Abstract
Co-thermal chemical conversion of coal and biomass is one of the important ways to realize efficient and clean utilization of coal. In this study, a typical Ningdong coal-Yangchangwan bituminous coal and cow manure were used to study the synergistic effect of intrinsic alkali, alkaline earth metals (AAEM) and organic matter on the co-gasification of coal and biomass by thermogravimetry analyzer (TG). The results showed that AAEM had obvious synergistic promotion effect on the gasification of a bituminous coal-cow manure mixture in the isothermal gasification (1000 ℃), whereas the organic matter will show the opposite effect on the process. To further investigate the effect of organic matter on the gasification process, the influence of organic matter on non-isothermal (25-1000 ℃) gasification reaction was investigated with heating rate of 10 ℃ /min, the kinetic parameters of the gasification reaction were obtained by Coats-Redfern method. The increase of biomass mass fraction in the sample facilitates the migration of alkali metals from the material to the solid phase. The possible mechanism of the synergistic effect of intrinsic AAEM/organic matter on the co-gasification process was proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
China’s energy and industrial structures are bound to be adjusted and optimized in the context of the “double carbon” target. However, judging from the results of the BP World Energy Statistics Yearbook 2021, it is difficult for China to shake the dominant position of coal utilization in the short term (Li et al. 2022). The traditional coal combustion utilization is inefficient and polluting. Seriously, and the utilization of biomass, the only renewable carbon source in the world, is also low, according to statistics, billions of tons of agricultural and forestry waste every year (Qin et al. 2016). Co-gasification technology of coal and biomass can not only solve the problem of massive waste of biomass resources, but also alleviate the greenhouse effect caused by the process of coal utilization (Mosqueda et al. 2019; Cong et al. 2017; Che et al. 2016). Therefore, Co-gasification technology of coal and biomass provides a prominent technological pathway to realize clean and efficient utilization of both carbon containing resources, which is an important initiative to respond to the “double carbon target”.
The process of preparing syngas (CO & H2) using both coal and biomass as gasification feedstock with a gasification agent (H2O/CO2/O2) and under certain conditions of temperature and pressure is called co-gasification of coal and biomass (Guo et al. 2018; Zemp et al. 2019). Up to now, many researches regarding the co-gasification of coal and biomass have been reported. From the point of view of operation parameters, Guo et al. (2017) researched the influence of temperature on the co-gasification reactivity of coal and biomass with Zhundong lignite and pine wood chips as feedstock. Terry et al. (2002) researched the impact of pressure on the char structure and found that pressure significantly affects the volatile matter yield and coal expansion rate during.
The de-volatilization process, which affects the structure and morphology of the produced char. Meesri and Moghtaderi (2002) studied the pyrolysis behavior of woody biomass/coal blends at low and high heating rate conditions from 200 to 1400 °C. The impact of different mass fractions of coal and biomass on product distribution and composition by using corn cob and rice straw, respectively (Ke et al. 2019; Wei et al. 2017). Ren et al. (2012) and Naidu et al. (2016) also found that increasing the biomass blending ratio is conducive to enhancing the synergistic promotion effect of co-gasification. Jeong et al. (2015) also pointed out that the synergistic promotion effect of co-gasification increases with the increase of biomass blending ratio and gasification temperature. Considering the feedstock type, Mishra et al. (2018) concluded that low-rank coal has higher gasification reactivity than high-rank coal through research because the concentration of active sites in the matrix of low-rank coal is higher. Zhang et al. (2016) examined the impact of coal type and biomass type on the synergistic behavior of co-gasification by using three types of coal (high ash lignite, low ash lignite, and bituminous coal) and four types of biomass (redwood, soybean straw, orange peel, and peanut shells). With respect to the effect of volatile fractions (AAEM and organic matter) on coal/biomass co-gasification, Li et al. (2020) studied the effect of biomass ash on pyrolysis process and char gasification reactivity of typical anthracite with two typical biomass ash additives: corn stalk ash (high K-Na, low Si) and poplar sawdust ash (high K-Ca, high Si), it was found that the addition of biomass ash slowed down the graphitization of anthracite during pyrolysis. Li et al. (2017) explored the impact of AAEM content in coal on the gasification process of coal char using Mongolia Baiyinhua lignite. Quyn et al. (2002) investigated the Na migration mechanism during Na-catalyzed coal gasification of coal and biomass using physical impregnation of NaCl into Victorian lignite. Li et al. (2020) investigated the deposition of alkali metals in coal char and the volatile fraction-coal char interactions during the co-gasification process of corn stover with anthracite coal. Shi et al. (2018) investigated the interaction of volatile fraction with volatile fraction by pyrolysis of two solid organic compounds (including one coal and four pure organic compounds) in programmed heating mode using crucibles with or without vertical baffles.
From the material composition point of view, intrinsic AAEM and organic volatile are the main reactive materials produced by the co-pyrolysis process of various carbonaceous materials, and the microstructural changes of the samples are centered on the interaction between these two components, but most of the studies investigating the influence of the synergistic behavior of coal-biomass co-gasification either do not differentiate the reactive sources (AAEM and organic volatile) to explore the synergistic effect The impact of alkali metals on the synergistic behavior has been investigated by loading, while almost no researches have been reported on the influencet of single intrinsic AAEM/organic matter on the co-gasification of coal and biomass. Co-gasification reaction of coal and biomass produces a large amount of organic matter under high temperatures, such as tar. The interaction between organic matter and char causes a number of problems, such as extremely low feedstock gasification efficiency and energy waste, which is also a major technical problem in the industrialization of coal and biomass co-gasification (Bates et al. 2017; Hughes 2000). If we can find the intrinsic mechanism of the effect of intrinsic organic matter on the co-gasification of coal and biomass, and then adopt a Efficient utilization method, it is of great value to improve the elemental and energy utilization.
Therefore, in this study, Ningdong Coal-Yangchangwan bituminous coal and cow manure were used as gasification samples, and the thermogravimetric analyzer was used to determine their gasification reaction activities under different treatment conditions, and then to analyze the synergistic effects of each of the intrinsic AAEM and organic matter on the co-gasification of coal and biomass, in order to provide basis for the equipment configuration and optimization of the operating parameters of industrial gasifiers using Ningdong Coal as feedstock.
2 Experimental methods and materials
2.1 Experimental materials
In this study, Yangchangwan bituminous coal (YCW) and cow manure (CM) were used as co-gasification raw materials. First, YCW and air-dried CM were crushed and screened to 100–120 μm and 100–150 μm respectively, then YCW and CM are placed in a thermostatic drying oven at 105 ℃ for 12 h. The elemental analyzer (Vario MACRO) and coal analyzer (5E-MACШ) were used to analyze the experimental samples for industrial analysis, elemental analysis and ash composition, and the result of the analysis are presented in Tables 1 and 2.
2.2 Experimental sample preparation
The main goal of this investigation is to explore the impact of the alkali and alkaline earth metals (AAEM, K, Ca and Na, etc.) and organic matter on the gasification reaction activity of bituminous coal and cow manure by using a thermogravimetric analyzer. Therefore, a single variable control approach was required to design the experiments. To investigate the influence of AAEM on the synergistic effect of co-gasification, the sample needs to de-volatile fraction with a tube furnace to obtain char. For exploring the impact of organic matter on the synergistic effect of co-gasification, the active AAEM in the feedstock needs to be removed by water washing, and there are four occurrence forms of AAEM in coal/biomass, acid-soluble, ion-exchange, water-soluble, and residue states (Chen et al. 2016), where AAEM in the water-soluble state (mainly including inorganic salts) and ion-exchange state has a catalytic effect on gasification reaction (Ma et al. 2022). In this study, we mainly exclude the effect of the water-soluble state of AAEM on the gasification reaction, and the water-soluble is generally alkali metals in the occurrence of inorganic salts, such as KCl, K2CO3 and GaCl2. After the samples of both were prepared, the gasification reaction activity of the samples was investigated separately using a thermogravimetric analyzer.
2.2.1 Preparation of char
A horizontal fixed bed reactor was utilized to prepare char. The experimental principle and system diagram is shown in Fig. 1. The process of preparing char was described below: about 10 g of sample was placed in an alumina ceramic crucible, and then the crucible is placed in quartz tubes in horizontal fixed-bed reactors. The reactor was heated to 1000 °C by programmed heating, and 200 mL/min of N2 was continuously offered during heating. When the temperature reached 1000 °C, the sample continued to stay for 30 min to remove most of the volatiles. The cow manure char (CMP) and Yangchangwan bituminous coal char (YCWP) were obtained respectively when the reactor had cooled to room temperature, the CMP and YCWP were thoroughly mechanically mixed according to the mass ratios of 3:1, 1:1, and 1:3, respectively, and the corresponding mixed char samples were marked as CMP: YCWP (3:1), CMP: YCWP (1:1), and CMP: YCWP (1:3).
2.2.2 Preparation of de-AAEM gasification samples
CM and YCW were placed in a beaker with deionized water mass fraction ratio of 1:10, respectively, and stirred under a magnetic stirrer for 12 h. Then they were repeatedly rinsed with deionized water and filtered by extraction, and the collected samples were dried at 105 ℃ for 12 h, then the solid-liquid mixture was filtered and rinsed repeatedly with deionized water, and the Na, K, Ga, and Mg content of filtrate was determined by the inductively coupled plasma emission spectrometer (ICP-OES). The measurements were repeated three times, and the water-soluble AAEM in the samples was considered to be completely removed if the content of four elements were all zero or nearly zero, and the residue is dried to obtain water-washed cow manure (wCM) and water-washed Yangchangwan bituminous coal (wYCW), respectively. wCM and wYCW were were fully mechanically mixed according to the mass ratios of 3:1, 1:1 and 1:3, and the corresponding mixed samples were marked as wCM: wYCW (3:1), wCM: wYCW (1:1) and wCM: wYCW (1:3).
2.3 Gasification reactivity test
Isothermal gasification (1000 ℃) and non-isothermal gasification (25-1000 ℃) of the samples under CO2 atmosphere were investigated using a thermogravimetric analyzer (NETZSCH STA 449 F3). For each TGA gasification experiment, about of 10 mg char was placed in an Al2O3 crucible and then placed in a TGA furnace. For the isothermal gasification reaction, the carrier gas was high-purity Ar (99.99%) at a flow rate of 200mL/min., and the reactor atmosphere was replaced with CO2 at a volume flow rate of 120 mL/min to eliminate the effect of external air diffusion on the gasification when the gasification reaction was complete. The experiment was stopped when the gasification reaction was complete, the weight loss curve no longer changed, and the data for weight changes could be obtained from the thermogravimetric analysis when the weight loss curve tended to be constant. For non-isothermal gasification, the heating furnace was purged with Ar for 20 min before starting the heating procedure, and then CO2 with a volume flow rate of 120 mL/min was offered, and the rate of heating is 10 ℃/min for non-isothermal gasification, and the temperature reached 1000 °C, and the sample continued to be kept for 30 min to stop the gasification experiment.
The carbon conversions X of the sample gasification can be utilized with the Eq. (1) (María et al. 2015):
In order to quantitatively characterize the gasification reactivity of the samples, the reactivity index R0.5 and R0.9 were applied (Ma et al. 2022).
To examine the synergistic behavior of the co-gasification process, a synergistic factor A was introduced (He et al. 2017) Eq. (2).
The carbon conversion (Xcal) calculated during the co-gasification of mixed samples can be expressed by Eq. (3) (Ren et al. 2012), assuming that the samples are not subjected to any interactions during the co-gasification process.
2.4 Reaction kinetic analysis
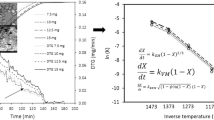
A non-isothermal method was used to study of gasification reaction properties of de-AAEM sample-CO2 by using a thermogravimetric analyzer at a heating rate of 10 ℃ /min. The coal gasification process is a heterogeneous gas-solid reaction. According to the conservation of mass, Arrehenius equation and the derivative method, he apparent reaction of the gasification process is: \(\frac{{\text{d}}{x}}{{\text{d}}{t}}=k\left(T\right){f}_{\left(a\right)}\), for simple reactions \({f}_{\left(a\right)}={\left(1-x\right)}^{n}\). The kinetic equation is generally expressed by the following Eq. (4):
Where k is the chemical reaction rate constant as a function of temperature T, n is the total number of reaction order, X is the carbon conversion, and T is the absolute temperature. The relationship between k and T can be shown by the Arrhenius Eq. (5):
Where Ea is the reaction activation energy, A stands for the pre-exponential factors, R represents the gas constant, and T is the absolute temperature of the reaction.
The rate of heating:
The coal gasification process is extremely complex, kinetic models are different for different coals or under different gasification conditions, such as the particle size, volatile fraction and heating rate of coal char. In gas-solid non-homogeneous reactions, there are more methods to obtain kinetic parameters by non-isothermal thermogravimetry, and the more classical ones are: Ozawa method, Kissinger method, DAEM, Coats-Redfern method, the first two apply to the combined heating rate method and the latter two to the single heating rate method (Li et al. 2001; Luo et al. 2006; Zhou et al. 2006). The Coats-Redfern method allows the whole reaction process to be divided into different stages for the calculation of the corresponding activation energies and pre-exponential factors.
Combining Eqs. (4), (5), (6), and using the Coats-Redfern method.
n ≠ 1
n = 1:
For the general reaction temperature interval, most \(\frac{{Ea}}{{RT}} \gg 1\)(Chen et al. 2016).
3 Results and discussion
3.1 Reactivity of sample gasification
3.1.1 Effect of AAEM
The variation of gasification carbon conversion with time for the char at 1000 ℃ is shown in Fig. 2. It is obvious that the experimental gasification curve of CMP is always higher than the experimental gasification reaction curve of YCWP during the whole gasification process, which indicates that the gasification reaction activity of CMP is better than that of YCWP. The CMP-YCWPcal is calculated by weighting of the gasification curve obtained from coal char gasification alone and the gasification curve obtained from cow manure char gasification alone. In the CMP-YCWP co-gasification process, the experimental reaction curves were always higher than the corresponding calculated reaction curves, and CMP-YCWP(3:1)exp > CMP-YCWP(1:1)exp > CMP-YCWP(1:3)exp, the larger the mass fraction of biomass in the mixed char sample the more active the gasification reaction is. The variation of dx/dt with carbon conversion of the mixed sample also verifies this point well, and the above reveal that there is a synergistic promotion in the co-gasification process between YCWP and CMP.
3.1.2 Effect of organic matter
The gasification curves of the samples wCM, wYCW and their mixed samples after the removal of the water-soluble AAEM at 1000 ℃ with respect to the gasification carbon conversion time are shown in Fig. 3. It can be fund that the gasification curve of wCM is always higher than that of wYCW throughout the gasification process, which indicates that the gasification reaction activity of wCM is stronger than that of wYCW. Samples with different mass fraction ratios of wCM and wYCW were used to investigate the influence of organic matter on the synergistic behavior of co-gasification of coal and biomass at different mass fraction, and the experimental gasification curve of the added cow manure sample was always lower than the corresponding calculated gasification reaction curves, which indicate that the presence of organic matter has a synergistic inhibitory effect on the co-gasification process. Especially, the experimental reaction gasification curves of wCM: wYCW(1:3) samples were not only lower than the calculated gasification reaction curves but even lower than the experimental reaction gasification curves of wYCW when the carbon conversion rate was lower than 0.7, which indicated that the organic matter seriously inhibited the co-gasification reaction. The experimental gasification curve was slightly higher than the calculated gasification curve when the carbon conversion exceeded 0.7, which indicated that the co-gasification reaction at this stage was again promoted synergistically. The dx/dt curves of the mixed samples with the carbon conversion consistent with the above phenomenon.
3.1.3 Synergistic behavior
The co-pyrolysis process is the first process that coal and biomass must undergo before the co-gasification reaction. This process includes the removal of volatile matter and AAEM migration behavior. Therefore, there will be complex interactions between coal and biomass, such as volatile-char interaction, intrinsic mineral-mineral interaction, and AAEM migration catalysis between coal and biomass particles. These factors will have an important impact on the physical and chemical structure of the sample, resulting in synergistic effects (synergistic promotion and synergistic inhibition) during the gasification process, leading to deviations in the theoretical co-gasification reaction activity (Wang et al. 2021; Guo et al. 2015; Yu et al. 2021). In order to quantify the reactivity of the co-gasification sample in the mid-term and overall reaction period, the gasification reaction activity index R0.5 and R0.9 were used. Figure 4 summarizes the gasification reaction activity index of coal and biomass blended in different proportions. The synergy factor A is used to quantify the changing characteristics of synergistic behavior in the co-gasification process, as shown in Fig. 5.
The R0.9 and R0.5 values of CMP are 3.77 and 3.99 times that of YCWP respectively. As the blending ratio of biomass char increases, both R0.9 and R0.5 show an increasing trend, indicating that the addition of biomass char is beneficial to improving the overall activity of the coal char gasification reaction. The order of reactivity of different gasified char is: CMP > YCWP-CMP (1:3) > YCWP-CMP (1:1) > YCWP-CMP (3:1) > YCWP. This is attributed to the fact that the active AAEM in biomass char promotes the cracking of the large aromatic ring structure of coal char into small ring structures during the gasification process, and at the same time helps to reduce the aromatic ring condensation reaction during the coal char gasification process (Tay et al. 2014). As the gasified carbon conversion rate increases, the A values of YCWP-CMP (1:3), YCWP-CMP (1:1) and YCWP-CMP (3:1) are respectively 0.007, 0.008 and 0.004 increased to 0.76, 0.58 and 0.47 when X from 10% to 90%. The continuous increase in the specific surface area during the coal char gasification reaction process leads to more developed pore structure characteristics, which enhances the retention capacity of active K from biomass char (Liu et al. 2015; Feng and Bhatia 2003), thus strengthening the catalytic co-gasification process and enabling continuous cooperative behavior enhance.
For the water-washed sample, the wYCW-wCM(1:3) sample proceeds with the co-gasification reaction. When X from 10% to 70%, the A value increases from − 0.34 to -0.05. When X from 80% to 90%, the A value increases from 0.07 increased to 0.13, and the A value changes of wYCW-wCM (1:1) and wYCW-wCM (3:1) showed the same trend, indicating that the synergistic inhibition effect of the co-gasification reaction process slowly decreased, and then X > 70% in the later stages of the reaction, this effect gradually transformed into enhanced synergistic promotion. This phenomenon is consistent with the changing trend of R0.5 and R0.9 of the water-washed co-gasification sample in Fig. 4. The experimental value of R0.5 is lower than the calculated value, while the experimental value of R0.9 is higher than the calculated value. According to research (Hu et al. 2017; Wall et al. 2002), organic volatiles produced during coal pyrolysis tend to be adsorbed on the in-situ char and further react with active components at high temperatures. Under the catalysis of inactive AAEM, it is specmolecules such as tar to generate PAHs or soot, this is the direct cause of the reduction in co-gasification that the interaction between volatile and char is more likely to cause the condensation and polymerization of organic ation reaction activity, and as the gasification reaction continues. As the PAHs on the surface of char particles are consumed, the inhibitory effect is weakened and the A value increases. In addition, although water washing can remove most of the water-soluble AAEM that plays an active catalytic role in the sample, a small amount of ion-exchanged sodium acetate and potassium acetate still remain. In the later stage of the reaction, X > 70% shows a limited synergistic promotion effect, and this synergistic effect was positively correlated with the biomass ratio.
3.2 Non-isothermal gasification reactivity
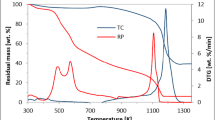
The mass loss (TG) and mass loss rate (DTG) distributions of the AAEM sample removed under non-isothermal gasification conditions at (25-1000 ℃) are given in Fig. 6. The DTG curve includes four mass loss peaks: The first stage is the increase from room temperature to 150 ℃, during which the moisture is mainly removed from the sample. The second stage was 150–790 ℃, which was mainly for removing the volatile from cow manure and the mixture of cow manure and coal, and it could be seen from the DTG curve that the weight loss rate increased with the increase of the mass fraction of cow manure in the sample. The third stage is 790–1000 ℃, which is the beginning of the gasification reaction. The fourth stage is 1000 ℃ constant temperature stage, in which the sample is completely gasified until the weight is no longer reduced. Figure 7 shows the carbon conversion curves of the water-washed co-gasification samples. The order of gasification activity is wYCW > wCM-wYCW(1:3) > wCM-wYCW(1:1) > wCM-wYCW(3:1) > wCM, while the experimental carbon conversion curves and theoretical carbon conversion curves completely overlap when the temperature is lower than 790 ℃, indicating that there is no synergistic behavior of gasification reaction in this temperature interval. From the above results, the volatile of organic matter does not participate in the CO2 gasification reaction at low temperature. When the gasification temperature exceeded 790 °C, the experimental carbon conversion curves of all mixed samples were lower than the calculated carbon conversion curves, which indicate a synergistic inhibition of the co-gasification process at this stage, the apparent reactivity of the gasification was lower than the theoretical value. It has been reported (Wijayanta et al. 2012) that this phenomenon occurs because the tar organic matter adhering to the surface of the char particles polymerizes to form PAHs, which preferentially react with the gasification agent CO2, resulting in lower reactivity of the co-gasification samples.
3.3 Kinetic parameters
Both biomass and coal are very complex substances in composition and structure, and their gasification reaction processes are correspondingly very complex. The parameters of kinetic calculation can be obtained by establishing the reaction kinetic model, which can better reflect the mass loss characteristics of the mixed samples. In this study, the co-gasification process of Yang Changwan bituminous coal and cow manure was investigated by using TGA. In the reaction temperature interval, Graphing 1/T using the \(\text{l}\,{\text{n}[\frac{{1 - {{(1 - X)}^{1 - n}}}}{{{T^2}(1 - X)}}}]\) shows more than a single linear trend. To facilitate the calculation of kinetic parameters, the gasification reaction process was divided into different reaction stages, the activation energy and pre-exponential factors of different reaction stage was obtained using the Coats-Redfern method. Table 3 shows the kinetic parameters obtained by fitting using the Coats-Redfern integral method. Where the mixed sample of \(\text{l}\,{\text{n}[\frac{{1 - {{(1 - X)}^{1 - n}}}}{{{T^2}(1 - X)}}}{]_\text{B}}\) is calculated according to Eq. (9), thereby yielding the calculated kinetic parameters.
B represents the mixture; C represents biomass; Y represents coal; and x represents the mass fraction ratio of biomass in the mixture sample.
It can be seen from Table 3 that in the entire reaction temperature range, the experimental activation energy of co-gasification reaction of wCM and wYCW is higher than the calculated value, and the higher the proportion of biomass in the mixed sample, the higher the activation energy of the corresponding gasification reaction. In the high temperature range, the experimental value of activation energy is not only higher than the calculated value, but the higher the proportion of biomass in the mixed sample, the greater the activation energy of the corresponding gasification reaction. For example, at 790–880 ℃, wCM-wYCW(1:3)exp, wCM-wYCW(1:1)exp and wCM-wYCW(3:1)exp correspond to activation energies of 75.21 kJ/mol, 68.78 kJ/mol, 62.34 kJ/mol, and these values are higher than the corresponding calculated values. The above evidence suggests that the organic matter hinders the co-gasification of coal and biomass, resulting in an increase in the activation energy of co-gasification, showing synergistic inhibitory effect. The change of conversion rate dx/dt with conversion rate X is shown in Fig. 8. It can be seen that the experimental conversion rate curve is always slightly lower than the calculated conversion rate curve, and the carbon conversion rate of biomass gasification is the fastest when the carbon conversion rate is low, and the carbon conversion rate of coal gasification is the smallest, and the increase of the proportional content of biomass in the mixed sample makes the conversion rate faster but still lower than the calculated conversion rate value. When the carbon conversion rate is high, the carbon conversion rate of biomass is the smallest, and the larger the proportion of biomass in the mixed sample, the smaller the carbon conversion rate of gasification, which corresponds well to the kinetic results analyzed in Table 3. Combined with the analysis on the effect of organic matter during isothermal gasification, this is due to the inhibitory effect of tar produced during coal/biomass co-gasification on its gasification reaction activity.
3.4 Mechanism of the synergistic effect of intrinsic AAEM/organic matter on the co-gasification process
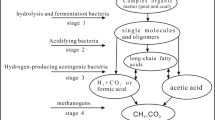
Synthesizing the research results of related investigation and the conclusions obtained from this study, in the co-gasification process of coal and biomass, the intrinsic AAEM and volatile fraction of organic matter are the main reactive substances produced by the co-pyrolysis process of various carbonaceous substrates, and the microstructural changes of the samples are closely related to the interaction between the two components. AAEM and organic matter play synergistic promotion and synergistic inhibition roles respectively. Based on this, the mechanisms of the respective effects of the intrinsic AAEM and organic matter on the synergistic behavior in the co-gasification process of coal and biomass are proposed, as shown in Fig. 9.
AAEM plays a synergistic role in the co-gasification of coal and biomass, most of the AAEM in the biomass char volatilizes into the gas phase during co-gasification of coal and biomass, and the alkali metal compounds of gas phase are adsorbed by the coal char, realizing the transformation of AAEM from the biomass char to the coal char in a gas-solid contact. Zhang et al. (2016), in their study of co-gasification of coal and biomass, found by SEM-EDX spectroscopy that no potassium was observed in the spectrum of the original coal char and a strong potassium peak appeared in the spectrum of the co-gasification coal and biomass, which offered persuasive evidence for the migration of potassium from biomass to the surface of coal char. Research shows that when coal and biomass are co-gasified, the oxygen-containing substances produced by the pyrolysis of biomass char are adsorbed and deposited on the surface of coal char, which increases the dispersion of oxygen elements on the surface of coal char and promotes the transfer of AAEM from biomass char to coal char (Hu et al. 2017), more alkali metals are transferred to coal char, thereby catalyzing the coal gasification reaction. During the co-gasification process, increasing of biomass mass fraction in the sample result in facilitating the migration of alkali metals from the material into the solid phase. However, it is noteworthy that the contribution of biomass to the co-gasification of coal and biomass process does not comply with a linear relationship with the proportion of biomass blended (Dai et al. 2021). Co-gasification of coal and biomass increases the reaction activity of coal, decreases the gasification temperature, and shortens the reaction time. However, under high temperature conditions, the active AAEM that originally remains in the solid phase will volatilize into the gas phase again, and in addition, active AAEM also tend to react with inert metals (Al, Si, etc.) under high temperature to produce inert salt materials, resulting in less active alkali metals remaining in the coal char leading to lower gasification reactivity in the gasification process (Ma et al. 2013).
Organic matter acts as a synergistic inhibition effect on the co-gasification process of coal and biomass. The interaction of the polycyclic aromatic hydrocarbon component of the organic matter with coal char is an important reason for the inhibition of the co-gasification reaction of coal and biomass. As shown in Fig. 9, the mechanism of inhibition of synergistic behavioral of organic matter on the co-gasification process of coal and biomass is revealed, where the volatile generated during co-pyrolysis first enters the gas phase and then the coal char macromolecular skeleton and interacts with each other. The oxygenated tar component produced by pyrolysis undergoes a series of reactions such as deoxygenation and dehydrogenation under the action of high temperatures and semi-char catalysis, and finally re-polymerizes on the surface of coal char to form polycyclic aromatic compounds (PAHs), which adhere to the coal char (Wijayanta et al. 2012), which attach to the pore structure of coal char particles, occupy the exposed active sites in the solid phase, and hinder the mass transfer between char and alkali metals in the gas-soild phase, thus inhibiting the co-gasification reaction. Ma et al. (2022) investigated volatile-char interactions in co-gasification of coal and biomass, found that oxygenated aromatic compounds generated from pyrolysis interacted and polymerized to form polycyclic aromatic hydrocarbons (PAHs) and occupied the char active sites, resulting in a decrease in the total amount of H/C and oxygenated functional groups in coal particles, which inhibited the co-gasification of coal and biomass. However, as the reaction temperature continues to increase, the catalytic cleavage of volatiles by the char becomes more and more intense, and the active sites on the char surface (oxygen-containing functional groups, pore structure distribution, AAEM, etc.) make the tar molecules adsorbed on the surface of the char crack (Song et al. 2012; Muradov et al. 2001), resulting in the reduction of the amount of tar molecules adhered to the surface of char as well as in the voids.
4 Conclusions
In this study, we investigated the synergistic behavior of intrinsic AAEM and organic matter on the co-gasification reaction during the co-gasification of Yangchangwan bituminous coal and cow manure using a thermogravimetry analyzer, and conducted a kinetic analysis of the non-isothermal gasification reaction process using the Coats-Redfern method, and obtained the following conclusions:
-
(1)
The active AAEM in cow manure will promote the co-gasification process of Yangchangwan coal char and cow manure char, and the larger the proportion of cow manure blending, the stronger the synergistic promotion effect at the late stage of the reaction when X > 70%, but the migration of active AAEM in cow manure to coal char is unfavorable under high temperature conditions to the extent that the gasification reaction activity is reduced.
-
(2)
The organic matter will inhibit the gasification reaction activity in the co-gasification reaction of Yangchangwan bituminous coal and cow manure. When the gasification temperature is less than 790 ℃, the organic matter does not participate in CO2 gasification, and the synergistic inhibition of CO2 gasification occurs mainly in the range of 0 < X < 70%, this is attributed to the fact that PAHs are preferentially consumed completely with gasification reactions.
-
(3)
The fitting curve for the co-gasification of coal and cow manure falls between the integral curves of coal and cow manure gasification alone, with a relatively good fitting effect observed when the reaction order N = 5. Moreover, at higher temperatures, the activation energy of the gasification reaction increases with the proportion of cow manure in the mixed sample.
References
Bates RB, Ghoniem AF, Jablonski WS, Carpenter DL, Altantzis F, Garg A, John L, Chen R, Randall P (2017) Field Steam-air blown bubbling fluidized bed biomass gasification (BFBBG):multi-scale models and experimental validation. AIChE J 63(5):1543–1565
Che DY, Sun YX, Li SH (2016) Exergy analysis of co-gasification process of pine sawdustand lignite in fluidized bed. Acta Energiae Solaris Sinica
Chen HD, Chen XL, Qiao Z, Liu HF (2016) Release and transformation characteristics of K and cl during straw torrefaction and mild pyrolysis. Fuel 167(MAR1):31–39
Cong HB, Zhao LX, Wang JC, Yao ZL (2017) Current situation and development demand analysis of rural energy in China. Transactions of the Chinese Society of Agricultural Engineering
Dai CY, Tian YS, Hu EF, Li MS, Ma DC, Shao S (2021) Research on co-pyrolysis characteristics of biomass and low-rank coal and its technical progress. Acta Energiae Solaris Sinica, 12–0326
Feng B, Bhatia S (2003) Variation of the pore structure of coal chars during gasification. Carbon 41(3):507–523
Guo P, Zhao HM, JIA TH, Wang MJ, Chang LP (2015) Effect of co-pyrolysis process on the oxidation reactivityof lignite char and biomass char. J Fuel Chem Technol 43(10):1188–1194
Guo FQ, Li XL, Wang Y, Wang Y, Liu Y, Li TT, Guo CL (2017) Characterization of Zhundong Lignite and biomass co-pyrolysis in a thermogravimetric analyzer and a fixed bed reactor. Energy 141(PT2):2154–2163
Guo QH, Wei JT, Gong Y, Yu GS (2018) Mechanism analysis of synergy behavior during blended char co-gasification of bituminous coal and rice straw. J Fuel Chem Technol 46(4):399–405
He Q, Wei JT, Gong Y, Ding L, Yu GS (2017) Experimental study on co-gasification reactivity of Shenfu bituminous coal char and MSW-based hydrochar. J Fuel Chem Technol, 10–1191
Hu J, Si Y, Yang H et al (2017) Influence of volatiles – char interactions between coal and biomass on the volatiles released, resulting char structure and reactivity during co-pyrolysis. Energy Conv Manag 152:229–238
Hughes E (2000) Biomass cofiring: economics, policy and opportunities. Biomass & Bioenergy, 19:457–465. 6
Jeong J, Hwang S, Hwang J (2015) Co-gasification of bituminous coal-pine sawdust blended char with H. Fuel 156:26–29
Ke P, He XM, Liu J, Feng DZ (2019) Characteristics of low temperature co-pyrolysis products of low-rank coal and Corncob. Biomass Chem Eng 53(4):26–30
Li RD, Yan JH, Li SQ (2001) Gasification kinetics of waste tire char with CO2. J Fuel Chem Technol 29(4):313–318
Li CL, Wang YG, Tian Z, Lin CX, Wu X (2017) Influence of inherent minerals on CO2 gasification of a lignite with high ash content. Journal Of Fuel Chemistry & Technology
Li XM, Zhang H, Liu MJ, ZHI LF, Bai J, Bai ZQ, Li W (2020) Investigation of coal-biomass interaction during co-pyrolysis by char separation and its effect on coal char structure and gasification reactivity with CO2. J Fuel Chem Technol, 8(48)
Li XM, Yang CF, Liu MJ, L W. Influence of different biomass ash additive on anthracite pyrolysis process and char gasification reactivity. Int J Coal Sci Technol, 2020(7).
Li HY, Zhang JQ, Chen JB, Chen WH, Zhao Y, Lin MZ, Li L, Xin Z, Dai XD (2022) Global energy transition faces challenges in 2021—Based on the Bp Statistical Review of World Energy. Information, Safety And Management, 6(40)
Liu L, Cao Y, Liu Q (2015) Kinetics studies and structure characteristics of coal char under pressurized CO. Gasif conditions. Fuel 146:103–110
Luo M, Zhang JM, Gao MS, Suo Y (2006) Comparison of different methods for the kinetic parameters calculation in activated char-CO_2 gasification. Journal of University of Shanghai for ence and Technology
Ma ZB, Bai J, LI W, Bai ZQ, Kong LX (2013) Mineral Transformation in Char and its effect on Coal Char Gasification Reactivity at high temperatures, part 1: Mineral Transformation in Char. Energy Fuels, 27(jul.-aug.):4545–4554
Ma M, Wang JF, Bai YH, Lv P, Song XD, SU WG, Yu GS (2022) Decoupling of volatile–char interaction in co-pyrolysis of cow manure and bituminous coal and deactivation mechanism of coal char reactivity. Energy 251:123891
María V, Riaza J, Lucía Á, Pevida G, Fernando R (2015) Biomass devolatilization at high temperature under N2 and CO2: Char morphology and reactivity. Energy 91(NOV):655–662
Meesri C, Moghtaderi B (2002) Lack of synergetic effects in the pyrolytics characteristics of woody biomass/coal blend sunder low and high heating rate regimes. Biomass & Bioenergy, 23:55–66.1
Mishra A, Gautam S, Sharma T (2018) Effect of operating parameters on coal gasification. Int J Coal Sci Technol, 018–0196
Mosqueda A, Wei JT, Medrano K, Ding L, Yu GS, Yoshikaw K (2019) Co-gasification reactivity and synergy of banana residue hydrochar and anthracite coal blends. Appl Energy 250:92–97
Muradov N (2001) Catalysis of methane decomposition over elemental carbon. Catal Commun 2(3–4):89–94
Naidu S, Aghalayam P, Jayanti S (2016) Synergetic and inhibition effects in carbon dioxide gasification of blends of coals and biomass fuels of Indian origin. Bioresour Technol 209:157–165
Qin YH, Zhao ZB, Tomasz W, Mashal A, Liang YN (2016) Investigation of co-gasification reactivity of torrefied jatrophaSeed cake with illinois #6 coal char. BioResources, 11(3)
Quyn DM, Wu HW, Li CZ (2002) Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of victorian brown coal. Part I. Volatilisation of na and cl from a set of NaCl-loaded samples. Fuel 81(2):143–149
Ren HJ, Zhang YQ, Fang YT, Wang Y (2012) Co-gasification properties of coal char and biomass char. J Fuel Chem Technol, 02–0143
Shi L, Chen XJ, Liu QY, Liu Z (2018) Reaction of volatiles from a coal and various organic compounds during co-pyrolysis in a TG-MS system. Part 2. Reaction of volatiles in the free gas phase in crucibles. Fuel 213:22–36
Song Y, Wang Y, Hu X, Hu S, Xiang J, Zhang L, Zhang S, Li CZ (2014) Effects of volatile-char interactions on in situ destruction of nascent tar during the pyrolysis and gasification of biomass. PartI. Roles of nascent char. Fuel 122:60–66
Tay H, Kajitani S, Wang S et al (2014) A preliminary Raman spectroscopic perspective for the roles of catalysts during char gasification. Fuel 121:165–172
Terry FW, Liu GS, Wu HW, Daniel GR, Kathy EB, Sushil G (2002) John a lucas, David J H. The effects of pressure on coal reactions during pulverised coal combustion and gasification. Progress In Energy and Combustion Science
Wall T, Liu G, Wu H et al (2002) The effects of pressure on coal reactions during pulverised coal combustion and gasification. Prog Energy Combust Sci 28(5):405–433
Wang M, Wan Y, Guo Q et al (2021) Brief review on petroleum coke and biomass/coal co-gasification: Syngas production, reactivity characteristics, and synergy behavior. Fuel 304:121517
Wei JT, Gong Y, Guo QH, Ding L, Wang FC, Yu GS (2017) Physicochemical evolution during rice straw and coal co-pyrolysis and its effect on co-gasification reactivity. Bioresour Technol 227:345e52
Wijayanta AT, Alam MS, Nakaso K, Jun F (2012) Numerical investigation on combustion of coal volatiles under various O2/CO2 mixtures using a detailed mechanism with soot formation. Fuel 93:p670–676
Yu J, Guo Q, Gong Y et al (2021) A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Process Technol 214:106723
Zhang Y, Zheng Y, Yang MJ, Song YC (2016) Effect of fuel origin on synergy during co-gasification of biomass and coal in CO2. Bioresource Technology Biomass Bioenergy Biowastes Conversion Technologies Biotransformations Production Technologies
Zemp M, Huss M, Thibert E, Eckert N, Mcnabb R, Huber J, Barandun M, Machguth H, Nussbaumer I, Gärtner-roer L, Thomson F, Paul F, Maussion S, Kutuzov JG (2019) Cogley Global glacier mass changes and their contributions to sea-level rise from 1961 to 2016. Nature 568(7752):382–386
Zhou ZJ, Fan XL, Zhang W, Wang FC, Yu ZH (2006) Char gasification kinetics using non-isothermal TGA. J Coal China Soc, 02–0219
Acknowledgements
The work was supported by the Outstanding Youth Science Foundation of Ningxia (2022AAC05016).
Author information
Authors and Affiliations
Contributions
Hongqiao Lu: Data curation, Investigation, Writing original draft. Meng Ma: Supervision, Software. Juntao Wei: Supervision. Yonghui Bai: Methodology, Validation, Writing - review & editing. Jiaofei Wang: Supervision, Visualization. Peng Lv: Investigation, Supervision. Xudong Song: Supervision, Visualization. Guanghua Lu: Supervision, Visualization. Guangsuo Yu: Validation, Writing review & editing, Project administration.
Corresponding author
Ethics declarations
Competing interests
The author declares that they have no interest in each other.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, H., Ma, M., Wei, J. et al. Study of synergistic behavior during bituminous coal-cow manure co-gasification: The role of intrinsic AAEM and organic matter. Int J Coal Sci Technol 11, 66 (2024). https://doi.org/10.1007/s40789-024-00694-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-024-00694-w