Abstract
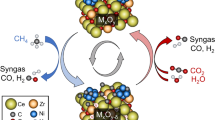
Coal catalytic hydrogasification (CCHG) is a straightforward approach for producing CH4, which shows advantages over the mature coal-to-CH4 technologies from the perspectives of CH4 yield, thermal efficiency, and CO2 emission. The core of CCHG is to make carbon in coal convert into CH4 efficiently with a catalyst. In the past decades, intensive research has been devoted to catalytic hydrogasification of model carbon (pitch coke, activated carbon, coal char). However, the chemical process of CCHG is still not well understood because the coal structure is more complicated, and CCHG is a combination of coal catalytic hydropyrolysis and coal char catalytic hydrogasification. This review seeks to shed light on the catalytic process of raw coal during CCHG. The configuration of suitable catalysts, operating conditions, and feedstocks for tailoring CH4 formation were identified, and the underlying mechanisms were elucidated. Based on these results, the CCHG process was evaluated, emphasizing pollutant emissions, energy efficiency, and reactor design. Furthermore, the opportunities and strategic approaches for CCHG under the restraint of carbon neutrality were highlighted by considering the penetration of “green” H2, biomass, and CO2 into CCHG. Preliminary investigations from our laboratories demonstrated that the integrated CCHG and biomass/CO2 hydrogenation process could perform as an emerging pathway for boosting CH4 production by consuming fewer fossil fuels, fulfilling the context of green manufacturing. This work not only provides systematic knowledge of CCHG but also helps to guide the efficient hydrogenation of other carbonaceous resources such as biomass, CO2, and coal-derived wastes.
Similar content being viewed by others
1 Introduction
Substituted natural gas (SNG), an important low-carbon and clean fuel, is characterized by a limited reserve compared to coal in Asian and European Union countries (Si et al. 2022). It is predicted that the SNG consumption in these countries will continuously increase, while more than 40% of them rely on importation (Xie 2021). For the security of energy structure, there is a great necessity for developing the technology of coal-to-SNG. Especially in recent years, the requirement for reducing greenhouse gas emissions motivates the low-carbon utilization of coal.
The coal-to-SNG technologies are often categorized into two-stage gasification (TSG), coal catalytic gasification (CCG), and coal hydrogasification (CHG) (Suuberg et al. 1980; He et al. 2013; Yuan et al. 2018; Chang et al. 2020). The TSG represented by the Lurgi gasifier has been commercialized, while the CCG and CHG are under demonstration (Li et al. 2021). TSG produces CH4 through various units, including coal gasification, water–gas shift reaction, and methanation. A long process characteristics TSG, and the endothermic and exothermic reactions are conducted separately, resulting in low thermal efficiency (61.9%) and high investment (Yuan 2020). CCG integrates the endothermic and exothermic reactions in one reactor with the aid of potassium/sodium catalysts, and thus, the energy efficiency is improved (71.4%) (Steinberg 2005). However, the methanation process in CCG is thermodynamically limited because all of the reactions in CCG take place at a relatively high temperature (~ 700 °C) (Hirsch et al. 1982). Therefore, CCG is characterized by high coal conversion (~ 95%) and low CH4 yield (~ 25%). From the perspective of CH4 production, CHG is the most appealing route, as it produces CH4 with a high yield (30%–50%) and thermal efficiency (79.6%) (Steinberg 2005; Yuan et al. 2018). However, the essence of CHG is a hydropyrolysis process. Suffering from the low C–H2 reactivity, a large amount of inert carbon remains unconverted, although harsh reaction conditions (850–1100 °C, 5–7 MPa) are adopted (Mısırlıoğlu et al. 2007). A large amount of char residue needs to be reused, which results in a high post-processing load and a low handling capacity.
Catalytic hydrogasification is an effective approach to accelerate the conversion of inert carbon under mild conditions (750–850 °C, 1–3 MPa) (Tomita and Tamai 1972; Haga and Nishiyama 1987; Qu et al. 2022). Since the 1980s, many research institutions have performed laboratory-scale investigations of catalytic hydrogasification with model carbon (graphite, activated carbon, pitch coke, and coal char) as the raw materials, and the alkali and transition metals were proved as the suitable catalysts (Casanova et al. 1983; Nishiyama 1986; Zoheidi and Miller 1987; Matsumoto et al. 1991). It is well accepted that catalytic hydrogasification of model carbon follows two crucial steps: supplying active hydrogen (H·) and activating C=C bonds (Tamai et al. 1977; Haga and Nishiyama 1983; Han et al. 2022). The catalysts mainly participate in these two processes to accelerate the C–H2 reaction. It is also well recognized that the reactivity of catalytic hydrogasification is determined by many factors, such as the nature of catalysts, including addition amount, dispersion, and component, and the properties of model carbon consist of surface area, ash/sulfur content, and degree of condensation (Tomita et al. 1983; Liu et al. 2017a, 2017b; Zhang et al. 2019; Saraceno et al. 2023). Disappointingly, although great progress had been made in catalytic hydrogasification of model carbon, the trail nearly ceased and did not extend to catalytic hydrogasification of coal because of the low economic attractiveness of SNG in the 1980–2000s. As a result, plenty of fundamental issues remain unsolved, such as the best configuration of catalyst, the crucial step for C–H2 catalytic reaction, the behavior of catalyst in catalytic hydrogasification of coal, et al. A clear elaboration of the above issues is worthwhile because it is not only helpful for the design of catalyst and feedstocks, but also provide strategies for regulating reaction process to tailor the generation of target products.
In the practical production process, raw coal instead of model carbon prefers to be used as the raw material. Compared to that of model carbon catalytic hydrogasification, coal catalytic hydrogasification (CCHG) has the following merits (Anthony and Howard 1976; Yan et al. 2017; Yuan et al. 2018): (1) Coal resource is abundantly distributed and ready for use; (2) Raw coal has less ordered carbon structure than that of the model carbon, which possesses relativity high reactivity itself; (3) CCHG produces additional high value-added HCL (hydrocarbon liquids) including ‘benzene, toluene, xylenes’ (BTX), ‘phenol, cresols, xylenols’ (PCX) and naphthalene. However, raw coal is a complex heterogeneous material with various chemical and physical properties, and catalysis in coal hydrogasification is significantly affected by coal properties (Yuan et al. 2015). In addition, the catalyst not only catalyzes hydrogasification of coal char, but also affects the hydropyrolysis of raw coal, which in turn shows enormous effects on coal char hydrogasificaion (Yan et al. 2018), making the CCHG process complicated. To date, most of the studies mainly concentrated on catalytic hydrogasification of model carbon, while the detailed catalysis process of coal hydrogasification remains ambiguous.
The interest in CCHG was stimulated in the past decade due to the rapid growth in price and demand for SNG. In addition, the endowment that CCHG produces CH4 and HCL simultaneously with high yield and high thermal efficiency (Table 1) under a mild reaction condition also contributes to the widespread attention (Gao et al. 2020; Yan et al. 2022). Efforts have been made to explore effective catalysts, proper reaction conditions, and catalytic mechanisms (Takarada et al. 1997; Qu et al. 2019; Sun et al. 2019). There appears to be a consensus that the recoverability of catalysts, the feedstock adaptability, and the availability of low-cost hydrogen are the crucial factors affecting the commercialization of CCHG. Fortunately, the first two issues have been preliminary addressed in recent years (Yan et al. 2017, 2021), which will be discussed in Sects. 3 and 4. In terms of H2, it can be produced from coal-based syngas, coke oven gas, chlorine alkali plants, or renewable energy (RE) based gasification or electrolysis (Saeidi et al. 2021). The last route has been proposed as a competitive H2 generation approach in the next ten years (Ipsakis et al. 2021), which can realize the insertion of RE into chemical and fuel production processes used in modern societies. Moreover, integrating RE-based H2 and CCHG is feasible to resolve the challenging H2 storage and transport process. Meanwhile, the existing infrastructure stores and transports SNG products easily, and the integrated process offers high flexibility to stabilize electricity grids with a high share of renewable resources, thus enabling long-term low-carbon running. Notably, China’s commitments to the international community regarding carbon peak and neutral targets set higher requirements for coal utilization. As CCHG has the potential to convert coal in a clean, efficient, and low-carbon manner, it is therefore proposed as an alternative technology for the traditional TSG and CCG in SNG production.
There have been many excellent reviews on converting coal to methane in the past through the approach of TSG, CCG, and CHG. (Hirsch et al. 1982; Li et al. 2021) summarized the technologies of CCG and TSG comprehensively. In these processes, H2O acts as the gasifying medium, and coal reacts with H2O to form CH4 with alkali/alkaline-based catalysts. When H2 acts as the gasifying medium, coal performs hydrogasification (CHG) to generate CH4 much more efficiently (Table 1), especially in the presence of hydrogenation catalysts. However, little review has been concentrated on this issue. (Saraceno et al. 2023) conducted a review of CHG, and the process layouts, hydrogasifiers, and catalysts were elaborated. Whereas, the catalytic mechanisms of coal hydrogasification with the addition of different catalysts (alkali-metal compounds, transition-metal compounds, and alkaline-earth-metal compounds) remain ambiguous. The profound configurations for the catalyst process (catalyst type, temperature, H2 pressure, and coal type) and approaches for enhancing CCHG are not well understood. Moreover, it is worthwhile to analyze the industrialization prospects of CCHG further and discuss the opportunities and strategies for CCHG under the restraint of carbon neutrality.
In the present work, we systematically conducted a review dealing with CCHG for SNG production. The acting behavior of catalysts, the effect of operating conditions, and reactor configuration for CCHG are investigated. Additionally, a comprehensive outline of the process enhancement is provided based on the reaction principle of CCHG. Additionally, a brief evaluation of the CCHG process was performed, emphasizing emissions and efficiency by adopting the existing experimental results. Furthermore, novel strategies for the penetration of renewables in CCHG are proposed and preliminary validated in the context of green manufacturing. This review can help gain insights into the CCHG process and be a reliable reference for scaling up CCHG in a pressurized fluidized bed.
2 The behavior of catalysts in CCHG
2.1 Catalytic hydrogasification of model carbon
CHG integrates coal hydropyrolysis and coal char hydrogasification. The rate-determining step of CHG is hydrogasification of coal char, and large quantities of studies concentrated on the catalytic hydrogenation of model carbon instead of raw coal in the 1980–2010s (Hiittinger 1981; Huttinger and Krauss 1981; Holstein and Boudart 1981; Baker et al. 1982; Haga and Nishiyama 1989; González et al. 2002; Cha et al. 2007). The core of catalytic hydrogasification is to explore the suitable catalyst with high activity, high recovery, and low cost. The catalyst commonly studied includes alkali compounds, transition compounds, and alkali-earth compounds, such as K-, Fe-, Co-, Ni-, and Ca- compounds (Qu et al. 2022). Figure 1 presents the activity of different catalysts in the hydrogasification of activated carbon (without ash, sulfur, or oxygen) at 850 °C and 1 MPa H2. The results show that the activity of Fe, Co, and Ni-based catalysts is much higher than that of K- and Ca-based catalysts at the loading amount of 2%. The detailed catalytic behaviors of different components are elaborated in the following sections.
Effect of different catalysts on hydrogasification of activated carbon (reaction conditions: 850 °C, 1 MPa H2) (Qu et al. 2022)
2.1.1 Alkali metals
It is well known that alkali metal salts are profound catalysts for coal gasification, with water vapor as the gasifying agent (Wood and Sancier 2006). Many researchers also adopted them for hydrogasification. K- and Na-based compounds were focused because of the abundantly available source and low cost. The activity of K- compounds is in the order of K2CO3 ≈ KHCO3 > K2SO4 > KCl, and the activity sequence is associated with the alkalinity of potassium catalyst (Cypres et al. 1984). (Skodras et al. 2016) found that K2PO4, K2CO3, CH3COOK, and KOH with higher alkalinity were more active for hydrogasification than KNO3, KBr, KCl, and KHSO4. The added amount of alkali metals influences its activity significantly. The suitable amount of K2CO3 for hydrogasification is 5%–20% (as potassium metal in coal), and the catalytic reactivity generally increases with the addition of catalysts in this range (Zhan et al. 2012). When the loading amount is below 5%, the catalytic effect is not obvious because there exist few active sites, consistent with the result in Fig. 1. When the loading amount is above 20%, large quantities of catalyst would block the pore structure and agglomerate on the surface of coal char, resulting in decreased reactivity. (Wang et al. 2019) conducted hydrogasification of coal char by blending biomass ash rich in alkali metals, and the results revealed that large quantities of K- and Na-based compounds migrated onto the coal char surface to promote hydrogasification. This research provides an economical and environment-friendly way for hydrogasification with alkali metal as the catalyst.
Different mechanisms were proposed for the alkali metal-catalyzed hydrogasification of model carbon. One mechanism involves the formation of M-(O)-C (M refers to K or Na) species during hydrogasification. (Zoheidi and Miller 1987) considered that the oxygen surface groups in coal char played an important role in determining K activity. The C=O carbonyl groups were possible candidates that would interact with K compounds to form K-(O)-C structures, which promotes CO generating and forming nascent active sites. Another mechanism is the direct reaction and interaction between model carbon and potassium compounds, as shown in Eqs. (1)–(3). (Liu et al. 2017a, b) reported that K2CO3 and Na2CO3 were reduced to K and Na metal by carbon, which then intercalated the carbon structure to restrain the graphitization process of coal char and facilitate the hydrogenation of carbon. Herein, it should be noted that the above two mechanisms might exist in hydrogasification simultaneously. (Martin and Toomajian 1992) impregnated 2%K2CO3 to the oxidized coal char, and a 100-fold increase in hydrogasification rate was observed. However, the same amount of K2CO3 results in little increase in the hydrogasification rate of activated carbon without oxygen, as shown in Fig. 1. The results hinter the interaction between K and oxygen surface groups, which promotes hydrogasification significantly. When it comes to coal char with little oxygen surface groups, the catalytic hydrogasification reactivity increases with the loading amount of catalyst, suggesting that the interaction between K and carbon plays the dominant role in this circumstance.
In the CCHG process, the reaction temperature is commonly at the scope 750–900 °C, which brings about severe evaporation of the alkali compounds. (Zoheidi et al. 1987) conducted catalytic hydrogasification under 865 °C. The results showed that more than 50% of K compounds would be lost when the carbon sample gasified to 47% conversion. (Song et al. 2019) evaluated the distribution of potassium during co-pyrolysis of coal and biomass at 500 °C. They found that 65% of potassium in biomass would migrate to the pyrolyzed coal char, 15.9% of potassium would evaporate to the gas phase, and the rest of the potassium would be retained in biomass char. Apart from evaporation, potassium tends to react with minerals in coal and form water-insoluble compounds, such as KAlSiO4, which is catalytically inactive and causes difficulties in the recovery of potassium (Masnadi et al. 2014, 2015). Therefore, the drawbacks of alkali compounds, including the need for a large amount of catalyst additions (5 wt%–20 wt% in metal), a tendency of alkali evaporation at high temperatures, and a possibility of reacting with mineral matter, would lead to catalyst losses, high catalyst recovery costs, and the corrosion of the tubes when considering the practical application. The integration of coal and biomass hydrogasification might solve the deficiencies of alkali catalysts, as biomass is commonly rich in alkali metals, which easily migrate to coal char to promote the C–H2 reaction. More importantly, the alkali compounds in biomass ash can be accumulated and reused cyclically without considering recovery, which might be cost-effective and fulfill the large loading amount of catalyst.
2.1.2 Transition metals
In the 1980s, (Tomita et al. 1972) first found that transition metals such as Rh, Ru, Ir, Pt, Ni, Pd, Co, and Fe possess activity towards hydrogasification of model carbon. Among them, the iron-group metals (Fe, Co, and Ni) appeal to many researchers due to their abundant resources and low price. Fe/Co/Ni supplies active hydrogen (H·), impairs the bond energy of C=C for the C–H2 reaction and exerts profound activity with the loading of 1%–5% (Haga et al. 1987; Yan et al. 2018). As shown in Fig. 1, the activity sequence of the iron-group metals is Co ≈ Ni > Fe. However, (Ohtsuka et al. 1987; Matsumoto et al. 1991) reported that the activity order is Co > > Ni > Fe. The iron-group metals show similar capability towards supplying active hydrogen, and the key factor determining their activity is their ability to activate and fracture C=C bonds in carbon structure (Tamai et al. 1977; Yan et al. 2017). The previous results commonly found that Fe has inferior activity than Co and Ni, suggesting Fe possesses a poor endowment of breaking C=C bonds.
For Co and Ni, they exert diverse activity sequences in different studies. As each research uses the same catalyst precursor, catalyst loading amount, and reaction conditions, the discrepancy in the Co and Ni activity sequence can be correlated with using different model carbon. The carbon species were usually demineralized prior to use, and the main factor affecting the catalyst’s activity might be the sulfur content. In the hydrogasification of model carbon, sulfur evolves in the form of H2S. Plenty of research reported that a ppm grade of H2S would suppress the activity of iron-group metals in hydrogasification because the electron in sulfur will strongly absorb in the empty d-orbit of Co and Ni, which restrained the disassociation of H2 and the activation of carbon structure by the catalyst (Tomita et al. 1983; Nishiyama et al. 1990). It can be seen in Fig. 2 that 50 ppm H2S decreases the activity of Co and Ni significantly at 850 °C. With increasing the reaction temperature to a higher temperature of 950 °C, the activity of Co is recovered remarkably, while the activity of Ni remains very low. This result suggests that a higher temperature is adverse to H2S adsorption on the catalyst surface, and the negative effect of H2S on the Ni is more obvious than Co because H2S is absorbed on the Ni surface more strongly.
Effect of 50 ppm H2S on the activity of Co (adapted from newly experimental data) and Ni (Tomita et al. 1983) for hydrogasification
The above discussions well explained the different activity sequences of Co and Ni in the literature. In Fig. 1, the carbonaceous material used for catalytic hydrogasification is sulfur-free, so the results present the instinct activity of Fe, Co, and Ni. (Ohtsuka et al. 1987; Matsumoto et al. 1991) use coal char as the raw material, which contains ~ 0.3% sulfur. A ppm grade of H2S would inevitably be generated during hydrogasification (Matsumoto and Walker 1989), which suppressed the activity of Ni to a greater extent. Thus, Ni shows an inferior activity than Co. In terms of Fe, (Huttinger et al. 1981) found that H2S reacted with Fe to form FeS directly instead of absorption on the Fe surface, and FeS showed no activity towards hydrogasification. For Co and Ni, no sulfide phase was formed because the Co and Ni sulfides were easily hydrogenated under a hydrogasification condition (Tomita et al. 1983; Yan et al. 2021). Instead, the generated H2S affected their activity through strong chemical adsorption. Therefore, care should be taken when evaluating the activity of transition metals for hydrogasification of sulfur-containing materials.
Apart from H2S, the dispersion state of iron-group metals also affects the catalytic activity, which is influenced by the loading approach, addition amount, the catalyst precursor, et al. (Tomita et al. 1972) loaded Ni catalyst onto the activated carbon through mechanical mixing and impregnation, respectively, and the latter approach showed higher activity due to the less extent of agglomeration. The agglomeration of catalysts during hydrogasification is attributed to the following two factors: (1) The hydrogasification temperature is usually above the Tamman temperature (TTamman = 0.5 Tmelting) of Fe/Co/Ni, and the bulk atoms of Fe/Co/Ni would migrate close to each other leading to agglomeration (Moulijn and Kapteijn 2001). (2) In the course of Fe/Co/Ni-catalyzed hydrogasification, the carbon gradually loses in the vicinity of catalysts, which promotes the chance of contact between the catalyst particles and results in agglomeration (Tomita et al. 1983). An increase in the loading amount of the catalyst generally contributes to the reactivity. Whereas the model carbon has a finite surface area, the agglomeration would be enhanced when the addition of catalyst exceeds the ideal amount, resulting in a decrease in reactivity. (Ohtsuka et al. 1987) investigated the effect of precursors on the dispersion of Fe catalysts. The results reveal that FeCl3 and Fe2(SO4)3 agglomerated seriously on the surface of model carbon, and the average crystallite size of Fe metals was above 100 nm. In the presence of Fe(NO3)3 and (NH4)3Fe(C2O4), Fe metals were well dispersed with an average crystallite size below 29 nm. It was especially pointed out that the catalytic activity of iron-group metals decreased drastically when the average size of crystallites grew above 30 nm (Asami and Ohtsuka 1993). Therefore, the chlorides and sulfates should not act as the precursor of the catalyst. The inferior effect of chlorides and sulfates on the dispersion of catalysts was also reported in other works (Inui et al. 1979; Jiang et al. 2017).
As the iron-group metals supply active hydrogen (H·) and activate C=C bonds for C–H2 reaction, two mechanisms exist in Fe/Co/Ni catalyzed hydrogasification of model carbon (Yan et al. 2017): (1) Active hydrogen spilling-over mechanism; (2) C=C bonds breakage mechanism, as depicted in Fig. 3. It had long been vague that which mechanism plays the crucial role in catalytic hydrogasification. Matsumoto et al. (1991) found that the hydrogasification rate increased enormously upon mixing a supported Ni catalyst with the catalyst-loaded char. The result hints that the spill-over of active hydrogen promoted catalytic hydrogasification, while the role of mechanism remains unaddressed. (Tamai et al. 1977) conducted catalytic gasification in different gasifying agents (H2O, CO2, or H2), and discovered that the activity sequence of different catalysts was independent of the reactant gas. The result holds that the catalyst-carbon interaction was more important than the catalyst-gas interaction, i.e., the catalytic fracturing of C=C bonds was the crucial step. As the chemisorption behavior of H2O, CO2, or H2 on different metals is quite different, the activity sequence should have exhibited a difference if the catalyst-gas interaction was more important than the catalyst-carbon interaction. Our previous work investigated Co-catalyzed hydrogasification of coal char at different pressures, and found that the C–H2 catalytic reaction is in zero order relative to H2 pressure at the reaction condition of above 800 °C and 1 MPa H2 (Yan et al. 2018). The result suggested that the supply of active hydrogen is adequate, and the catalytic fracture of C=C bonds was demonstrated to be the crucial step in the catalytic hydrogasification of model carbon. The above explorations provide meaningful theory guidance to promote catalytic hydrogasification via mediating the condensation of carbon structure instead of increasing the supply of active hydrogen under appropriate reaction conditions.
Tracing back to the instinct activity sequence of Fe, Co, and Ni, the type of interaction between the catalyst and carbon might account for their different activity, as the supply of active hydrogen is adequate at an appropriate reaction condition. Many researchers suggested that the carbide formation or carbon dissolution in Fe/Co/Ni metal might be an attractive explanation for the interaction as such carbons possessed high reactivity (Tamai et al. 1977; Holstein and Boudart 1981; Qu et al. 2019). In the catalytic hydrogasification process, Fe3C is often found in the catalyzed coal chars, whereas no Co or Ni carbide was formed; instead, they existed in the metallic state (Yan et al. 2017; Yuan et al. 2017a, b). (Zhang et al. 2019) reported that Fe3C is an active phase for catalytic hydrogasification, but the activity of Fe is low because the carbon atoms combined with Fe in the form of an ionic bond, which is too strong to be rapidly hydrogasified. However, in terms of Co and Ni, carbon atoms cover the catalyst surface in a dissolution state, which is readily hydrogenated by active hydrogen, and thus, Co and Ni exhibit superior activity than Fe (Haga et al. 1992; Yan et al. 2022). Equations (4)–(6) presents the principle of Fe/Co/Ni-catalyzed hydrogasification of model carbon (Yan et al. 2017).
2.1.3 Alkali earth metals
Alkali earth metals such as CaO performs well in catalytic gasification with CO2 and H2O as the gasifying agent (Lahijani et al. 2013; Yu et al. 2020; Liu et al. 2021). In the presence of oxidizing agents, CaO was able to interact with carbon in coal and form highly reactive Ca–O–C species (González et al. 2013; Zhang et al. 2021). However, in the presence of H2, CaO exhibited little activity toward hydrogasification of model carbon (Fig. 1). The researchers applied CaO to hydrogasification because it exerts catalytic effects on the cleavage of methyl groups in aromatic rings and accelerates the rupture of rings including BTX, PCX, indole, and benzofuran during cracking of coal tar in N2 (Banerjee et al. 1998; Jia et al. 2004). The absence of activity for CaO-catalyzed hydrogasification might be attributed to: (1) Model carbon such as coal char and graphite has much more condensed carbon structures than the above-mentioned model chemicals. The dissociation energies of C=C bonds at the edge of condensed aromatic rings is very high because of the electron delocalization effect (Yan et al. 2022). Hence, the catalytic fracture ability of CaO may only suit the carbonaceous materials with less ordered carbon structure; (2) In the H2 atmosphere, CaO is not able to provide active hydrogen or interact with condensed carbon, and thus it shows little activity for C–H2 reaction (Suzuki et al. 1998).
Herein, it should be emphasized that when the model carbon is less ordered in carbon structure or contaminated by some Fe-containing minerals, the experimental result would reveal that CaO exerts profound activity toward hydrogasification. Jiang et al. (2016) conducted CaO-catalytic hydrogasification with demineralized lignite char as the raw material, and the result revealed that CaO promoted hydrogasification and restrained the graphitization process of coal char. Jiang et al. (2017) found that CaO had no activity toward hydrogasification of bituminous coal char. However, when the bituminous coal char contains a small amount of Fe, CaO would migrate adjacent to Fe, and they cooperate to exert a catalytic effect. With the ever-ordering carbon structure to a graphite state, neither CaO nor CaO–Fe works well for hydrogasification. Casanova et al. (1983) impregnated CaO onto graphite and pretreated the specimen in an H2O atmosphere at 600 °C prior to hydrogasification. The result revealed that CaO catalyzed the depolymerization of graphite to a more reactive form, which exhibited high hydrogasification reactivity, as shown in Eqs. (7)–(8). The above researches provide valuable strategies for enhancing CaO-catalyzed hydrogasification of model carbon, such as reducing the ordering of carbon structure, adding a definite amount of Fe, and introducing a proper content of H2O. Anyway, it would be safe to conclude that CaO exhibits inferior activity towards hydrogasification of model carbon free of any heteroatoms.
2.1.4 Transition & alkali earth bimetallic metals
The above discussions in this section indicate that the iron-group metals have advantages over the other two types of catalyst because Fe/Co/Ni possess high instinct activity for C–H2 reaction at a low loading amount (1%–5%), and Fig. 1 shows the experimental data intuitively. However, in practical manufacturing, the carbonaceous materials (coal, coal char, pitch coke et al.) have a low surface area and a definite sulfur content. The low surface area makes iron-group metals agglomerate to form large particles, and the evolved H2S poisons the catalyst easily, which results in the activity loss of Fe/Co/Ni towards the C–H2 reaction.
Many efforts have been devoted to solving the sintering and poisoning problems. Among them, one efficient approach is to add alkaline-earth metals as additives (Haga and Nishiyama 1987; Dziembaj et al. 1996). Ca compound itself has little activity for hydrogasification of carbon. However, when the Ca compound acts as the additive, it not only promotes the distribution of iron-group metals on the carbon surface, but also reacts with H2S to prevent Fe/Co/Ni from being poisoned (Yuan et al. 2015; Zhang et al. 2023). (Haga and Nishiyama 1983) investigated the effect of Ca, Mg, Al, and Ba compounds on Ni-catalyzed hydrogasification of pitch coke. The results showed that the Ca compound showed a superior promoting effect, and the ideal addition amount was 1%. Calcium nitrate and acetate have the same promoting effect, while calcium chloride shows no promoting effect.
A detailed analysis of the promoting behavior of Ca compounds revealed that the impregnated calcium nitrate/acetate reacts with carbon and forms CaO(COO) species upon heating in H2 (Haga and Nishiyama 1983). Subsequently, CaO(COO) decomposed into CO2 and CaO above 650 °C, which are the vital components promoting the activity of Fe/Co/Ni with Fe/Co/Ni and Ca nitrates/acetates as the precursor of the binary catalysts. (Haga et al. 1992) reported that the CO2 liberated from the CaO(COO) would be in-situ captured by the adjacent Ni–C structure. As a result, a new Ni–(O)–C species was formed, which was responsible for the high dispersion of Ni and high reactivity of carbon. In terms of CaO, the group led by (Liu et al. 2017a, b) found that CaO not only retards Fe sintering and poisoning, more importantly, Ca also triggered Fe-catalyzing hydrogasification of low-reactive amorphous/graphite carbon. Very recently, we mechanically mixed CaO with cobalt (Co)-impregnated anthracite (Yan et al. 2021). The results showed that the CaO particles migrated to the surface of char and exhibited an enormous promoting effect on the Co-catalyzed hydrogasification of graphite carbon. When CaCO3 was used instead of CaO, the initial reaction stage was promoted to a greater extent, attributing to the role of CO2 evolved from CaCO3. These experimental results validate the promoting mechanism of Ca compound proposed by (Jiang et al. 2017; Haga et al. 1992).
Therefore, the use of Ca and Fe/Co/Ni nitrates/acetates as binary catalysts solves the bottle-neck problem of iron-group metals in hydrogasification of carbonaceous resources, and the Ca compounds exhibit the following effects: (1) making Fe/Co/Ni disperse well on carbon surface; (2) retarding the poisoning of Fe/Co/Ni; (3) mediating the Fe/Co/Ni-C interactions to facilitate the catalytic hydrogenation of unreactive carbon (graphite/amorphous carbon), as shown in Eqs. (9)–(14) (Yuan et al. 2017a, b; Yan et al. 2021, 2022).
where M, C−, and C* represent Fe/Co/Ni metal, graphite/amorphous carbon, and active carbon, respectively.
2.2 Catalytic hydrogasification of coal
Section 2.1 indicates that the rate-determining step of CHG can be well addressed by adding desirable catalysts. The behavior of different catalysts for the C–H2 reaction is presented in Table 2. It is appreciated to observe that the binary catalyst composed of an iron-group metal and a Ca compound is superior to other catalyst types. In the course of CHG, coal particles experience devolatilization (R1.1), the secondary reaction of volatiles (R1.2), the intermediate reaction of radicals (R2), hydrogenation of active carbon, and hydrogenation of graphite/amorphous carbon (R3), as shown in Fig. 4. The previous researches on hydrogasification of model carbon primarily focused on R3. However, when it comes to CHG, the reaction process is more complicated. The catalyst not only promotes R3, but also exhibits effects on R1 and R2, which determines the reactivity of pyrolyzed coal char and the subsequent generation of target products, including CH4, C2–C3, and HCL. The researches on this issue are relatively rare because few works conducted catalytic hydrogasification with raw coal as the specimen until the past decade. In this section, the acting role of Fe/Co/Ni and Ca in the binary catalysts on CHG is elaborated with reference to the catalytic hydrogasification of model carbon and the previous works of our group on catalytic hydrogasification of coal.
Reaction process of coal hydrogasification (Canel et al. 2005)
2.2.1 Catalytic hydropyrolysis of coal
Plenty of works proved that Fe/Co/Ni supplied active hydrogen and impaired C=C bonds in the hydrogasification of coal char. During the hydropyrolysis of coal, the iron-group metals exerted similar effects. Two mechanisms were involved in the catalysis process, as depicted in Fig. 5. On one hand, the catalyst promoted the cleavage of chemical bonds in coal and catalyst-dissociated active hydrogen stabilized the radicals in volatiles and coal char, by which the coal devolatilization process (R1) was facilitated, and the formation of C− in R2 was suppressed. This action was referred to as the catalytic depolymerization and hydrogenation mechanism (Cat-DH), which conduced to generating more volatiles and the high hydrogasification reactivity of pyrolyzed coal char (Yan et al. 2018). On the other hand, the Fe/Co/Ni-char was a strong Lewis acid structure, and it showed an enormous catalytic hydrocracking (Cat-HC) effect on the volatiles (Han et al. 2014), which decreased the tar yield and increased CH4 yield. The combination of Cat-DH and Cat-HC effect generally gives rise to the increase of CH4 yield, while the tar and HCL yields depend on the behavior of catalysts, as shown in Fig. 6. In the presence of Fe or Ni, tar and HCL increased with the addition of catalyst, suggesting the Cat-DH effect plays a more important role than the Cat-HC effect. Whereas, in terms of Co, the yield of tar and HCL decreased, attributing to the extremely high acid of the Co-char structure that resulted in a severe Cat-HC effect (Yan et al. 2017; Han et al. 2014).
Schematic diagram of the catalysis process of coal hydropyrolysis (Yuan et al. 2015)
With the addition of 1%Ca, the tar and HCL yields for 5%Fe, 5%Ni, and 5%Co further increased. However, when 1%Ca existed alone, the tar and HCL yield decreased. This result indicates 1%Ca promoted 5% Fe/Co/Ni-catalyzed coal hydropyrolysis, with the promoting effect on Cat-DH being more evident than that on Cat-HC, and thus contributed to the yield of target products such as HCL and CH4. The promoting mechanism of Ca in Fe/Co/Ni-catalyzed hydropyrolysis can be deduced with reference to the role of Ca in Fe/Co/Ni-catalyzed hydrogasification. It was recognized in Sect. 2.1.4 that Ca promoted the dispersion of iron-group metals, which contributed to the supply of active hydrogen and the interaction efficiency between the catalyst and coal. Additionally, Ca triggered the catalytic hydrogenation effect of Fe/Co/Ni catalyst on low-reactive amorphous/graphite carbon, suggesting the chemical bonds fracturing ability of Fe/Co/Ni catalyst was strengthened. The above roles of Ca favored the Cat-DH effect of the catalyst in hydropyrolysis; as a result, more volatiles were generated and hydrogenated to form CH4, HCL, and tar (Yan et al. 2022).
2.2.2 Catalytic hydrogasification of pyrolyzed coal char
The interaction between catalyst and coal in the pyrolysis stage not only affects the formation behavior of gaseous and liquid products, but also influences the carbon structure of pyrolyzed coal char, which determines its subsequent gasification reactivity (Zhu et al. 2017; Liu et al. 2019). To facilitate coal char gasification in H2O/CO2, many studies impregnated catalysts onto coal surfaces and conducted catalytic pyrolysis prior to catalytic gasification. The results proved that the catalyst changed the evolution pathway of coal structure during pyrolysis. The pyrolyzed coal char was less ordering, and rich in pore structures and surface functional groups, which contributed to the high gasification reactivity (Zhang et al. 2017; Śpiewak et al. 2021). When conducting catalytic gasification in H2, a similar role of catalyst existed. Our previous work conducted catalytic pyrolysis (in N2) and catalytic hydropyrolysis (in H2) of subbituminous coal in a pressurized fluidized bed, and the results proved that the coal char generated from catalytic hydropyrolysis had higher hydrogasification reactivity, as shown in Fig. 7 (Yan et al. 2018). With the coexistence of the Co–Ca catalyst and H2 in the pyrolysis stage, the chemical bonds could be fractured and hydrogenated rapidly, which had less chance to undergo ring condensation, and thus, the pyrolyzed coal char possessed more reactive sites. When H2 was replaced by N2 in the catalytic pyrolysis stage, the fractured chemical bonds could not be hydrogenated, which would recombine, and the condensing of coal char was inevitable; thus, the pyrolyzed coal char showed relatively low reactivity.
Effect of coal pyrolysis on catalytic hydrogasification of pyrolyzed coal char (reaction condition: 850 °C, 3 MPa H2; py: pyrolysis, hypy: hydropyrolysis, hyga: hydrogasification) (Yan et al. 2018)
For the coal char generated after Co–Ca–H2 (hypy), its subsequent catalytic hydrogasification was zero order relative to hydrogen pressure at temperatures above 800 °C and H2 pressure above 1.0 MPa (Yan et al. 2018; Qu et al. 2022), suggesting the supply of active hydrogen is adequate for C–H2 reaction, and the crucial step is the catalytic cleavage of C=C bonds. The catalytic cleavage and hydrogenation process of C=C bonds has been proposed with respect to the catalysis principle of Co and the established mechanism for hydrogasification of model carbon (Calderón et al. 2016, 2017), as shown in Fig. 8. The Co catalyst embedded into the aromatic rings to facilitate the controlling step of 1,2-hydrogen migration (S2 → S3), which favored the cleavage of C1–C3 bonds and the formation rate of CH4. In the binary catalyst system, CaO is a Lewis base, while the Co–C structure is Lewis acid. CaO appealed to migrating close to Co, making Co well dispersed and mediating the Co–C interaction, and thus, the Co-catalyzed hydrogasification of coal char was promoted. Further works are deserved to validate the stepwise catalytic cleavage and hydrogenation process of C=C bonds and reveal the interacting mechanism between CaO and Co–C structure with the aid of theory calculation.
The catalytic cleavage process of C=C bonds in aromatic rings (Yan et al. 2018)
2.3 Comparison of catalytic hydrogasification of model carbon and coal
Based on the above discussions, the characteristics of model carbon and coal catalytic hydrogasification are summarized and compared in Table 3. It can be seen that the reaction of coal catalytic hydrogasification is more complicated than that of model carbon. During this process, the superior Fe/Co/Ni–Ca bimetallic can be adopted to achieve a high CH4 yield. According to the reaction condition, it is indicated that the hydrogasification reactivity of model carbon is much lower than that of raw coal, because higher temperature, H2 pressure, and significantly longer particle residence time are required for model carbon to achieve a desirable CH4 yield. Regarding CH4 production, coal catalytic hydrogasification results in less CO2 emission because the coal structure contains an additional amount of H; thus less ‘grey’ H2 is required for CH4 formation. Whereas, these additional amounts of H cause a higher heating value of coal than that of model carbon, which lowers the energy efficiency of coal catalytic hydrogasification. In terms of catalytic mechanisms, the catalysts spill over active hydrogen and impair C–C bonds for C–H2 reaction during model carbon hydrogasification. When it comes to coal hydrogasification, the catalysts play an additional catalytic depolymerization role during the hydropyrolysis of coal. This action contributes to a higher subsequent hydrogasification reactivity of coal char and higher yields of high-value-added HCL compounds. Consequently, coal catalytic hydrogasification shows higher potential to produce CH4 due to the relatively mild reaction conditions, higher reactivity, lower CO2 emissions, and abundant reserves, despite the energy efficiency being somewhat lower than that of model carbon hydrogasification.
3 Effect of experimental variables on CCHG
Coal catalytic hydrogasification includes coal catalytic hydropyrolysis and coal char catalytic hydrogasification, which are greatly affected by the reaction conditions such as catalyst component and amount, temperature, hydrogen pressure, coal property, et al. A clear understanding of the effect of these factors will be helpful for the design of specimen, reactor, and tailoring the generation behavior of target products (CH4 and HCL). In the past decade, numerous researchers have concentrated on this topic, and the main results are listed in Table 4.
3.1 Catalyst type and loading
The catalyst type and loading are important parameters determining the formation efficiency of CH4 in CCHG. The design of a catalyst should consider the activity, economy, the catalysis mechanism, et al. The selection of suitable catalyst configurations based on the above criteria and a clear understanding of the catalysis mechanisms is of significance for further researches on CCHG. It is indicated from Sect. 2.1 and Table 4 that Fe–Ca, Co–Ca, and Ni–Ca are suitable catalysts for CCHG, because they are cheaper than the noble catalysts (Pt, Ru, Rh et al.). In addition, they achieve a high yield of CH4 in a short particle residence time (< 60 min) due to the synergy of the binary components. The role of Fe/Co/Ni is to supply active hydrogen and impaired C=C bonds in coal structure. With increasing the loading of the metals, the above behaviors will be strengthened, and the result in Fig. 9a presents that the appropriate amount might be 5%. In terms of Ca, it retards the sintering and poisoning of Fe/Co/Ni and mediates the Fe/Co/Ni-C interaction. With increasing the addition of Ca from 0% to 1%, the promoting effect increases significantly (Fig. 9b). Whereas, further increasing Ca addition from 1% to 2% decreases its promoting effect, attributing to the fact that the small surface area of coal particles restricts the accommodation of catalysts. Thus, a high addition of Ca gives rise to the agglomeration of Fe/Co/Ni metals (Yuan et al. 2017a, b). It is intuitively in Fig. 9 that the activity sequence of the binary catalyst is Co–Ca > Ni–Ca > Fe–Ca. The higher activity of Co–Ca might be correlated with its superior ability to fracture C=C bonds and stronger tolerance towards H2S during hydrogasification, as has been discussed in Sect. 2.1.2.
CH4 is the main gaseous product in CCHG, and the increase of catalyst loading to a proper amount (Fe/Co/Ni: 5%; Ca: 1%) generally facilitates CH4 formation rate and yield. In the binary catalyst system, the increase of Fe/Co/Ni facilitates reactions (Eqs. (4)–(6)), while the increase of Ca promotes (Eqs. (9)–(14)), and thus they cooperate to accelerate the whole coal-H2 reaction process. Herein, it should be noted that although adding 2%Ca has an inferior effect on the dispersion of Fe/Co/Ni than 1%Ca, the maximum CH4 formation rate of 2%Ca was much higher than that of 1%Ca. This result is because the chemical-promoting effect of Ca on Fe/Co/Ni–C interaction was more important than the physical-promoting effect on Fe/Co/Ni dispersion (Yan et al. 2022). In the hydropyrolysis stage, Ca-promoted Fe/Co/Ni–C interaction or Fe/Co/Ni dispersion boosted the cleavage of chemical bonds in coal structure, resulting in the generation of more volatiles and the formation of pyrolyzed coal char with abundant active sites. In the hydrogasification stage, the highly dispersed Fe/Co/Ni alone was not able to catalyze hydrogenation of graphite carbon, while the Ca-promoted Fe/Co/Ni–C interaction triggered the gradual hydrogenation of graphite carbon.
In terms of liquid products such as water, tar, and HCL, their yields depend on the addition of a catalyst. The water originates from catalytic hydropyrolysis of coal and hydrogenation of nitrate/acetate catalyst, and its yield increases as the Fe/Co/Ni or Ca amount because of the hydrogenation of additional oxygen-containing catalyst precursors (Yan et al. 2018). Tar and HCL come from catalytic hydropyrolysis of coal, and the Cat-DH and Cat-HC effects dominate their yields (Yan et al. 2017). In the binary catalyst system, the two effects coexist. A high loading amount of Co (~ 5%) contributes to the Cat-HC effect due to the high acid of the Co–C structure, which decreases tar and HCL yield. A high loading amount of Fe/Ni (~ 5%) conduces to the Cat-DH effect because the acid of Fe/Ni-C structure is moderate, which increases tar and HCL yield. With increasing Ca addition to a proper amount (1%), the Fe/Co/Ni are well dispersed, and the Cat-DH and Cat-HC effects are strengthened, with Cat-DH being enhanced to a greater extent, which favors the yield of tar and HCL.
3.2 Temperature
Reaction temperature affects the generation behavior of volatiles during the hydropyrolysis of coal, the formation rate of CH4 during the hydrogasification of coal char, and the interaction between catalyst and coal during the reaction process. Elaborating on the effect of reaction temperature on these issues will help to tailor the CCHG process. In the hydropyrolysis stage, a high temperature simultaneously promotes the Cat-DH and Cat-HC processes (Yan et al. 2022). The previous results presented that a temperature higher than 600 °C decreases the tar yield drastically because the Cat-HC effect was intensified to a greater extent, as shown in Fig. 10a. In the hydrogasification stage, the hydrogenation of carbon is the primary reaction, which is reversible and exothermic. It is consensus that a high reaction temperature is unfavorable for C–H2 reaction due to the thermodynamic limit. However, the results in Fig. 11 show that a high temperature benefits catalytic hydrogasification, manifesting that the C–H2 catalytic reaction locates in the kinetic-controlling region instead of the thermodynamic-controlling region at the temperature range of 600–900 °C. When the reaction temperature reaches 850 °C, more than 80% of carbon conversion (CH4 yield) is achievable.
Reaction temperature plays two important roles in CCHG. On the one hand, a high temperature promotes the dissociation of hydrogen and the cleavage of chemical bonds. On the other hand, increasing reaction temperature facilitated the diffusion of catalyst in coal structure, which boosted the interaction frequency between catalyst and carbon; as a result, more carbon in coal was catalytically hydrogasified in much reactive form characterized by the activation energy (Yan et al. 2022). Therefore, elevating reaction temperature in the range of 600–900 °C facilitated CH4 formation rate significantly. In general, conducting CCHG above 750 °C ensures a high coal conversion and CH4 yield at the expense of HCL yield, as shown in Figs. 10a and 11.
3.3 Pressure
Model carbon hydrogasification belongs to a volumetric reduction reaction, and increased H2 pressure generally promotes carbon conversion and CH4 yield. When it comes to coal catalytic hydrogasification, H2 pressure not only affects the C–H2 reaction, but also influences the secondary hydrogenation of volatiles during coal pyrolysis. Elaborating the effect of H2 pressure on these issues will help to enhance CH4 and HCL formation. As shown in Fig. 12, the increase of H2 pressure promotes model carbon and coal catalytic hydrogasification, and most of the researchers found that a desirable CH4 yield could be achieved by elevating H2 pressure to 3 MPa. In the presence of a superior Co-based catalyst, a mediate pressure of 3 MPa attains a high carbon conversion of ~ 90%. Herein, it is worth noting that high pressure itself could not greatly facilitate coal conversion. The acting role of pressure should be accompanied by an appropriate temperature. For instance, (Yuan et al. 2017a, b) performed Fe-Ca-catalyzed coal hydrogasification at 700 °C. The results showed that carbon conversion of coal increased slightly from 42.2% to 48.0% with elevating H2 pressure from 1 to 3 MPa. Whereas, (Jiang et al. 2016, 2017) conducted Fe–Ca-catalyzed hydrogasification of coal char at 800 °C, and carbon conversion increased significantly from 5.0% to 70.3% with H2 pressure rising from 0.1 to 2.25 MPa. Our group also found that at a high temperature of 850 °C, the elevation of H2 pressure from 0.6 to 3 MPa increased carbon conversion significantly from 62.1% to 91.3% for Co–Ca catalyzed hydrogasification of coal (Yan et al. 2022). The promoting effect of elevated H2 pressure on CH4 formation rate and yield attributes to the following facts: (i) From a kinetic point of view, large quantities of active hydrogen concentrated around the coal surface, which is appreciable for accelerating the attacking of carbon at the edge of coal; (ii) From a thermodynamic perspective, a high concentration of H2 dilutes the CH4 product; as a result, the reversible C–H2 reaction proceeds to the formation of CH4 more thoroughly.
In addition to CH4, H2 pressure also affects the generation of HCL, tar, CO, CO2, et al. In the catalytic hydropyrolysis stage, high pressure, on the one hand, promoted the three-phase interaction of catalyst-coal-H2, by which the Cat-DH effect was enhanced (Yan et al. 2022). On the other hand, rising pressure hindered the release of volatiles from the coal particle. It increased the residence time of volatiles in the high-temperature region, intensifying the Cat-HC (Zhang et al. 2014, 2016). The overall effect would give rise to the variation of HCL and tar yields in different reaction systems. When CCHG was conducted at 700 °C, the elevation of H2 pressure promoted HCL yield while decreasing tar yield. Whereas, at a reaction temperature of 850 °C, the elevation of H2 pressure promoted HCL yield and tar yield simultaneously (Fig. 10b), appearing that the Cat-DH was more evident than Cat-HC. This result is mainly attributed to the fact that a high temperature stimulated the diffusion of catalyst in the bulk structure of coal, and the catalytic cleavage and hydrogenation of chemical bonds during hydropyrolysis were strengthened greatly, conducing to the effect of Cat-DH. In conclusion, high H2 pressure increased the yield of HCL (Fig. 10b), while the yield of tar depends on the reaction temperature. In terms of CO and CO2, a high H2 pressure promoted the methanation and reversal water–gas shift reactions, and thus, the CO and CO2 as byproducts decreased at the elevated pressure.
3.4 Coal property
The above reviews mainly concentrated on catalytic hydrogasification characteristics of model carbon or idealized coal with low ash, low caking index, low sulfur content, and medium–low rank. However, in the practical production process, the exploited coal usually has one or more of the abovementioned properties, which might influence the instinctive activity of catalysts. For instance, Ni-catalyzed hydrogasification of biomass char achieved a high carbon conversion of 95% at a moderate reaction condition of 850 °C and 0.1 MPa H2 (González et al. 2002). Whereas, for Ni-catalyzed hydrogasification of pitch coke at 850 °C and 1 MPa H2, only ~ 10% carbon conversion was achievable (Haga and Nishiyama 1987). The discrepancy in Ni activity mainly arose from the carbonaceous specimen’s different carbon structures or sulfur content. (Jiang et al. 2017) performed Fe-catalyzed hydrogasification of coal char prepared from the same bituminous coal. The results revealed that the un-thoroughly demineralized char showed higher reactivity as the retained CaO-containing ash promoted Fe activity greatly. Therefore, elaborating the effect of coal properties on CCHG and exploring the process mediating approaches are important for the further application process.
To address this issue, a comprehensive investigation of catalytic hydrogasification of coals with high ash, caking propensity, high rank, or sulfur content was conducted in a pressurized fluidized bed very recently (Yan et al. 2021). The results in Fig. 13 show that CCHG can adapt well to medium–low rank coals with low caking index and low sulfur content directly, and the high ash content has limited residence to the activity of the catalyst. However, a suitable mediation approach should be adopted to realize a high reactivity in terms of high caking, high rank, or high sulfur-containing coal.
Catalytic hydrogasification characteristics of coals with diverse properties in a pressurized fluidized bed (reaction condition: 850 °C, 3 MPa H2) (Yan et al. 2021)
For CCHG of high caking ZJX coal, the blending of a definite amount of medium–low rank FG coal or YM coal not only well addressed its caking propensity due to the physical separation effect, but also greatly promoted the overall CCHG reactivity because of the volatile-catalyst-coal interactions as shown in Fig. 14a, correspondingly, Eqs. (7), (8), (12)–(14) in Sect. 2.1 depicted the interaction process. When it comes to CCHG of high-rank YQ anthracite and high sulfur-containing SHM subbituminous coal, the mechanical blending of a suitable amount of CaO/CaCO3 promotes the reactivity enormously. On the one hand, CaO/CaCO3 migrated into the pore structures of coal to trigger the activity of iron-group metals towards hydrogenation of inert carbon in coal; on the other hand, CaO/CaCO3 captured H2S during CCHG to retard the poisoning of iron-group metals, as depicted in Fig. 14b. The promoting role of CaO/CaCO3 can also be interpreted by the proposed mechanism in Eqs. (9)–(14).
Schematic diagram of probable mechanisms for catalytic hydrogasification of a Caking coal and b High rank/sulfur coal (Yan et al. 2021)
3.5 Impurities in gasifying agent
It is noteworthy that in a practical production process, CCHG in a continuous gasifier might not implemented in pure H2, but in the mixture of H2, H2O, CO2, CO, H2S, et al., because coal-generated H2O, CO2, CO, and H2S affects CCHG. (Feng et al. 2023a) investigated the effect of steam on Co–Ca-catalyzed coal hydrogasification, and the results demonstrated that the presence of 5% steam in the gasifying agent significantly inhibited Co–Ca activity toward hydrogasification. The negative effect of steam could be relieved by elevating the reaction temperature to 900 °C and total pressure. However, other researchers evidence the promoting effect of steam on CCHG. For instance, (Casanova et al. 1983) found that water vapor initiated the CaO-catalyzed depolymerization of graphite/amorphous carbon; thus, carbon in coal existed in a more reactive form and was easily hydrogenated. (McKEE 1974) demonstrated that water vapor in H2 significantly promoted Fe-catalyzed hydrogasification of graphite. The discrepancy in the effect of H2O on CCHG might attributed to the type of catalyst and reaction conditions, and further researches should be conducted to clarify the effect of H2O comprehensively. In terms of CO and CO2, (Gil and Smoliński 2015) proved that adding 10% CO2 to H2 considerably enhanced char hydrogasification. Our previous work (Feng et al. 2022) conducted CCHG in H2 + CO2, and the results demonstrated that Co–Ca containing char improved the CH4 selectivity of CO/CO2 methanation, thus significantly increasing coal-based CH4 production. When it comes to H2S, it is consensus that the ppm grade of H2S poisons Fe/Co/Ni catalysts during CCHG (Matsumoto and Walker 1989; Yan et al. 2021). The negative effect of H2S can be mitigated by elevating the reaction temperature to above 900 °C or adding AAEMs additives (Huttinger and Krauss 1981; Tomita et al. 1983; Yan et al. 2021). A high temperature restrains the strong absorption of H2S on the catalyst surface, and the AAEMs are conductive to react with H2S to form sulfates, by which the poisoning effect of H2S could be eliminated. The above experimental results provide theory guidance to modulate hydrogasification reactivity when conducting CCHG in complicated gasifying conditions.
3.6 Methods for promoting CH4 and HCL in CCHG
In the context of CCHG, it has been noted that the generation of target products (CH4 and HCL) closely correlates with the reaction conditions, including the catalyst type, catalyst loading, reaction temperature, H2 pressure, coal property, etc. In terms of catalyst, 5%Co–1%Ca showed higher activity than that of 5%Ni–1%Ca and 5%Fe–1%Ca as cobalt had a superior performance for activating C=C bonds in coal structure, which facilitated coal depolymerization to boost HCL formation and enhanced coal char hydrogasification to accelerated CH4 formation. For reaction conditions, it is consensus that a high temperature is not beneficial for CH4 yields because a positive standard Gibbs free energy change (ΔG⊖) will emerge. However, plenty of research conducted CCHG at a high temperature of around 800–1000 °C, and high CH4 yields could be obtained (Mısırlıoğlu et al. 2007; Yan et al. 2002; Fan et al. 2023). Herein, it is of interest to ascertain how reaction conditions affect C–H2 reaction. Figure 15 shows the thermodynamic results for the effect of temperature and pressure on the C–H2 methanation reaction. It can be seen that a high temperature decreases CH4 yield in all cases. Nevertheless, when a high temperature is coupled with high pressure, CH4 yield can be boosted to a great extent. Especially at a high C:H2 ratio of 1:4 under 5 MPa, achieving a 100% CH4 yield at 900 °C is possible. This result can be interpreted by Eqs. (15)–(16). At a high reaction temperature, ΔG⊖ is a positive value. When a high hydrogen pressure or C:H2 molar ratio is accompanied, the term RT InJ would be negative because the value of J will be much less than 1. As a result, a negative value of ΔG can be obtained under a high temperature. The thermodynamic results explain well why CCHG experiments are usually implemented under high temperatures and H2 pressure. A high temperature is beneficial to accelerate the methanation reactions, while a high H2 pressure or high C:H2 feeding ratio is conduced to achieve a desired equilibrium state for CH4 yield.
wherein, ΔG is Gibbs free energy change at certain reaction conditions, J/mol; R is the universal gas constant, J/(mol K); T is the reaction temperature, K; J is the reaction quotient; \({P}_{{{\text{CH}}_{{4}} }}\) is CH4 partial pressure, Pa; \({P}_{{{\text{H}}_{{2}} }}\) is H2 partial pressure, Pa; Pθ is standard pressure, Pa.
Figure 16 depicts the Van Krevelen diagram of various hydrogasification feedstocks. The results show that the feedstocks with higher atomic O/C and H/C ratios perform superior reactivity. The higher atomic O/C ratio is suggestive of abundant oxygen-containing species in carbon structure (Zoheidi and Miller 1987; Zhao et al. 2020), and a higher atomic H/C ratio represents that more edge carbon persists (Tomeczek and Gil 2010), and they are commonly recognized as active sites for hydrogasification or catalytic hydrogasification (Zoheidi and Miller 1987; Yan et al. 2018). Therefore, when considering CCHG feedstocks, medium–low rank coals (lignite and subbituminous coal) and biomass with high atomic O/C and H/C ratios are preferred. Apart from these feedstocks, recent works also demonstrated that CCHG could be applied to coals with high ash/caking/rank/sulfur properties. Through the modulating approach of blending low-rank coal or CaO/CaCO3, high carbon conversion (~ 90%) and CH4 yield (~ 80%) were achievable in a short particle residence time of 1.0 h (Yan et al. 2021). These results manifested that the technology of CCHG has the potential to act as a universal method for carbonaceous resources (coal, pitch coke, biomass, solid wastes, et al.)-to-SNG. The effects of experimental variables on CCHG are summarized in Table 5, and the results might provide valuable proposals for regulating target products and dealing with different kinds of carbonaceous feedstocks.
4 Preliminary evaluation of CCHG process
In the CCHG process, the recovery of the catalyst, the emission of the pollutants, the thermal efficiency of the reaction, and the scaling-up perspective are important issues to be concerned. To date, the abovementioned subjects have yet to be addressed comprehensively. This section discussed the Co–Ca-catalyzed coal hydrogasification, focusing on the recycling of Co, the generation behavior of nitrogen/sulfur-containing species, the thermal analysis, and the scaling-up calculation of the whole reaction process preliminarily.
4.1 Recycling of catalyst
In the Co–Ca-catalyzed coal hydrogasification process, Co catalyst existed in metallic form without reacting with the inherent minerals (Qu et al. 2019). Hence, the expensive Co can be recovered by a simple acid leaching method. The result in Fig. 17 shows that a high recovery of 99.9% can be realized for Co, and the recycled catalyst obtained comparable activity with the fresh catalyst. For the laboratory scale of CCHG, the nitrates commonly act as the precursor of catalysts. However, when it comes to the commercial scale of CCHG, large quantities of HNO3 and nitrates would be used. In China, the commercial scale of utilizing nitrates may be prohibited in policy due to the susceptive of preparing explosives; thus, another alternative catalyst for nitrate should be explored.
The schematic diagram of recycling Co catalyst for CCHG (Yan et al. 2017)
Tracing back to the catalyst loading process, the Co salts should be impregnated onto the coal to attain a good dispersion, while the Ca compound could be loaded by wet-impregnation or mechanically mixing (Yan et al. 2021). Therefore, it requires that the Co salts are water-soluble. In addition to nitrates, relatively cost-effective salts such as cobalt acetate, halides, and sulfates are optional. However, (Inui et al. 1979; Feng et al. 2023b) reported that the halides and sulfates easily agglomerated and showed low activity during hydrogasification; thus, only cobalt acetate is available. Figure 18 shows the characteristics of CCHG with acetates with the catalyst. Compared to 5%Co–1%Ca-nitrate, 3%Co–1%Ca-acetate has a comparable carbon conversion, CH4 formation rate, and yield. In terms of tar and HCL, their yields are 7.8% and 2.2%, respectively, much higher than that of 4.04% and 1.5% for 5%Co–1%Ca-nitrate. Therefore, using acetates as a catalyst precursor in CCHG has an advantage over the nitrates as a lower loading amount of Co achieves a higher yield of target products.
The Co catalyst in the char residue can be recovered through acid leaching-precipitation-acid dissolving, as shown in Fig. 18c. The recovery of Co in this procedure also reaches 99.9%, and the recovered Co(Ac)2 and Ca(Ac)2 can be re-impregnated onto the coal to conduct CCHG. The results in Fig. 18a and b show that the activity of the recovered catalyst changes insignificantly. Herein, it is worth noting that the recycling of the catalyst was conducted only once, far less than the required cycles for the practical production process. In the long run, some inherent coal minerals will inevitably be dissolved into the catalyst precursors and then influence Co–Ca activity. To address this issue, our recent work investigated the effect of seventeen mineral impurity elements on Co–Ca-catalyzed coal hydrogasification (Feng et al. 2023b). The results demonstrated that a high Co recovery of 99.7% could be obtained after six cycles, while the activity of Co–Ca catalyst was not able to be maintained because Al and S-containing compounds performed a negative effect on Co–Ca activity. Relevant results will guide the optimization of catalyst recycling and the reusing process by removing the impurities.
For instance, the negative effect of Al can be eliminated by modulating the catalyst recovery process. (Feng et al. 2023b) penetrated the sodium jarosite precipitation technology into the Co recovery process, as shown in Fig. S1. The results prove that more than 95% of Al will be removed into jarosite at a pH of 4.0. As a result, the activity of the recovered catalyst nearly rebounded to the level of the fresh catalyst, which showed great potential for application. In terms of S-containing compounds, they mainly reacted with Ca salts and thus lowered the synergy catalytic effect between Co and Ca. This effect could be mitigated by adding extra CaO/CaCO3 into the CCHG system, as it had been demonstrated that the physically mixed CaO/CaCO3 could migrate into the cobalt-loaded char and promote the activity of Co enormously (Yan et al. 2021).
4.2 The emission of sulfur/nitrogen-containing species
SOx, H2S, NOx, HCN, et al. are the commonly reported pollutants in coal gasification, with steam, CO2, or O2 as the gasifying agent (Yuan et al. 2012; Duan et al. 2017). However, under a catalytic hydrogasification condition, the formation behavior of sulfur and nitrate species is not well documented, especially in the presence of a catalyst. For sulfur-containing species, (Tomita et al. 1983; Liang et al. 2016) reported that sulfur in coal was mainly generated in the form of H2S during CCHG, which poisoned the iron-group metals. Very recently, our group conducted Co–Ca-catalyzed hydrogasification of high-sulfur coal (Yan et al. 2021). The results revealed that the mechanically mixed CaO in a fluidized bed could capture the evolved H2S, and the gaseous products contained no sulfur-containing species detected by the mass spectrometry, suggesting that the CCHG process possesses an important characteristic of generating no sulfur-containing pollutants in the gaseous products.
In terms of nitrogen-containing species, it can be seen in Fig. 19 that nitrogen in coal and nitrate catalysts mainly evolved in the form of N2 and NH3, tar-N and char-N during CCHG, while no NOx was detected by using the equipment of NOx analyzer (NOx5210). In the presence of a Co–Ca catalyst, more than 95% of N was converted into N2 and NH3 with an NH3 selectivity of 28.8%, and the detailed analysis of products found that nearly all NH3 dissolved in the condensed water. Preliminary experimental results revealed that the CCHG-to-SNG process is an environmentally friendly process without generating nitrogen or sulfur-containing pollutants in gaseous products. Moreover, the nitrogen in coal and catalyst precursors can be resourced into a valuable product of ammonia, which can potentially complement the ammonia synthesis process.
The mass balance of nitrogen in CCHG (reaction condition: 850 °C, 3.0 MPa H2; Total nitrogen = nitrogen in coal + nitrogen in nitrate catalyst; ‘Tar–N’, ‘NH3–N’, ‘N2–N’, and ‘Char–N’ refer to the distribution of nitrogen in Tar, NH3, N2, and coal char, respectively) (Yan et al. 2022)
4.3 Thermal efficiency
Based on the available experimental data for CCHG, the thermal efficiency can be preliminarily evaluated. The basic data is presented in Table 6, and the following calculation procedure is adopted with reference from (Steinberg 2005):
-
(1)
Catalytic hydrogasification of FG bituminous coal
$$\begin{aligned} & {\text{CH}}_{{0.{74}}} {\text{O}}_{{0.{14}}} + {1}.{\text{31H}}_{{2}} = 0.{\text{8CH}}_{{4}} + 0.0{\text{35CO}} + 0.0{\text{25CO}}_{{2}} + 0.0{43}\left( {{\text{CH}}} \right) + 0.0{\text{97C}} + 0.0{\text{55H}}_{{2}} {\text{O}} \\ & \Delta {\text{H}}_{{1}} = - {16}.0{\text{6kcal}}/{\text{mol}} \\ \end{aligned}$$(17)wherein, the automatic ratio of C, H, and O is calculated based on the ultimate analysis; the CH4 is assumed to be the sum products of CH4 and C2–C3 in Table 6; (CH) is assumed to be the tar; C is referred to as char.
In terms of reaction heats, the coal pyrolysis stage is assumed to be 1.14 kcal/mol, while the heat released for generating CH4 in the hydrogasification stage is − 17.2 kcal/0.8 mol CH4, calculated based on the stoichiometric reaction of C + H2 = CH4 (∆H = − 21.5 kcal/mol). Therefore, the overall reaction heat for CCHG is − 16.06 kcal/mol (∆H1).
-
(2)
Hydrogen production
$$0.0{\text{35CO}} + 0.0{\text{35H}}_{{2}} {\text{O}} = 0.0{\text{35CO}}_{{2}} + 0.0{\text{35H}}_{{2}} \quad \Delta {\text{H}}_{{{2} - {1}}} = 0{\text{kcal}}/0.0{35}\;{\text{mol}}$$(18)$$0.{\text{571CH}}_{{0.{74}}} {\text{O}}_{{0.{14}}} + {1}.0{\text{63H}}_{{2}} {\text{O}} = {1}.{\text{275H}}_{{2}} + 0.{\text{571CO}}_{{2}} \quad \Delta {\text{H}}_{{{2} - {2}}} = {14}.{\text{35kcal}}/0.{571}\;{\text{mol}}$$(19)wherein, 1.31 mol H2 for CCHG comes from the water–gas shift reaction and coal gasification; The raw material is 0.035 mol CO generated from CCHG and 0.571 mol FG bituminous coal, respectively. The overall reaction heat for H2 production is 14.35 kcal/mol (∆H2).
-
(3)
Lower heating value (LHV) of CH4 product
$$0.{\text{77CH}}_{{4}} + {\text{O}}_{{2}} = 0.{\text{77CO}}_{{2}} + 0.{\text{385 H}}_{{2}} {\text{O}}\quad \Delta {\text{H}}_{{3}} = - {163}.{2}\;{\text{kcal}}/0.{77}\;{\text{mol}}$$(20)wherein, it is assumed that 0.03 mol CH4 of equivalent energy is used for catalyst recovery; thus the net CH4 production is 0.77 mol.
-
(4)
LHV of raw coal
$${1}.{\text{571CH}}_{{0.{74}}} {\text{O}}_{{0.{14}}} + {1}.{\text{752O}}_{{2}} = {1}.{\text{571CO}}_{{2}} + 0.{\text{581H}}_{{2}} {\text{O}}\quad \Delta {\text{H}}_{{{4} - {1}}} = - {187}.{3}\;{\text{kcal}}/{1}.{571}\;{\text{mol}}$$(21)$$0.{\text{123CH}}_{{0.{74}}} {\text{O}}_{{0.{14}}} + 0.{\text{137O}}_{{2}} = 0.{\text{123CO}}_{{2}} + 0.0{\text{46H}}_{{2}} {\text{O}}\quad \Delta {\text{H}}_{{{4} - {2}}} = - {14}.{67}\;{\text{kcal}}/0.{123}\;{\text{mol}}$$(22)wherein, the 1.571 mol CH0.74O0.14 is supplied for hydrogasification and hydrogen production, while the combustion of 0.123 mol CH0.74O0.14 is used for compensating the endothermic gasification reaction in procedure 2).
-
(5)
Overall thermal efficiency
The ratio of output energy to input energy is the overall thermal efficiency, which can be calculated as follows:
$${\text{Thermal}}\;{\text{efficiency}} = (\Delta {\text{H}}_{{1}} + \Delta {\text{H}}_{{2}} + \Delta {\text{H}}_{{3}} )/(\Delta {\text{H}}_{{{4} - {1}}} + \Delta {\text{H}}_{{{4} - {2}}} ) = {81}.{8}\%$$
Preliminary results reveal that compared to other coal-to-SNG technologies in Table 1, CCHG not only harvests a higher yield of CH4, but also has higher thermal efficiency that in turn mitigates CO2 emission. Therefore, scaling up CCHG is feasible to reduce the emissions (SOx, NOx, and CO2) for SNG production, which fulfills the context of green manufacturing.
4.4 Suitable gasifier for CCHG
Table 7 presents the characteristics of different reactors commonly applied to coal gasification, including fixed bed gasifier, fluidized bed gasifier, entrained bed gasifier, and plasma gasifier (Chanthakett et al. 2021; Midilli et al. 2021). A fixed bed reactor is usually adopted for the fundamental research of CCHG, because it is capable of dealing with a small dose of coal sample to ensure the reproducibility of the results, collecting the gas–liquid-solid three-phase products, and attaining the instinct reaction kinetics. Whereas, the fixed bed reactor might not be the suitable gasifier, as CCHG treats pulverized coal (< 6 mm) instead of lump coal (6–50 mm) for impregnating catalyst. In addition, CCHG is a strongly exothermic reaction, and the large quantities of reaction heat might be hard to remove in situ, resulting in the flying of temperature and the sintering of the catalyst.
When it comes to the fluidized bed reactor, pulverized coal can be used, and the reaction temperature of 800–1000 °C, pressure of 10–30 bar, and minute-level particle residence time can all be suited to CCHG. The pulverized coal particles used for impregnation of catalysts, an appropriate reaction temperature and H2 partial pressure (850 °C, 3 MPa) favor the activity of catalysts, and the minutes of reaction time is necessary for achieving a high carbon conversion and CH4 yield. It is consensus that the fluidized bed has a profound transferring endowment, which promises good temperature-controlling ability and makes CCHG proceed fluently (Xia et al. 2021). Moreover, as indicated in Sects. 3 and 4, CCHG in a pressurized fluidized bed can be well adapted to coals with diverse properties, and it produces SNG with little toxic emissions. Therefore, CCHG in a fluidized bed gasifier overcomes the drawback of a fixed bed gasifier; simultaneously, the advantages of the entrained bed gasifier (flexibility on coal types) and plasma gasifier (low emission of carbon and toxins) are also taken.
4.5 Coupling of reaction and fluidization process underlying scaling-up of CCHG
When conducting CCHG in a fluidized bed gasifier, H2 acts as not only the gasifying agent, but also the fluidization medium, which leaves open questions about the integration of reaction and fluidization. That is, in the context of a scaling-up process, the feeding H2 should make the coal particles well fluidized and thoroughly converted by obeying the following criteria: (1) the fluidizing number (N) locates in 2–5; (2) the ratio of dense-phase bed height (H) to the inner diameter of fluidized bed (D) is 2–5; (3) the mass of H2 to the mass of coal is 0.25–0.67 to ensure a sufficient supply of H2 and high utilization of hydrogen atom. Wherein, H2/Coal (mass ratio, referred to as ‘r’) of 0.25 is the stoichiometric hydrogen for the C–H2 methanation reaction, while r of 0.67 is the value used for generating the products in Table 6, and it is considered as the upper-limit for supplying H2. Thus, N and H/D are associated with fluidization, while the value of r correlates with the reaction, and these values should be well coupled.
In the context of scaling-up of CCHG in a fluidized bed, there are many independent variables to be ascertained to fulfill the above-mentioned requirement, consisting of coal particle diameter (dp), reactor diameter (D), fluidizing number (N), and mass feeding rate of coal (W). By taking the results in Table 6 as the basis for scaling up CCHG, the known experimental variables and the restrictions for designing the fluidized bed are listed in Table 8. In the commercial scale of CCHG, the value of dp is estimated to be 2 mm for pulverized coal, while the value of D is determined as 3.6, 3.0, and 2.5 m in the calculation process. The calculating procedure is shown as follows:
1. Minimum fluidization velocity (umf)
The Ergun equation is used for calculating the minimum fluidization velocity of coal particles (Chitester et al. 1984):
wherein, \(\rho_{\text{g}}\) is H2 density, kg/m3; μg is H2 viscosity, Pa·s; k2/2k1 = 28.7, 1/k1 = 0.0494, and Ar is the Archimedes constant.
2. Fluidization velocity (u) and mass feeding rate of coal (W)
Generally, W can be determined to produce a definite amount of SNG per year from CCHG. For instance, Table 6 indicates that 1.2 Nm3 CH4 can be generated from 1 kg coal in CCHG. Accordingly, to produce 2 billion cubic CH4 from CCHG per year, 5400 t/d coal should be handled. Based on the determined W, the u and r can be determined with varying N (2–5). Meanwhile, the value of H can also be ascertained according to Eqs. (26)–(28).
3. Bed expanding height
(Babu et al. 1978) proposed an equation taking into account high-pressure expansion data shown as follows:
wherein, Hmf is the bed expanding height at the minimum fluidization velocity, m; ρp is the coal particle density, kg/m3; t is the residence time of coal particle, h; \(\varepsilon_{\text{mf}}\) is the bed voidage at the minimum fluidization velocity; Remf is the Reynolds number at the minimum fluidization velocity.
Figure 20 presents the calculating results for scaling-up of CCHG in a fluidized bed using Eqs. (23)–(28). It can be seen that with the increase of D, the capacity of the fluidized bed increases at the precondition that the values of N, r, and H/D are located in the appropriate range of 2–5, 0.25–0.67, and 2–5, respectively. For the diameter of 2.5 m, the handling of 1800 t/d coal is capable as N, r, and H/D are well coupled (Fig. 20a). With increasing the capacity to 2700 t/d, the value of H/D exceeds the range of 2–5 (Fig. 20b), which might be inappropriate. Figure 20c and d show that the reactor diameter of 3.0 m can deal with 2700 t/d coal, while unable to handle 5400 t/d coal due to the high H/D ratio. 3.6 m is the upper limit for designing the diameter of a fluidized bed reactor in practical application, and this value makes the reactor capable of handling 2700–5400 t/d coal, as shown in Fig. 20e and f. This result might be striking because it means that 2 billion cubic SNG can be produced in one gasifier dealing with 5400 t/d coal.
Tracing back to the coal-to-SNG technologies in Table 1, CCG and CCHG have attracted wide attention in recent years because of their high thermal efficiency, high CH4 yield, and short process. In the commercial coal-to-SNG process, the production of 2 billion cubic SNG /year is the minimum limit, which requires 12 fluidized bed gasifiers (2 gasifiers for standby) for CCG, and 5 fluidized bed gasifiers for CCHG with 2 gasifiers for generating SNG (1 gasifier for standby) and 3 gasifiers for supplying H2 (1 gasifier for standby). The much fewer gasifiers needed for CCHG attributes to the fact that much more CH4 is produced from an equal amount of coal as compared to CHG, which exhibits a great perspective for the investment of facilities. If demonstrated, CCHG in a pressurized fluidized bed with pulverized coal as the raw material has the potential to act as a complement for the mature two-stage gasification (Lurgi technology) with lump coal as the raw material.
5 Opportunities and strategic approaches for CCHG
5.1 Opportunities
While there are numerous pressures with the utilization of fossil fuels in the context of carbon neutralizing, there exist many opportunities for innovative gasification technologies that are clean, thermally efficient, and able to reduce the environmental impact. Of the existing coal-to-SNG technologies, the release of CO2 in the CCHG process is estimated to be 1.71 kg/Nm3 CH4, much lower than that of commercialized Lurgi technology (4.03 kg/Nm3 CH4) and demonstrated CCG technology (3.22 kg/Nm3 CH4) from a reaction point of view (Chen et al. 2017). Moreover, CCHG, with a higher efficiency of up to 81.8%, may have an additional contribution to reducing greenhouse gas emissions. From an environmental perspective, CCHG generates little nitrogen/sulfur-containing pollutants in gaseous products, and thus, applying CCHG for SNG production might minimize the damage to nature.
The conventional Lurgi process generates large amounts of ash and waste exposed in products other than gases, needing further disposal (Yang et al. 2017). When the SNG production process is done with CCHG, the solid and liquid products can be used as resources. For instance, there remains 8.7 wt% carbon unable to be converted in the CCHG process (Table 6), and detailed analysis of the remaining carbon reveals that it is characterized by large amounts of reactive sites and dominated mesoporous structure with a high surface area of 409 m2/g after recovering of the catalyst (Qu et al. 2019). These properties make it possible to be used as a profound raw material, catalyst, or carrier in the field of tar upgrading (Chen et al. 2020), flue gas desulfurization (Song et al. 2017), catalytic graphitization (Liu et al. 2013), CO/CO2 methanation (Ipsakis et al. 2021), microwave absorbents (Liang et al. 2022), et al. In terms of liquid products, CCHG is able to produce 3.36 wt% HCL as a byproduct (Table 6), and it can be used as valuable chemicals. In addition, the dissolved NH3 in the water product (Sect. 4.2) can also be resourced. It can be estimated that CCHG generates ~ 66,000 tons of HCL and ~ 16,478 tons of NH3 along with 2 billion cubic SNG per year in one fluidized bed gasifier, which may be an important aspect making the economy of the process favorable.
5.2 Strategic approaches
The investment of CCHG strongly depends on the availability of low-cost hydrogen. Coal gasification is the mature H2 production process in China and other countries mainly relying on imported natural gas, which generates a large amount of CO2 (~ 1 kg CO2/Nm3 H2) (Jin et al. 2022). It is noteworthy that today, partly H2 can be obtained from renewable sources, including biomass, wind, and solar (Bargiacchi et al. 2021; Valizadeh et al. 2022; Lu et al. 2023; Ghodke et al. 2023). Renewable energy-initiated electrolysis has a modest effect on the environment, and the CO2 emission is below 0.18 kg CO2/Nm3 H2 (Tosti et al. 2016), much lower than that of the coal gasification-to-H2 process. (Glenk and Reichelstein 2019) established a model and predicted that in the next ten years, the power to gas technologies would produce renewable H2 with costs competitive to the gasification process. The model also predicted that the policy incentives could make renewable H2 more economical in places like Texas and Germany. In this regard, the renewable H2 acts as an intermediate “energy vector” for CH4, and CCHG produces SNG with an extremely low CO2 emission of ~ 0.4 kg CO2/Nm3 CH4. As a result, CCHG-based SNG is not only an attractive, versatile energy carrier for coal, but also stores surplus power from renewables in off-peak hours, which promotes the penetration of renewables and fossils in future energy systems.
Figure 21 presents a schematic description of the future possibilities of CCHG technology. The “green” H2 generated through renewable energies is an important medium for hydrogenating carbonaceous resources, including coal, biomass, CO2, etc. By the approach of CCHG, the carbonaceous resources can be converted into useful chemicals and materials such as SNG, synthesis gas, NH3, HCL, and activated carbon with mesoporous structure. The recovered catalyst and condensed water can be recycled for impregnation and electrolysis. Herein, it is noteworthy that anticipated by the global urgent for reducing CO2 emissions and the market need for SNG in Asian and EU countries, the CCHG technology is not only a service for merely one carbonaceous resource, but may be able to deal with multi-carbonaceous resources, such as co-CCHG of coal and biomass, co-CCHG of coal and CO2, et al. To date, the catalytic hydrogenation of individual coal, biomass, or CO2 has already been studied (Zhou et al. 2020; Wang et al. 2022). Whereas, few researches, to the best of our knowledge, have provided an analysis on the coupled CCHG of coal and biomass, or coal and CO2, with H2 as the gasifying agent. It may be meaningful to gain some insights into this issue because implementing renewables and the penetration of CO2 utilization in coal catalytic hydrogasification helps mitigate carbon emissions in the context of SNG production. To this end, our group investigated co-catalytic hydrogenation of coal and biomass/CO2 in a pressurized fluidized bed very recently (Yan et al. 2021; Feng et al. 2022), and preliminary results are shown in Figs. 22 and 23.
Experimental results for co-CCHG of FG bituminous coal and biomass (corn stalks, CS) in a fluidized bed (reaction condition: 850 °C, 3 MPa H2) a Product distribution b The calculated and experimental yields of CO and CO2 with blending different fraction of biomass c CH4 formation rate with adding 20% biomass d CH4 formation rate with adding 30% biomass
Co-catalytic hydrogenation characteristics of FG bituminous coal and CO2 in a pressurized fluidized bed (reaction condition: Fe/Co/Ni–Ca catalyst, 850 °C, 3 MPa, 80%H2–20%CO2 with H2 flow rate 12 L(STP)/min and CO2 flow rate 3 L(STP)/min) a Carbon balance of the reaction system b Product yields on the basis of carbon in coal c Main gaseous product formation behavior d The yield of CO and CH4 form CO2 catalytic hydrogenation (‘Blank’ refers to CO2 hydrogenation without the existence of catalyst)
5.2.1 Co-CCHG of coal and biomass
Biomass is a carbon–neutral feedstock with high reactivity, and it can be hydrogasified without adding a catalyst (Tian et al. 2021). We preliminarily conducted biomass hydrogasification and co-CCHG of biomass and coal in a fluidized bed, and the experimental details are presented in Methodology S2. As shown in Fig. 22, biomass hydrogasification achieves a high conversion of 90.1% and generates a considerable amount of CO (27.5%), SNG (30.6%), and tar (3.88%). Despite the profound results, the large-scale hydrogasification of biomass is impractical due to the low energy density and the restricted seasonal availability. In this regard, co-CCHG of coal and biomass provides a strategy for clean and efficient utilization of fossil fuel and renewable energy.
As shown in Fig. 22a, the penetration of 20 wt%–30 wt% biomass into CCHG yields comparable results with coal catalytic hydrogasification. The results in Fig. 22b show that adding 20 wt%–30 wt% biomass into CCHG greatly decreases the generation of byproducts, including CO and CO2, reaffirming co-CCHG is a low-carbon-emission process. Detailed analysis of CH4 formation behavior in Fig. 22c and d reveals that biomass promotes coal catalytic hydrogasification remarkably. Especially at the addition of 30%, the maximum CH4 formation rate for the experimental result is 1.94 times that of the linear result, indicating an intensive interaction between biomass and catalyst-containing coal during co-CCHG. The promoting effect might be attributed to the role of alkali & alkaline earth metals in biomass or the transferring of large quantities of radicals with low molecules from biomass to coal (Ellis et al. 2015; He et al. 2021; Wang et al. 2021; Wu et al. 2021). Further researches are needed to elaborate the interaction mechanism clearly, which will help provide theory guidance for the design of specimens and enhance the CCHG process by adopting a cheap and disposable Fe-Ca catalyst.
5.2.2 Co-catalytic hydrogenation of coal and CO2
The transformation of CO2 to valuable chemicals such as CO and CH4 using renewable H2 has proven to be an emerging solution to mitigate climate change and global warming (Saeidi et al. 2021). The in-depth research on this process is consistent with the European policy with the target of reducing 80% of greenhouse gas emissions compared to 1990 levels by 2050 (Ipsakis et al. 2021), Chinese policy to peak greenhouse gas emissions by 2030 and reach carbon–neutral by 2060 (Xie 2021), respectively. It is worth mentioning that in CO2 utilization, a hydrogen-rich condition is needed, and expensive supported catalysts with the configuration of complicated physicochemical structures are required (Saeidi et al. 2021). In addition, restricted by the thermal equilibrium of CO2 methanation, the reaction temperature should not surpass 350 °C to achieve a considerable yield of CH4, which results in a low kinetic process and low CO2 utilization capacity (generally less than \({5}.{9}\;{L}_{{{\text{CO}}_{{2}} }} /{\text{h}} \;{\text{g}}_{{{\text{cat}}}}\)) (Lee et al. 2021; Shao et al. 2021).
To promote CO2 utilization efficiency economically, attaining a high conversion rate of CO2 in a short gas residence time with the use of recyclable and non-noble metals is desired. To this end, developing a coupled process combining CO2 catalytic hydrogenation and coal catalytic hydrogasification in one fluidized bed reactor could be a solution. Wherein, the hydrogen-rich atmosphere endows CO2 hydrogenation, the relatively high temperature and pressure accelerate the CO2 conversion rate, and the impregnated Fe/Co/Ni-Ca not only acts as the catalyst for coal hydrogasification, but might also promote CO2 hydrogenation and methanation as the in-situ generated catalyst containing char is rich in mesopore structures and active sites. If this integrated process simultaneously realized a high conversion of coal and CO2 in one pressurized fluidized bed under the same reaction condition, the CH4 yield and H2 utilization efficiency could be boosted greatly, along with the reduced capital investment, operation cost, and energy consumption.
Our group has conducted a pioneering study to integrate coal catalytic hydrogasification and CO2 catalytic hydrogenation in a fluidized bed (Additional file 1: Methodology S2). Part of the updated data is presented in Fig. 23. It can be seen that CH4 and CO are the main products with coupling CCHG and CO2 hydrogenation (Fig. 23a). The catalyst evaluation results demonstrated that Co–Ca catalyst exerts superior activity from the aspects of carbon conversion of coal (92.4%) and CH4 yield (247%, calculated based on carbon in coal). Remarkably, the CH4 yield is much higher than that of CCHG in pure H2 (77.3% in Table 6), suggesting that in the CCHG-to-SNG process, coal resource can be partly substituted by CO2, because CO2 catalytic methanation contributes to a high share of CH4 (more than 50%) when it is coupled with coal catalytic hydrogasification.
Figure 23c shows that Co–Ca catalyzed hydrogenation of coal and CO2 proceeds to a steady state at 60–80 min, wherein carbon conversion of coal reaches 92.4%, and CO2 is the only carbon resource converted to CO and CH4 with the Co–Ca containing char residue acting as the catalyst. Detailed results in Fig. 23d reveal that Co–Ca containing char possesses profound activity towards CO2 methanation with CO2 conversion and CH4 yield respectively achieved to be 77.8% and 34.5%, which approaches the equilibrium results (79.2% for CO2 conversion and 36.2% for CH4 yield). Therefore, although a high temperature is adverse to the methanation of CO2, the CO2 conversion rate can be greatly accelerated, making the required residence time for achieving a considerable CO2 conversion well coupled with the short gas residence time of ~ 4 s for CCHG in a pressurized fluidized bed. Especially in the presence of Co–Ca containing char as the superior catalyst, the methanation of CO2 reaches equilibrium in the gaseous residence time of ~ 4 s, ensuring that large quantities of CO2 can be dealt with to generate SNG when CO2 catalytic hydrogenation is integrated with coal catalytic hydrogasification in a pressurized fluidized bed. According to the preliminary experimental results, it can be calculated that the CO2 utilization capacity is \({17}.{7}\;{L}_{{{\text{CO}}_{{2}} }} /{\text{h}} \;{\text{g}}_{{{\text{cat}}}}\), while the CO2-based CH4 space–time yield is \({7}.{6}\;{L}_{{{\text{CH}}_{{4}} }} /{\text{h}} \;{\text{g}}_{{{\text{cat}}}}\), far surpassing the reported results for catalysis of CO2 methanation as summarized in Additional file 1: Table S1.
Herein, it is noteworthy that the capacity of dealing \({17}.{7}\;{L}_{{{\text{CO}}_{{2}} }} /{\text{h}} \;{\text{g}}_{{{\text{cat}}}}\) is subversive in the progress of CCHG when considering scaling up. As estimated in Sect. 4.5, 5400 t/d coal can be progressed to produce 2 billion cubic SNG/year, wherein 8.7% char residue acts as the catalyst for CO2 hydrogenation. On this basis, it is calculated that ~ 5.5 million tons of CO2 can be dealt with, amounting to 0.015% and 0.048% of the total CO2 emission in 2022 of the world and China, respectively. This result is striking because approximately 8.8% of CO2 can be neutralized in China by applying the technology of CCHG to produce 366 billion cubic SNG/year (SNG consumption in 2022). Therefore, the coupling of coal catalytic hydrogasification and CO2 catalytic hydrogenation in a pressurized fluidized bed provided a novel strategy for SNG production in the context of green manufacturing. Further researches are deserved to investigate the detailed mechanism of catalyst-containing char on CO2 methanation under CCHG conditions, and also, the acting behavior of CO2 hydrogenation on coal catalytic hydrogasification can be explored. A clear elaboration of these issues will provide theory guidance for designing reaction conditions and tailoring product distribution in a practical continuous-fed fluidized bed.
Table 9 summarizes the existing approaches for manufacturing CH4 from CH4 production, H2 consumption, and CO2 emission perspectives. The detailed calculation procedures for the data and stoichiometric reactions are given in Additional file 1: Methodology S1 and Table S3 of support information, respectively. The results show that CCHG, Co-CCHG of coal and Biomass, and Co-CCHG of coal and CO2 are emerging approaches for boosting CH4 production with fewer coal consumptions and CO2 emissions, which shows advantages over mature TSG and CCG technologies. Herein, it is interesting to find that biomass hydrogasification is a carbon-sinking process with CH4 as the end of the carbon trajectory; whereas, CH4 production from CS hydrogasification is unsatisfied. Therefore, the coupling of CS hydrogasification and CCHG might be preferred because the penetration of biomass not only generates additional CH4, but also mitigates CO2 emission. Herein, it should be noted that the calculations in Table 9 are based on the supply of ‘grey’ H2 (generated from coal gasification), which releases large quantities of CO2 (11 kg CO2/kg H2). Once the ‘green’ H2-based technologies from biomass pyrolysis/gasification or wind/solar-induced water electrolysis were matured, CO2 emissions would be decreased drastically, especially for Co-CCHG of coal and CO2 and CO2 catalytic methanation technologies.
6 Conclusions and outlooks
Among the existing coal-to-SNG technologies, coal catalytic hydrogasification seems promising due to a high CH4 yield, high thermal efficiency, and low emissions. In addition to CH4, other gas–liquid-char products such as ammonia, HCL, and activated carbon can also be resourced as valuable chemicals and materials. In the past decade, pioneer researches have well performed with a focus on the design of catalyst, the catalysis process of model carbon/coal hydrogasification, and the effect of experimental variables. This paper reviews the above topics systemically, and the critical issues concerning process analysis, opportunities, and strategic approaches for CCHG under the constraint of carbon neutrality are also discussed preliminarily. The main conclusions and future directions are as follows:
-
(1)
The binary catalysts composed of 1%–5% Fe/Co/Ni and 1%Ca show profound activity toward model carbon/coal hydrogasification. The iron group metals supply active hydrogen (H·) and impair C–C bonds for coal-H2 reaction, while the calcium compounds promote Fe/Co/Ni distribution, retard Fe/Co/Ni poisoning, and mediate Fe/Co/Ni-C interaction to initiate the Cat-DH effect during pyrolysis and catalytic hydrogenation of graphite carbon (rate determining step) during gasification. The prior findings are meaningful for understanding the macro reaction characteristics, but remain kind of superficial. Comprehensive insights about the active center and the detailed interaction between CaO and Fe/Co/Ni–C structure during hydropyrolysis and hydrogasification are paramount for catalyst design and product tailoring. Accordingly, the in-situ characterization techniques (XRD, TEM, X-ray absorption) and DFT calculations should be employed to reveal the catalysis process on a molecule scale.
-
(2)
The elevation of temperature and H2 pressure under 600–900 °C and 0.1–8 MPa generally promotes coal conversion and CH4 yield, wherein CCHG is dynamically controlled. CCHG can be suited to coals with high ash content/caking propensity/high rank/sulfur content properties. The findings provide strategies for mediating the reaction process, such as step-wise hydrogenation to boost CH4 and HCL yield simultaneously, blending low-rank coals to suppress agglomeration and promote overall reactivity, and mechanically mixing CaO/CaCO3 to fix H2S and accelerate CH4 formation. A universal instinct reaction kinetics for catalytic hydropyrolysis and catalytic hydrogasification of coal with diverse properties should be further established to provide a basis for the precise design of a gasifier.
-
(3)
The pressurized fluidized bed might be the suitable gasifier for CCHG from the viewpoint of millimeter/micrometer-sized particles (< 5 mm), minute-level particle residence time, and high mass/heat transfer efficiency. In the presence of a superior Co–Ca catalyst, it is estimated that 2 billion cubic SNG /year, along with ~ 66,000 tons HCL and ~ 16,478 tons NH3, can be produced in one gasifier with an inner diameter of 3.6 m and coal capacity of 5400 t/d. In addition, the CCHG process generates CH4 with a high thermal efficiency of 81.8%, little NOx or SOx pollutants, and small CO2 emission (1.71 kg/Nm3 CH4), which exhibits a distinguishing advantage over the commercialized Lurgi technology and the demonstrated CHG technology. Further researches should perform CCHG in a kilogram-fed continuous fluidized bed to determine the proper coupling parameters for reaction and fluidization, including N, r, and H/D. In addition, the mathematical model of reaction-transfer-fluidization for CCHG deserves to be established through the approach of CFD, which will be helpful for exploring the scaling-up characteristics of the fluidized bed gasifier.
-
(4)
There are numerous opportunities and strategic approaches for CCHG. Once the “green” H2 acts as an intermediate “energy vector” for CH4, the emission of CO2 in CCHG greatly reduces to ~ 0.4 kg CO2/Nm3 CH4. In addition, the CCHG of coal can be integrated with biomass hydrogasification or CO2 hydrogenation, by which CCHG-to-SNG is strengthened, and fossil feedstock is partly substituted by renewable resources. Preliminary trials have proved that the coupled CCHG process is reliable in transforming biomass and CO2 into valuable fuels and chemicals, which would be helpful to circulate carbon in order to diminish the greenhouse effect. Especially for co-catalytic hydrogenation of coal and CO2, the CO2 capacity and CO2-based CH4 space–time yield amounts to \({17}.{7}\;{L}_{{{\text{CO}}_{{2}} }} /{\text{h}} \;{\text{g}}_{{{\text{cat}}}}\) and \({7}.{6}\;{L}_{{{\text{CH}}_{{4}} }} /{\text{h}} \;{\text{g}}_{{{\text{cat}}}}\), respectively, far surpassing the reported results for catalysis of CO2 methanation in the literature. As a future work, the interaction mechanism between biomass/CO2 hydrogenation and CCHG can be elaborated, and conducting a detailed process and economic analyses to establish an optimized configuration of the integrated process.
The outcomes of this paper can lay the theory foundation, provide basic data, and create incentives for further researches about designing efficient catalysts and configuring reaction processes for converting carbonaceous resources such as coal, biomass, CO2, wastes et al. to value-added products environmentally and efficiently. The bottleneck problem for commercializing CCHG might be the design of efficient and recyclable catalysts. In terms of the expensive Co-based catalyst, how to recover Co from CCHG completely and cycle Co in CCHG with little activity loss is urgent to be addressed. When considering the in-expensive Fe-based catalyst, developing a modulated process for enhancing Fe activity towards the C–H2 reaction will be of significance.
Availability of data and materials
The data that support the findings of this review are available on request from the corresponding author.
Abbreviations
- AAEMs:
-
Alkali–alkaline earth metals
- Ar:
-
Archimedes constant
- BTX:
-
Benzene, toluene, and xylenes
- C2–C3:
-
C2H6, C2H4, C3H8, and C3H6
- Cat-DH:
-
Catalytic depolymerization and hydrogenation
- Cat-HC:
-
Catalytic hydrocracking
- CCHG:
-
Coal catalytic hydrogasification
- CCG:
-
Coal catalytic gasification
- CHG:
-
Coal hydrogasification
- Co–Ca:
-
Cobalt–calcium binary catalyst
- co-CCHG:
-
Combining coal cataytic hydrogasification and biomass hydrogasification
- CS:
-
Corn stalks
- D:
-
Inner diameter of fluidized bed (m)
- DFT:
-
Density functional theory
- d p :
-
Coal particle diameter (m)
- FG:
-
Fugu bituminous coal
- H:
-
Height of dense phase bed (m)
- HCL:
-
Hydrocarbon liquids
- LHV:
-
Lower heating value
- N:
-
Fluidization number
- P θ :
-
Standard pressure (Pa)
- \({P}_{{{\text{CH}}_{{4}} }}\) :
-
CH4 partial pressure (Pa)
- PCX:
-
Phenol, cresols, and xylenols
- \({P}_{{{\text{H}}_{{2}} }}\) :
-
H2 partial pressure (Pa)
- PRT:
-
Particle residence time
- PS:
-
Particle size
- r :
-
H2/coal mass ratio
- R :
-
Universal gas constant (J/mol K)
- Re:
-
Reynolds number
- Remf :
-
Reynolds number at the minimum fluidization velocity
- RE:
-
Renewable energy
- RTZ:
-
Reaction zone temperature
- SHM:
-
Shanghaimiao high sulfur sub-bituminous coal
- SNG:
-
Substituted natural gas
- STP:
-
Standard temperature and pressure
- T :
-
Temperature (K)
- t :
-
Residence time of coal particle (h)
- TEM:
-
Transmission electron microscopy
- TSG:
-
Two-stage gasification
- J :
-
Reaction quotient
- u :
-
Gas velocity (m/s)
- u mf :
-
Minimum fluidization velocity (m/s)
- \({V}_{{{\text{CH}}_{{4}} }}\) :
-
CH4 formation rate (mL/g min)
- VM:
-
Volatile matter
- W :
-
Mass feeding rate of coal (kg/h)
- XRD:
-
X-ray diffraction
- \({\text{Y}}_{{{\text{CH}}_{{4}} }}\) :
-
CH4 yield (%)
- YM:
-
Yimin lignite
- YQ:
-
Yangquan anthracite
- ZJX:
-
Zhujixi bituminous coal
- ΔG⊖ :
-
Standard Gibbs free energy change (J/mol)
- ΔG:
-
Gibbs free energy change at certain reaction conditions (J/mol)
- ∆H:
-
Reaction heat (kcal/mol)
- ρ g :
-
H2 density (kg/m3)
- ρ p :
-
Coal particle density (kg/m3)
- μ g :
-
H2 viscosity (Pa s)
- ℇ mf :
-
Bed voidage at the minimum fluidization velocity
References
Anthony D, Howard J (1976) Coal devolatilization and hydrogasification. AlChE J 22(4):625–656
Asami K, Ohtsuka Y (1993) Catalytic behavior of iron in the gasification of coal with hydogen. Stud Surf Sci Catal 77:413–416
Babu S, Talwalkar A, Shah B (1978) Fluidization correlation for coal gasification materials-minimun fluifization velocity and fluidized bed expantion ratio. AlChE 74:176–186
Baker R, Sherwood R, Derouane E (1982) Further-studies of the nickel-graphite-hydrogen reaction. J Catal 75(2):382–395
Banerjee D, Nagaishi H, Yoshida T (1998) Hydropyrolysis of Alberta coal and petroleum residue using calcium oxide catalyst and toluene additive. Catal Today 45:385–391
Bargiacchi E, Candelaresi D, Valente A, Spazzafumo G, Frigo S (2021) Life cycle assessment of substitute natural gas production from biomass and electrolytic hydrogen. Int J Hydrog Energy 46(72):35974–35984
Calderón L, Chamorro E, Espinal J (2016) Mechanisms for homogeneous and heterogeneous formation of methane during the carbon–hydrogen reaction over zigzag edge sites. Carbon 102:390–402
Calderón L, Chamorro E, Espinal J (2017) Understanding the kinetics of carbon-hydrogen reaction: insights from reaction mechanisms on zigzag edges for homogeneous and heterogeneous formation of methane. Carbon 118:597–606
Canel M, Mısırlıoğlu Z, Sınağ A (2005) Hydropyrolysis of a Turkish lignite (Tunçbilek) and effect of temperature and pressure on product distribution. Energy Convers Manag 46:2185–2197
Casanova R, Cabrera A, Heinemann H, Somorjai G (1983) Calcium oxide and potassium hydroxide catalysed low temperature methane production from graphite and water comparison of catalytic mechanisms. Fuel 62:1138–1144
Cha W, Baek I, Jang H (2007) Hydrogasification of various carbonaceous sources using pressure change properties. Korean J Chem Eng 24(3):532–536
Chang G, Wu W, Shi P, Ma J, Guo Q (2020) A promising composite bimetallic catalyst for producing CH4-rich syngas from bitumite one-step gasification. Energy Convers Manag 205:112408
Chanthakett A, Arif M, Khan M, Oo A (2021) Performance assessment of gasification reactors for sustainable management of municipal solid waste. J Environ Manag 291:112661
Chen Z, Dun Q, Shi Y, Lai D, Zhou Y, Gao S et al (2017) High quality syngas production from catalytic coal gasification using disposable Ca(OH)2 catalyst. Chem Eng J 316:842–849
Chen Z, Wang D, Li C, Yang H, Wang D, Lai D et al (2020) A tandem pyrolysis-upgrading strategy in an integrated reactor to improve the quality of coal tar. Energy Convers Manag 220:113065
Chitester D, Kornosky R, Fan L, Danko J (1984) Characteristics of fluidization at high-pressure. Chem Eng Sci 39(2):253–261
Cypres R, Ghodsi M, Feron D (1984) Influence of added alkaline salts on hydrogenation kinetics of coal. Thermochim Acta 81:105–112
Duan Y, Duan L, Anthony E, Zhao C (2017) Nitrogen and sulfur conversion during pressurized pyrolysis under CO2 atmosphere in fluidized bed. Fuel 189:98–106
Dziembaj R, Makowski W, Chmielarz L (1996) Synergistic effects in the transition metal catalysed hydrogenation of commercial graphites promoted by Ca(NO3)2 and pretreated with O2 or CO2. Carbon 34:913–916
Ellis N, Masnadi M, Roberts D, Kochanek M, Ilyushechkin A (2015) Mineral matter interactions during co-pyrolysis of coal and biomass and their impact on intrinsic char co-gasification reactivity. Chem Eng J 279:402–408
Fan J, Gao K, Zhang P, Dang Y, Ding Y, Zhang B (2023) Direct transformation of fossil carbon into chemicals: a review. J Energy Chem 77:247–268
Feng J, Yan S, Zhang R, Gu S, Qu X, Bi J (2022) Characteristics of Co–Ca catalyzed coal hydrogasification in a mixture of H2 and CO2 atmosphere. Fuel 324:124486
Feng J, Gu S, Zhang R, Qu X, Bi J (2023a) Effect of steam on cobalt–calcium catalyzed coal hydrogasification. Fuel Process Technol 249:107868
Feng J, Yan S, Zhang R, Gu S, Qu X, Bi J (2023b) Recycling and reuse performance of cobalt catalyst for coal hydrogasification. Fuel 335:126939
Gao R, Dou B, Chang Q, Xu J, Dai Z, Yu G et al (2020) Effect of temperature and hydrogen on product distribution and evolution of char structure during pyrolysis of bituminous coal in a drop tube furnace. Fuel 267:117078
Ghodke P, Sharma A, Jayaseelan A, Gopinath K (2023) Hydrogen-rich syngas production from the lignocellulosic biomass by catalytic gasification: a state of art review on advance technologies, economic challenges, and future prospectus. Fuel 342:127800
Gil S, Smoliński A (2015) Experimental study of hydrogasification of lignite and subbituminous coal chars. Sci World J 2015:1–9
Glenk G, Reichelstein S (2019) Economics of converting renewable power to hydrogen. Nat Energy 4(3):216–222
González J, Ramiro A, Sabio E, Encinar J, González C (2002) Hydrogasification of almond shell chars. Influence of operating variables and kinetic study. Ind Eng Chem Res 41(15):3557–3565
González J, Mondragón F, Espinal J (2013) Effect of calcium on gasification of carbonaceous materials with CO2: a DFT study. Fuel 114:199–205
Haga T, Nishiyama Y (1983) Promotion of nickel-catalyzed hydrogasification of carbon by alkaline-earth compounds. J Catal 81(1):239–246
Haga T, Nishiyama Y (1987) Promotion of iron-group catalysts by a calcium salt in hydrogasification of carbons at elevated pressures. Ind Eng Chem Res 26(6):1202–1206
Haga T, Nishiyama Y (1989) Promotion of iron-group catalysts by a calcium salt in hydrogasification of coal chars. Ind Eng Chem Res 28(6):724–728
Haga T, Ozaki J, Suzuki K, Nishiyama Y (1992) Role of MgO and CaO Promoters in Ni-catalyzed hydrogenation reactions of CO and carbon. J Catal 134(1):107–117
Han J, Wang X, Yue J, Gao S, Xu G (2014) Catalytic upgrading of coal pyrolysis tar over char-based catalysts. Fuel Process Technol 122:98–106
Han G, Zhang P, Scholzen P, Noh H, Yang M, Kweon D et al (2022) Extreme enhancement of carbon hydrogasification via mechanochemistry. Angew Chem Int Ed Engl 61(18):e202117851
He C, Feng X, Chu K (2013) Process modeling and thermodynamic analysis of Lurgi fixed-bed coal gasifier in an SNG plant. Appl Energ 111:742–757
He Z, Sun Y, Cheng S, Jia Z, Tu R, Wu Y et al (2021) The enhanced rich H2 from co-gasification of torrefied biomass and low rank coal: the comparison of dry/wet torrefaction, synergetic effect and prediction. Fuel 287:119473
Hiittinger K (1981) Kinetics of hydrogasification of coke catalysed by Fe, Co and Ni. Fuel 60(11):1005–1012
Hirsch R, Gallagher J, Lessard R, Wesslhoft R (1982) Catalytic coal gasification: an emerging technology. Science 215(4529):121–127
Holstein WL, Boudart M (1981) Hydrogenolysis of carbon and its catalysis by platinum. J Catal 72(2):328–337
Hong B, Wang X, Zhou Z, Yu G (2013) A comparison of the gas-product-release characteristics from coal pyrolysis and hydrogasification. Energy Technol 1(8):449–456
Huttinger K, Krauss W (1981) Catalytic activity of coal minerals in hydrogasification of coal. Fuel 60(2):93–102
Inui T, Ueno K, Funabiki M, Suehiro M, Sezume T, Takega Y (1979) Synergistic effect of composite catalysts on the direct hydrogenation of carbon. J Chem Soc Farad T 75:1495–1508
Ipsakis D, Varvoutis G, Lampropoulos A, Papaefthimiou S, Marnellos GE, Konsolakis M (2021) Τechno-economic assessment of industrially-captured CO2 upgrade to synthetic natural gas by means of renewable hydrogen. Renew Energy 179:1884–1896
Jia Y, Huang J, Wang Y (2004) Effects of calcium oxide on the cracking of coal tar in the freeboard of a fluidized bed. Energy Fuels 18:1625–1632
Jiang J, Liu Q, Liu Z (2016) Superior catalytic effect of calcium oxide on the hydrogasification of char for CH4. Fuel 180:737–742
Jiang J, Liu Z, Liu Q (2017) Synergetic catalysis of calcium oxide and iron in hydrogasification of char. Energy Fuel 31(1):198–204
Jin Y, Hu S, Zhang Z, Zhu B, Bai D (2022) The path to carbon neutrality in China: a paradigm shift in fossil resource utilization. Resour Chem Mater 1:129–135
Lahijani P, Zainal Z, Mohamed A, Mohammadi M (2013) CO2 gasification reactivity of biomass char: catalytic influence of alkali, alkaline earth and transition metal salts. Bioresour Technol 144:288–295
Lee WJ, Li C, Prajitno H, Yoo J, Patel J, Yang Y et al (2021) Recent trend in thermal catalytic low temperature CO2 methanation: a critical review. Catal Today 368:2–19
Li W, Yu Z, Guan G (2021) Catalytic coal gasification for methane production: a review. Carbon Resour Convers 4:89–99
Liang Y, Wang F, Zhang H, Wang J, Li Y, Li G (2016) A ReaxFF molecular dynamics study on the mechanism of organic sulfur transformation in the hydropyrolysis process of lignite. Fuel Process Technol 147:32–40
Liang L, Gao X, Gao F, Mao L, Zhu B, Li G (2022) Preparation and composition optimization of the composite Ni/carbon-based microwave absorbents from coal hydrogasification semicoke. J Fuel Chem Technol 50:896–903
Linares-Solano A, Hippo EJ, Philip L, Walker J (1985) Catalytic activity of calcium for lignite char gasification in various atmospheres. Fuel 65:776–779
Liu Y, Liu Q, Gu J, Kang D, Zhou F, Zhang W et al (2013) Highly porous graphitic materials prepared by catalytic graphitization. Carbon 64:132–140
Liu X, Xiong B, Huang X, Ding H, Zheng Y, Liu Z et al (2017a) Effect of catalysts on char structural evolution during hydrogasification under high pressure. Fuel 188:474–482
Liu X, Xiong B, Huang X, Li J, Zheng Y, Liu Z et al (2017b) Development of Zhundong subbituminous coal char structure during hydrogasification under high pressure. Int J Hydrog Energy 42(8):4935–4942
Liu Y, Guan Y, Zhang K (2019) Toward understanding the reactivity and catalytic mechanism of coal pyrolysis with metal chloride modification. J Anal Appl Pyrol 138:196–202
Liu F, Yu G, Yu D, Chen S, Han J, Yu X et al (2021) Calcium catalytic behavior during in-situ gasification of different aged coal chars in CO2 and steam. Fuel 287:119803
Lu Q, Yuan S, Chen X, Xie X et al (2023) The effect of reaction condition on catalytic cracking of wheat straw pyrolysis volatiles over char-based Fe-Ni-Ca catalyst. Energy 263:125722
Martin E, Toomajian M (1992) Effect of oxidation and other treatments on hydrogasification rate of coal char. Fuel 71:1055–1061
Masnadi M, Habibi R, Kopyscinski J, Hill J, Bi X, Lim C et al (2014) Fuel characterization and co-pyrolysis kinetics of biomass and fossil fuels. Fuel 117:1204–1214
Masnadi M, Grace J, Bi X, Lim C, Ellis N (2015) From fossil fuels towards renewables: Inhibitory and catalytic effects on carbon thermochemical conversion during co-gasification of biomass with fossil fuels. Appl Energy 140:196–209
Matsumoto S (1991) Catalyzed hydrogasification of Yallourn char in the presence of supported hydrogenation nickel catalyst. Energy Fuel 5(1):60–63
Matsumoto S, Sakagami S (1993) Catalytic gasification activity of iron enhanced by spilt-over hydrogen. Stud Surf Sci Catal 77:409–412
Matsumoto S, Walker P (1989) Catalysis of the concurrent C–H2O–H2 and methanation reactions-Inhibition by sulfur. Carbon 27(3):395–402
McKee DW (1974) Effect of metallic impurities on the gasification of graphite in water vapor and hydrogen. Carbon 12(4):453–464
Midilli A, Kucuk H, Topal M, Akbulut U, Dincer I (2021) A comprehensive review on hydrogen production from coal gasification: challenges and opportunities. Int J Hydrog Energy 46(50):25385–25412
Mısırlıoğlu Z, Canel M, Sınağ A (2007) Hydrogasification of chars under high pressures. Energy Convers Manag 48(1):52–58
Moulijn J, Kapteijn D (2001) Catalyst deactivation is it predictable? What to do? Appl Catal A Gen 212:3–16
Nishiyama Y (1986) Catalytic behavior of iron and nickel in coal-gasification. Fuel 65(10):1404–1409
Nishiyama Y, Haga T, Tamura O, Sonehara N (1990) A kinetic feature of catalytic gasification of carbons—activation of nickel and iron catalysts during gasification. Carbon 28(1):185–191
Ohtsuka Y, Tamai Y, Tomita A (1987) Iron-catalyzed gasification of brown coal at low-temperatures. Energy Fuel 1(1):32–36
Qu X, Yan S, Yan X, Zhang J, Zheng Q, An Y (2019) Role of cobalt and calcium on coal catalytic hydrogasification in a pressurized fluidized bed. Fuel 239:347–356
Qu X, Wang Q, Yan S, Feng J, Zhang J, Zhang R, Bi J (2022) The behavior of the different catalysts for model carbon hydrogasification. J Fuel Chem Technol 50:1–10
Saeidi S, Najari S, Hessel V, Wilson K, Keil FJ, Concepción P et al (2021) Recent advances in CO2 hydrogenation to value-added products—current challenges and future directions. Prog Energ Combust 85:100905
Saraceno E, Ruocco C, Palma V (2023) A review of coal and biomass hydrogasification: process layouts, hydrogasifiers, and catalysts. Catalysts 13:417
Shao B, Hu G, Alkebsi K, Ye G, Lin X, Du W et al (2021) Heterojunction-redox catalysts of FexCoyMg10CaO for high-temperature CO2 capture and in situ conversion in the context of green manufacturing. Energy Environ Sci 14(4):2291–2301
Si X, Lu R, Zhao Z, Yang X, Wang F, Jiang H et al (2022) Catalytic production of low-carbon footprint sustainable natural gas. Nat Commun 13:258
Skodras G, Panagiotidou S, Kokorotsikos P, Serafidou M (2016) Potassium catalyzed hydrogasification of low-rank coal for synthetic natural gas production. Open Chem 14(1):92–102
Song X, Ma X, Ning G, Gao J (2017) Pitch-based nitrogen-doped mesoporous carbon for flue gas desulfurization. Ind Eng Chem Res 56(16):4743–4749
Song Y, Li Q, Li F, Wang L, Hu C, Feng J et al (2019) Pathway of biomass-potassium migration in co-gasification of coal and biomass. Fuel 239:365–372
Śpiewak K, Czerski G, Porada S (2021) Effect of K, Na and Ca-based catalysts on the steam gasification reactions of coal. Part I: type and amount of one-component catalysts. Chem Eng Sci 229:116024
Steinberg M (2005) Process for conversion of coal to substitute natural gas (SNG). New York, HCEI-8-05-001r2
Sun H, Zhang F, Bi J, Qu X, Yan S, Zhang J et al (2019) Study of Cu–Ni–Ca composite catalysts in catalytic hydrogasification of char. Energy Fuel 33(10):9661–9670
Sun H, Wang J, Bi J, Zhang R, Qu X, Zhang J et al (2021) Investigating the Cu-based catalysts for char catalytic hydrogasification and its recovery. Fuel 294:120567
Suuberg E, Peters W, Howard J (1980) Product compositions in rapid hydropyrolysis of coal. Fuel 59:405–412
Suzuki T, Iwasaki J, Tanka K, Okazaki N, Funaki M, Yamada T (1998) Influence of calcium on the catalytic behavior of nickel in low temperature hydrogasification of wood char. Fuel 77(7):763–767
Takarada T, Onoyama Y, Takayama K, Sakashita T (1997) Hydropyroylysis of coal in a pressurized powder-particle fluidized bed using several catalysts. Catal Today 127–136
Tamai Y, Watanabe H, Tomita A (1977) Catalytic gasification of carbon with steam, carbon-dioxide and hydrogen. Carbon 15(2):103–106
Tian Y, Li J, Wei W, Zong P, Zhang D, Zhu Y et al (2021) Parametric effect of biomass partial hydropyrolysis process in a downer reactor to co-produce high-quality tar and syngas. Bioresour Technol 320:124401
Tomeczek J, Gil S (2010) The kinetics of coal chars hydrogasification. Fuel Process Technol 91:1564–1568
Tomita A, Tamai Y (1972) Hydrogenation of carbons catalyzed by transition-metals. J Catal 27(2):293–300
Tomita A, Kimura A, Ohtsuka Y, Tamai Y (1983) Sulfur poisoning in the nickel catalyzed gasification of activated carbon in hydrogen. Carbon 21(3):225–229
Tosti S, Spazzafumo G, Capobianco D, Buceti G, Pozio A, Bartucca S (2016) EU scenarios of renewable coal hydro-gasification for SNG production. Sustain Energy Technol 16:43–52
Valizadeh S, Jang S, Hoon G, Lee J, Loke P, Ali M et al (2022) Biohydrogen production from furniture waste via catalytic gasification in air over Ni-loaded ultra-stable Y-type zeolite. Chem Eng J 433:133793
Wang X, Yao K, Huang X, Chen X, Yu G, Liu H et al (2019) Effect of CaO and biomass ash on catalytic hydrogasification behavior of coal char. Fuel 249:103–111
Wang M, Wan Y, Guo Q, Bai Y, Yu G, Liu Y et al (2021) Brief review on petroleum coke and biomass/coal co-gasification: syngas production, reactivity characteristics, and synergy behavior. Fuel 304:121517
Wang G, Guo Y, Yu J, Liu F, Sun J, Wang X et al (2022) Ni-CaO dual function materials prepared by different synthetic modes for integrated CO2 capture and conversion. Chem Eng J 428:132110
Wood B, Sancier K (2006) The mechanism of the catalytic gasification of coal char: a critical review. Catal Rev 26(2):233–279
Wu Z, Zhang J, Fan Y, Zhang B, Guo W, Zhang R et al (2021) Synergistic effects from co-pyrolysis of lignocellulosic biomass with low-rank coal: a perspective based on the interaction of organic components. Fuel 306:121648
Xie K (2021) Reviews of clean coal conversion technology in China: Situations & challenges. Chin J Chem Eng 35:62–69
Xia Z, Yan S, Chen C, Qu X, Bi J (2021) Three-dimensional simulation of coal catalytic hydrogasification in a pressurized bubbling fluidized bed. Energy Convers Manag 250:114874
Yan S, Bi J, Qu X (2017) The behavior of catalysts in hydrogasification of sub-bituminous coal in pressured fluidized bed. Appl Energy 206:401–412
Yan S, Zhang J, Yan X, Pan D, Ren H, Qu X (2018) Catalytic coal hydrogasification by cobalt–calcium catalyst in a pressurized fluidized bed: Role of hydropyrolysis and catalysis process. J Anal Appl Pyrol 135:251–259
Yan S, Qu X, Xia Z, Chen C, Bi J (2021) Catalytic hydrogasification characteristic of coal with diverse properties in a pressurized fluidized bed. Fuel 296:120652
Yan S, Qu X, Xia Z, Chen C, Bi J (2022) Effect of experimental variables on coal catalytic hydrogasification in a pressurized fluidized bed. Fuel 307:121761
Yang S, Qian Y, Liu Y, Wang Y, Yang S (2017) Modeling, simulation, and techno-economic analysis of Lurgi gasification and BGL gasification for coal-to-SNG. Chem Eng Res Des 117:355–368
Yu G, Yu D, Liu F, Han J, Yu X, Wu J et al (2020) Different impacts of magnesium on the catalytic activity of exchangeable calcium in coal gasification with CO2 and steam. Fuel 266:117050
Yuan S (2020) Research and development of production of substituted natural gas by hydrogasification. Mod Chem Res 3:5–14
Yuan S, Zhou Z, Li J, Wang F (2012) Nitrogen conversion during rapid pyrolysis of coal and petroleum coke in a high-frequency furnace. Appl Energy 92:854–859
Yuan S, Qu X, Zhang R, Bi J (2015) Effect of calcium additive on product yields in hydrogasification of nickel-loaded Chinese sub-bituminous coal. Fuel 147:133–140
Yuan S, Zhang N, Qu X, Bi J, Cao Q, Wang J (2017a) Promoted catalysis of calcium on the hydrogasification reactivity of iron-loaded subbituminous coal. Fuel 200:153–161
Yuan X, Fan S, Choi S, Kim H, Lee K (2017b) Potassium catalyst recovery process and performance evaluation of the recovered catalyst in the K2CO3 - catalyzed steam gasification system. Appl Energy 195:850–860
Yuan S, Bi J, Qu X, Lu Q, Cao Q, Li W et al (2018) Coal hydrogasification: Entrained flow bed design-operation and experimental study of hydrogasification characteristics. Int J Hydrog Energy 43(7):3664–3675
Zhan S, Wang X, Hong B, Yu G, Wang F (2012) Experimental study on catalytic hydrogasification of lignite. J Fuel Chem Technol 40(1):8–14
Zhang J, Wang X, Wang F, Wang J (2014) Investigation of hydrogasification of low-rank coals to produce methane and light aromatics in a fixed-bed reactor. Fuel Process Technol 127:124–132
Zhang J, Zheng N, Wang J (2016) Two-stage hydrogasification of different rank coals with a focus on relationships between yields of products and coal properties or structures. Appl Energy 173:438–447
Zhang X, Wang J, Liu Q, Te G, Ban Y, Wang Y et al (2017) The Effects of sodium and alkalinity on the microcrystalline structure and the steam gasification performance of Shengli lignite. J Anal Appl Pyrol 125:227–233
Zhang F, Sun H, Bi J, Qu X, Yan S, Zhang J et al (2019) The evolution of Fe and Fe-Ca catalysts during char catalytic hydrogasification. Fuel 257:116040
Zhang J, Tang J, Liu L, Wang J (2021) The evolution of catalytically active calcium catalyst during steam gasification of lignite char. Carbon 172:162–173
Zhang Y, Zhu J, Wang X, Liang P, Jiao T, Li X (2023) Mechanism of sulfur migration and conversion in pyrolysis/combustion staged conversion process of high sulfur coal. J Anal Appl Pyrol 172:106026
Zhao D, Liu H, Zhu D, Qin M (2020) Effect of O-containing functional groups produced by preoxidation on Zhundong coal gasification. Fuel Process Technol 206:106480
Zhou X, Yang X, Li J, Zhao J, Song S, Hao Z et al (2020) High-pressure rapid hydrogasification of pinewood for methane production using calcium looping concept. Energy Convers Manag 203:112247
Zhu H, Yu G, Guo Q, Wang X (2017) In situ raman spectroscopy study on catalytic pyrolysis of a bituminous coal. Energ Fuel 31(6):5817–5827
Zoheidi H, Miller D (1987) Role of oxygen-surface groups in catalysis of hydrogasification of carbon-black by potassium carbonate. Carbon 25(6):809–819
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (22308170), A Project Supported by Scientific Research Fund of Zhejiang Provincial Education Department (Y202250270), the Key research and development project of Shanxi Province (202102090301029), Scientific Research Incubation Program of Ningbo University of Technology (2022TS12), Scientific Research Project Funded by Ningbo University of Technology (2022KQ04).
Author information
Authors and Affiliations
Contributions
Shuai Yan: Investigation, Writing-Original Draft; Jun Feng: Methodology, Validation; Shenfu Yuan: Visualization, Writing-Review & Editing; Zihong Xia: Validation, Writing-Review & Editing; Fengshuang Han: Formal analysis; Xuan Qu: Supervision, Project administration, Writing-Review & Editing; Jicheng Bi: Conceptualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, S., Feng, J., Yuan, S. et al. A critical review on direct catalytic hydrogasification of coal into CH4: catalysis process configurations, evaluations, and prospects. Int J Coal Sci Technol 11, 32 (2024). https://doi.org/10.1007/s40789-024-00677-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-024-00677-x