japanese Ministry of Health, Labor, and Welfare. Basic concept for medical information database usage. June 9, 2017. https://www.pmda.go.jp/files/000218531.pdf. Accessed 13 Sep 2018 (in Japanese).
Suvarna V. Phase IV of drug development. Perspect Clin Res. 2010;1(2):57–60.
Narukawa M. Research on the situation and implications of the post-marketing all-case surveillance study in Japan–considerations based on a questionnaire survey. Reg Sci Med Prod. 2014;4(3):199–206.
The Japanese Ministry of Health, Labor, and Welfare. Ministerial ordinance on standards for investigation and testing after manufacture and sales of pharmaceuticals. October 26, 2017. https://www.pmda.go.jp/files/000220721.pdf. Accessed 17 Aug 2018 (in Japanese).
Japanese Society for Pharmacoepidemiology. Survey of Japanese databases in Japan available for clinical/pharmaco- epidemiology. 2017. http://www.jspe.jp/mt-static/FileUpload/files/JSPE_DB_TF_E.pdf. Accessed 18 Aug 2018.
Hall GC, Sauer B, Bourke A, Brown JS, Reynolds MW, LoCasale R. Guidelines for good database selection and use in pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2012;21(1):1–10.
Pharmaceuticals and Medical Devices Agency. Guidelines for pharmacoepidemiological studies using health information databases for drug safety assessments. https://www.pmda.go.jp/safety/surveillance-analysis/0032.html. Accessed 13 Sep 2018 (in Japanese).
Katada H, Yukawa N, Urushihara H, Tanaka S, Mimori T, Kawakami K. Prescription patterns and trends in anti-rheumatic drug use based on a large-scale claims database in Japan. Clin Rheumatol. 2015;34(5):949–56.
Hirano Y, Asami Y, Kuribayashi K, Kitazaki S, Yamamoto Y, Fujimoto Y. Possibility of database research as a means of pharmacovigilance in japan based on a comparison with sertraline post-marketing surveillance. Value Health Reg Issues. 2018;15:1–5.
Watanabe J, Ito Y, Ohsumi S, Mizutani M, Tashiro H, Sakurai K, Takahashi M, Saito T, Tsurutani J, Mukai H, Yoshinami T, Takao S, Yamamoto Y, Matsuoka T, Iwase H, Iwata H, Nakamura S, Saeki T. Safety and effectiveness of eribulin in Japanese patients with locally advanced or metastatic breast cancer: a post-marketing observational study. Invest New Drugs. 2017;35(6):791–9.
Tsurutani J, Sakata Y, Matsuoka T. Chemotherapy-induced peripheral neuropathy in breast cancer patients treated with eribulin: interim data from a post-marketing observational study. Breast Cancer. 2019;26:235–43.
Miyazaki M, Shimodera M. For a change of pharmacovigilance activities, from a viewpoint of a pharmaceutical company. Jpn J Pharmacoepidemiol. 2015;20(1):17–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eisai Co. Ltd., Tokyo, Japan.
Conflict of interest
YS, HT, TM, SO, and MI are/were employees of Eisai Co. Ltd., Tokyo, Japan. TK and TT are employees of Clinical Study Support, Inc., Nagoya, Japan, and were involved in the study based on the contract with Eisai Co. Ltd.
Ethical approval and informed consent
For this retrospective database study, all data for analysis were extracted from a pre-existing anonymized database. For the usage of de-identified secondary data, ethical approval and informed consent do not apply, according to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Data availability
The MDV data that support the findings of this study are available from Medical Data Vision Co., Ltd. (MDV; Tokyo, Japan) upon purchase. Restrictions do apply to the availability of these data, and are thus unavailable to the public. For inquiries on the dataset analyzed in this study, please contact MDV (https://www.mdv.co.jp/). The PMS data that support the findings of this study are available upon request from the corresponding author, YS. The data are not publicly available due to the absence of consent from the participating institutions.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sakata, Y., Matsuoka, T., Ohashi, S. et al. Use of a Healthcare Claims Database for Post-Marketing Safety Assessments of Eribulin in Japan: A Comparative Assessment with a Prospective Post-Marketing Surveillance Study. Drugs - Real World Outcomes 6, 27–35 (2019). https://doi.org/10.1007/s40801-019-0150-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-019-0150-8