Abstract
The article presents the results of experimental studies on the gasification of mixtures of brown coal and polyethylene (up to 20 wt% fraction) in a laboratory reactor. The work aims to study the agglomeration process during the heating and oxidation of the mixtures. The measurement results (gas composition, pressure drop) provide indirect information on the dynamics of thermal decomposition and structural changes in the fuel bed. We have shown that the interaction between polyethylene and a coal surface leads to the formation of dense agglomerates, in which the molten polymer acts as a binder. Clinkers form as a result of interfacial interactions between components and filtration flow rearranging. The hydrogen/carbon ratio in the solid residue of coal-polyethylene co-gasification increases from 0.07–0.2 to 1.11, indicating the formation of stable hydrocarbon compounds on the carbon surface. The conducted research makes it possible to identify possible interactions between chemical reactions and transfer processes that lead to agglomeration in mixtures of coal with polyethylene.
Similar content being viewed by others
1 Introduction
Co-thermochemical conversion of coal and artificial polymers is usually considered in connection with the disposal of municipal solid waste (in which the proportion of plastics is quite high) (Wong et al. 2015; Lopez et al. 2018; Qin et al. 2019; Zakharyan et al. 2020; Ding et al. 2022; Akanksha et al. 2023). Polyethylene (PE), as the simplest polymer, is most often used to determine basic patterns of co-conversion, which may help to improve this technology.
Experimental studies of the composition of tarry and gaseous products of the coal and plastics co-conversion were carried out in several works (Havelcova et al. 2016; Kriz and Bicakova 2011; Straka and Bicakova 2014). The reaction product composition often is non-additive (Mastellone 2010; Onay and Koca 2015; Wu et al. 2022), and incomplete degradation of polymers leads to a high yield of tarry compounds (Mastellone et al. 2012). The authors (Glushkov et al. 2020; Vershinina et al. 2022; Antonov et al. 2022) studied combustion of composite liquid fuels with the addition of coal and polymer wastes.
Gasification of coal and plastics can, in some cases, be more efficient than incineration (for example, due to a more efficient power cycle or easier flue gas cleaning compared to incineration) (McIlveen-Wright et al. 2006). The effect of adding small amounts of polymer-containing waste to coal at large power plants has been considered, for example, by authors (Sugiyama et al. 2005; Cormos et al. 2015).
The processes of coal and plastic co-pyrolysis and co-gasification under the conditions of thermogravimetric analysis were studied by Zhou et al. (2009), Melendi-Espina et al. (2015), Wu et al. (2021a, b) and Xinjie et al. (2021). The interaction between the components and decomposition products in the crucible and the gas phase was considered by Shi et al. (2018). Non-additive behaviour was found in both cases (in mass loss and gas release kinetics). Direct measurements show changes in solid residue characteristics when coal and polymers decompose together, including spin concentration (Wu et al. 2021b) and reactivity (Zhang et al. 2021). Detailed kinetic models of the processes of coal and polymers co-pyrolysis and co-gasification do not quantitatively explain the observed effects, although there has been some recent progress in this area (Ranzi et al. 2016; Hong et al. 2021).
Small-scale coal gasification can provide an alternative to combustion for off-grid power systems (Singh and Tirkey 2022). The paper (Du et al. 2021) proposed a mathematical model of the coal and polymers co-gasification process, which did not consider the interaction of fuel particles. A statistical model for the coal and plastic waste co-gasification was proposed by Hasanzadeh et al. (2022).
One of the problems of fixed-bed conversion of polymer-containing systems is agglomeration, which occurs due to the melting and sintering of polymer particles. Agglomeration and associated defluidization has been reported in several papers on fluidized-bed processing of polymers (Mastellone and Arena 2004). Therefore, existing waste processing plants usually include force-mixing systems (rotating sections, screws) that prevent agglomeration (Al-Salem et al. 2009; Chen et al. 2014). Another option, although more energy-consuming, is high-temperature conversion, for example, plasma or solar energy assisted (Janajreh et al. 2013; Piatkowski et al. 2011; Cudjoe and Wang 2022).
In connection with the subject of this article, the work on the fixed bed conversion of coal-plastic mixtures is of interest. Authors of Salganskaya et al. (2010, 2013) report the results of experiments on the filtration combustion (gasification) of charcoal with PE, where they observed the instability of the oxidation front at a polymer content of more than 20%. The authors attribute this to the polymer melting and blockage of the reaction zone: the melt flows onto the combustion front, reducing the oxygen supply. Similar phenomena occur during filtration combustion of viscous hydrocarbons (Zaichenko et al. 2017). In (Sahu and Vairakannu 2022), an allothermic gasification of an equally composed mixture in CO2 flow was carried out. The authors studied the interaction between the components and found that the carbon content in ratty products decreases with increasing heating temperature. The authors suggested that PE decomposes on the coal surface yielding light hydrocarbons and oxygenates. Agglomeration was not reported in this work.
Thus, in previous works, the conditions were selected to avoid bed agglomeration. In general, agglomeration is considered an unfavourable circumstance, and experimental results under these conditions are often discarded in the analysis. However, these results make it possible to investigate the causes of agglomeration. Unlike previous works, we tried to establish the patterns of coal-PE mixtures agglomeration by indirect observations of structural changes in a reacting bed (namely, pressure fluctuations and gas composition) and relating them to the process conditions. Experimental evidence observed under agglomeration conditions is not discarded but is the main result of the work. Our experimental work aims to consider agglomeration in terms of the cooperative influence of heat transfer, chemical reactions and interfacial processes.
The review shows that there have been no systematic studies of fixed bed gasification of coal with polymers with varying coal and plastics grades and combustion conditions. In our work, we study the oxidative conversion of coal with PE under fixed bed conditions. The study aims to determine the conditions for agglomeration during the gasification process in laboratory conditions, as well as to identify the causes of agglomeration.
2 Materials and methods
Azeyskiy brown coal (Irkutsk region, Russia) was used in experiments. The particle size was chosen so that PE granules and coal particles were approximately the same, so a fraction of 5–10 mm was used. Before the experiment, the coal was held in laboratory conditions for a long time, so its humidity corresponds to the air-dry state.
We used LDPE FL-7000 in the form of 5–10 mm granules manufactured by Uz-Kor Gas Chemical. The density of the granules is 0.956 g/cm3, and the softening point according to ASTM D 1525 is 124 °C. Table 1 presents the results of the proximate and ultimate analysis of coal and PE.
Thermogravimetric (TG) curves of co-oxidation of Azeyskiy coal and PE were obtained using a Netzsch STA 449 F1 Jupiter STA instrument. Coal and PE were ground up to 100–200 μm. The samples of about 30 mg were used (the composition of the mixture varied with a step of 20% by weight). The height of the sample layer in the crucible varies from 2 mm (one particle size for fuel particles with an average diameter of 0.13 mm) to 3 mm (practically equal to the internal height of the crucible). The sample weight was chosen so that the amount of oxidizer (air) supplied to the furnace was sufficient for its complete oxidation (however, the complete oxidation was not observed).
Samples were heated from room temperature to 1000 °C at a rate of 30 °C/min. The study was carried out in an oxidizing environment (air flow rate is 70 mL/min, and the protective gas flow rate is 20 mL/min) in corundum crucibles for differential scanning calorimetry (DSC). During the experiment, the qualitative and quantitative composition of the gaseous decomposition products was monitored using a Netzsch QMS 403 C Aeolos quadrupole mass spectrometer. The energy of electron impact is 70 eV. The same technique was used in our previous work (Donskoy et al. 2020).
Samples were heated from room temperature to 1000 °C at a rate of 30 °C/min. The study was carried out in an oxidizing environment (air flow rate is 70 mL/min, protective gas flow rate is 20 mL/min) in corundum crucibles for DSC. During the experiment, the qualitative and quantitative composition of the gaseous decomposition products was monitored using a Netzsch QMS 403 C Aeolos quadrupole mass spectrometer. The energy of electron impact is 70 eV. The same technique was used in our previous work (Donskoy et al. 2020).
Experiments on the co-conversion of coal and PE were carried out using a laboratory fixed-bed reactor unit (Fig. 1) (Donskoy et al. 2022). The laboratory unit consists of a batch reactor for fixed bed conversion of solid fuels, a system for coarse and fine tar capture, a gas chromatograph, and a control and measuring system for determining temperatures (T1–T4) and pressure drop (P). The conversion reactor has a cylindrical shape with a diameter of 150 mm and a height of 350 mm. A heater (1''') and thermal insulation (1'') are installed. Maximum wall temperatures in experiments were up to 700 °C (this temperature allows us to conduct experiments in a reasonable time). The reactor also has a grate through which the oxidizer is supplied (air flow rate 14 × 103 mL/min). It should be noted that the air flow rate does not determine directly gasification stoichiometry, because the oxidation degree is determined by reaction rates whose occurrence, in turn, depends on flow regimes (heat transfer, filtration, etc.).
Before loading, the coal particles and PE granules were mixed to create a homogeneous (as possible) mass. The fuel was loaded in such a way as to, on the one hand, maintain an acceptable experiment time (of the order of a couple of hours) and, on the other hand, ensure a sufficient bed height for gasification reactions to develop in it. The weight of the filling was about 2 kg, with a bed height of about 10–15 cm. The experiments were carried out at a PE mass fraction in a mixture with coal of 0%, 10% and 20 wt%. Granular mixtures are coarse systems, so small differences in composition are not suitable. Literature review and our studies show that the range of 10%–20% wt of plastic is critical for agglomeration (Donskoy 2023). Authors of Uwaoma et al. (2022) reported on briquetting coal with waste plastic as a binder (mass fraction of 5%–30%).
During the experiments, thermocouple temperature measurements were carried out (to control the thermal regime). Gas was sampled for chromatographic analysis (INFICON Micro GC Fusion gas chromatography). The volume concentration of the main components of the gas mixture was measured, namely O2, H2, CH4, CO, CO2, C2H4, and C2H6. The gas sampling for analysis began after reaching a temperature of 400 °C when a noticeable weight loss begins (due to coal pyrolysis). Gas composition measurement error is about 3%–7%. The pressure drop was measured with a water pressure gauge. After the experiments, the reactor was cooled down, and its contents were removed and subjected to additional studies. Elemental analysis of solid products of incomplete coal and PE gasification was carried out using a CHNS/O analyzer (FLASH EA 1112, Italy), as well as using thermal analysis combined with mass spectrometry according to the method described in Kozlov et al. (2015). Elemental analysis error is less than 3%. Measurement errors were preliminary estimated by calibration.
Liquid products of thermochemical conversion were not collected or analyzed: tar traps were used only to clean gas before gas chromatographic analysis. The main gas flow with entrained tar went into the stack, where loose depositions were observed after experiments.
Uncertainty in experimental data is associated mainly with the errors of measuring devices. The range of deviations in temperature measurements varies from 2 to 5 °C; the range of deviations in flow measurements is 2.5%; the response time of the measuring equipment is up to 0.5 s. On the scale of experimental values, these deviations can be neglected. The sources of uncertainty in thermoanalytical measurements were considered by Kozlov et al. (2015). The fluctuations associated with the stochastic nature of the agglomeration process significantly exceed the indicated permissible deviations.
3 Results and discussion
In experiments without the addition of PE, an updraft coal gasification process occurred: the content of combustible components in dry gas is up to 35%–40 % (vol), which is close to the "ideal" thermodynamic reaction conditions (see, for example, the classical experiments of Kolodtsev and Nicholls (Nicholls 1934; Predvoditelev et al. 1949). Due to the wall heating, the conversion process becomes allothermic, and the conditions for the gasification reactions improve.
Mass loss is almost linear with respect to time in all cases, with slight deviations at the first stage of the unit heating (Fig. 2). With the addition of PE, the gasification regime changes dramatically: the fuel particles intensively sinter, forming very dense agglomerate (clinker). In this case, of course, intensive reactions fade out: the measured bed temperature is close to the wall temperature, however, judging by the gas composition, the slow decomposition and oxidation of the fuel continues.
Coal without PE additives steadily reacts with the oxygen producing a high-quality gas (containing CO, CH4 and H2 as main components). The volume fraction of hydrogen and methane first increases, then gradually decreases due to their exhaustion in the process of devolatilization (Fig. 3a). At a PE mass fraction of 10%, the gasification process becomes unstable: decomposition and oxidation of the fuel occur, as can be seen from the low oxygen concentration in the products, but CO2 turns out to be the main gaseous product. It can be assumed that, due to agglomeration, fuel oxidation occurs mainly in a narrow gas near the wall. Due to excess air, the oxidation of combustible components proceeds deeper than in the previous case. Finally, when the mass fraction of PE is 20%, the stable gasification process becomes impossible. Despite the heat supply through the walls, the oxygen concentration in the exhaust gases reaches 19 vol%, which is close to the atmospheric content.
Changes in gas composition give some indirect information about processes in the bed. We assume that CO and CH4 are products of competing processes: CO is formed during oxidative decomposition, and CH4 is a result of thermal decomposition. Oxidation requires sufficient access of oxidizer to fuel surface and releasing heat allows polyolefin chains to break with hydrocarbon formation. In this regard, the symbatic formation of CO and CH4 is a result of the interaction between coal and PE at the reactor scale. A detailed investigation of this interaction is out of the scope of this work.
During agglomeration, the pressure drop and its fluctuations increase, which indicates a deterioration in the gas permeability of the bed (Fig. 4). During coal gasification, the pressure drop increases monotonically on average due to the gradual conversion of coal and a decrease in mean particle size. With PE addition, the pressure drop evolution becomes irregular. The structure of the bed of particles changes due to chemical and phase transformations; as a result of these stochastic processes, pressure bursts are observed, which can be used to trace the onset of intense agglomeration. At a PE mass fraction of 10%, sharp peaks of pressure drop are observed towards the end of the experiment, while at a PE mass fraction of 20%, higher pressure peaks occur at the beginning of the experiment, after which the process intensity drops. Among the conversion products, products of incomplete decomposition and oxidation of PE appear, which are deposited in gas ducts in the form of a loose, rusty mass. The appearance of the clinkers is shown in Fig. 5: as can be seen, the clinkers have the shape of a reactor, while they have high mechanical strength compared to agglomerates obtained by burning PE and sawdust (Donskoy et al. 2022). Interestingly, the bottom part of clinkers is cone-shaped, which is a result of slow decomposition near walls, where temperature and oxygen concentration are high enough to maintain oxidation.
With a PE mass fraction of 20%, the oxidation process fades out, and the stationary conversion becomes impossible. In this regard, it was considered inexpedient to carry out experiments at high PE content on used equipment due to low bed permeability.
Interesting results were obtained in the elemental analysis of solid samples taken from different bed zones after the experiments (see Table 2). During coal gasification without PE, the hydrogen and oxygen content are reduced to the values typical for coal chars. The compositions of solid residues from the middle of the bed and the near-wall region are very close. The addition of PE leads to a significant difference in the compositions of different bed regions: the hydrogen content increases in the core of the clinker, while in the near-wall region, the organic mass burns out deeper than in experiments without the addition of PE. This fact can be explained by changes in filtration flow. When a clinker blocks the central part of the reactor, air flows in the gap between the wall and the clinker. PE forms stable compounds with the coal surface, acting as a binder. As our previous studies have shown, agglomeration leads to deterioration in heat transfer between the heating wall and the fuel. Tarry products of PE decomposition were detected as deposits on the grids and in gas ducts. But a significant part of PE remains in the space between the coal particles, so the hydrogen content in the clinker increases.
Table 2 shows that H/C relations in the agglomerate core are similar for 10% PE and 20% PE mixtures. This similarity may be a result of coal surface saturation by hydrocarbon chains. Another explanation is the higher sensitivity of H/C relation to measurement errors (hydrogen has the lowest atomic mass). One can compare the elemental composition of solid residue with published experimental data obtained under similar conditions (Sahu and Vairakannu 2022): authors report on char residue with high O/C relation, which may be a result of encapsulation of coal particles in melted polymer.
We propose two reasons for the strong agglomeration of coal in a mixture with PE. The first is the wettability of the coal surface by hydrocarbons, which are produced during the melting and thermal decomposition of PE. The second is the low reactivity of coal (compared to PE). As was shown in our previous work (Donskoy et al. 2020), the decomposition and oxidation of wood promote the decomposition of hydrocarbons. But the decomposition and oxidation of coal begin at temperatures closer to the decomposition temperature of PE. In this case, PE does not allow the oxidation of coal to develop, both chemically (by inhibiting reaction chains) and mechanically (forming a film on the surface that prevents the access of an oxidizing agent).
The chemical interaction between coal and polymers during thermal decomposition has been studied in several works, for example, by Sharypov et al. (2007) and Hong et al. (2021). Hydrocarbon polymers can act as hydrogen donors (Dominguez et al. 2001), initiating reaction chains at high temperatures and inhibiting them at low temperatures due to radical stabilization, which leads, among other things, to an increase in the yield of liquid products (Wen et al. 2023).
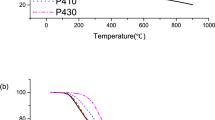
Thermogravimetric analysis shows that the PE decomposition is delayed in the presence of coal, which is associated with the interaction between the components (Fig. 6a). The heating rate (30 K/min) was chosen so that the superposition of the stages of decomposition of coal and PE was more noticeable. However, in this case, the oxidation of coal proceeds in a diffusion mode: the stages of devolatilization and char oxidation practically merge, and a decrease in the coal mass fraction leads to a decrease in the overall reaction time. Low-temperature exothermic oxidation of PE is suppressed by coal addition, but the cracking of PE (corresponding to a significant endothermic peak in the DSC curve at 700–750 K) does not significantly reduce the rate of coal oxidation (Fig. 6b). The temperature of the crucible is likely somewhat higher than the temperature of the heating gas.
In the fixed-bed reactor, unlike thermogravimetric apparatus, we do not control reaction temperature (only wall temperature). In this regard, it would be not correct to relate the mass loss rate presented in Figs. 2 and 6. Thermal decomposition observed in Fig. 6 also occurs at the fixed bed reactor, but due to heat and mass transfer limitations, this decomposition is very uneven across a bed section. It is well-known that coal interacts with polyolefins, but this phenomenon was studied mainly at the micro-scale. Agglomeration is induced by surface interaction, but its development becomes possible due to the effects of heat transfer and filtration flow.
The shapes of the clinkers can be compared with the result of numerical simulation from works (Tanoue et al. 2014; Donskoy 2022). However, the experimental agglomeration does not unambiguously correlate with the calculation results. Under experimental conditions, heat was not only supplied to the reactor through the walls, but also released in the oxygen zone near the grate. The thermal decomposition of the clinker is limited by the oxidizing agent supply rate and the specific surface area of the reacting carbonaceous material. Melted and decomposed polymer binder coal particles, filling and blocking the porous space between them and covering their surface. Therefore, the reacting surface becomes close to the geometric surface of the clinker. External heating becomes ineffective since most of the input heat leaves the reactor with air, which, under agglomeration conditions, flows mainly in the gap between the wall and the clinker surface. The exothermic process becomes unstable due to a decreasing oxidizer residence time and fuel surface. The described above scheme of coal and PE interaction leading to agglomeration is presented in Fig. 7. Exothermic oxidation reaction enhance melting which results in melt covering reacting surface, showing some kind of a negative feedback. It should be noted, that non-uniformity of temperature is a crucial factor for presented agglomeration scenario: wall heating leads to forming the zones with varying permeability (and, therefore, oxygen access) across the section.
Presented results can not be discussed in a context of detailed chemical mechanisms, for several reasons. Firstly, we do not have realistic comprehensive mechanisms for co-decomposition of coal with polymers. Secondly, even if we had, it would be extremely difficult to split physical and chemical effects in such a complex system as an agglomerating bed: Fig. 7 shows that interaction of phases and chemical reactions manifests itself already on a level of qualitative explanations. Quantitative studies and comparison of physical and chemical scales requires deeper investigation.
There are also issues related to coal and PE composition and condition. Thermochemical conversion is the main way to utilize waste plastic, which undergoes ageing due to weather conditions, low-temperature air oxidation and biodegradation (Gulmine et al. 2003; Hakkarainen and Albertsson 2004). Coal also may change its properties during long-term storage and transportation (Zakharov et al. 2018).
Finally, some options should be listed that would help to mitigate agglomeration during the co-conversion of coal and PE. The first and the most obvious is a separate conversion of coal and PE with heat integration (i.e. using coal combustion heat to convert PE into hydrocarbon products). Further, the content of plastic in waste depends on many factors, but given its average fraction (usually about 10%), it is possible to restrict mixture composition to prevent agglomeration. The third option is stirring of bed or using rotary furnaces (Zaichenko et al. 2010). An interesting option in the context of the present work may be using organized packing (Zhang et al. 2022).
4 Conclusions
Fixed bed co-gasification of coal and PE leads to agglomeration caused by several factors. New data were obtained on fixed-bed agglomeration dynamics, which allow us to obtain information about physicochemical and flow transitions caused by fuel components' interaction. The experimental study allows us to clarify some features of agglomeration:
-
(1)
Due to its good wettability with respect to the coal surface, the molten PE acts as a binder for the coal particles. The reactive surface of the coal decrease due to covering, and the self-sustaining oxidation process stops.
-
(2)
The degradation of the macroporous fuel bed structure leads to intense pressure fluctuations during air filtration. The heating conditions promote clinker formation, so the main fraction of the air flows in the gap between the wall and the bed, which acts as thermal protection for the agglomerate.
-
(3)
The chemical analysis shows that the inner region of the agglomerate is saturated with PE decomposition products, so the H/C ratio is higher than that of the original coal. Thermal analysis shows that the decomposition of PE in a mixture with coal is delayed, which may also be associated with surface interaction.
-
(4)
The agglomeration of coal and PE mixtures during heating and oxidation is a complex phenomenon, which, in addition to chemical interactions, also includes heat and mass transfer conditions (in this case, air filtration and heat transfer between the wall and the bed).
The information obtained may be useful in the development of the thermochemical co-conversion processes of coal with plastic-containing waste.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Akanksha M, Shalini G, Tripurari S (2023) Gasification kinetic studies of low volatile weakly caking coal. Int J Coal Sci Technol 10(1):25. https://doi.org/10.1007/s40789-023-00587-4
Al-Salem SM, Lettieri P, Baeyens J (2009) Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manage 29:2625–2643. https://doi.org/10.1016/j.wasman.2009.06.004
Antonov D, Glushkov D, Paushkina K, Kuznechenkova D, Ramanathan A (2022) A mathematical model of industrial waste-derived fuel droplet combustion in high-temperature air. Appl Sci 12:12273. https://doi.org/10.3390/app122312273
Chen D, Yin L, Wang H, He P (2014) Pyrolysis technologies for municipal solid waste: a review. Waste Manage 34:2466–2486. https://doi.org/10.1016/j.wasman.2014.08.004
Cormos A-M, Dinca C, Cormos C-C (2015) Multi-fuel multi-product operation of IGCC power plants with carbon capture and storage (CCS). Appl Thermal Eng 74:20–27. https://doi.org/10.1016/j.applthermaleng.2013.12.080
Cudjoe D, Wang H (2022) Plasma gasification versus incineration of plastic waste: energy, economic and environmental analysis. Fuel Proc Tech 237:107470. https://doi.org/10.1016/j.fuproc.2022.107470
Ding L, Yang M, Dong K, Vo DVN, Hungwe D, Ye J, Ryzhkov A, Yoshikawa K (2022) Mobile power generation system based on biomass gasification. Int J Coal Sci Technol 9(1):34. https://doi.org/10.1007/s40789-022-00505-0
Dominguez A, Blanco CG, Barriocanal C, Alvarez R, Diez MA (2001) Gas chromatographic study of the volatile products from co-pyrolysis of coal and polyethylene wastes. J Chromatogr A 918:135–144. https://doi.org/10.1016/S0021-9673(01)00736-1
Donskoy I (2022) Influence of heating conditions on formation and development of agglomerates in a reactive porous medium. Heat Transfer Res 53(12):25–36. https://doi.org/10.1615/HeatTransRes.2022038756
Donskoy I (2023) Particle agglomeration of biomass and plastic waste during their thermochemical fixed-bed conversion. Energies 16:4589. https://doi.org/10.3390/en16124589
Donskoy IG, Kozlov AN, Kozlova MA, Penzik MV, Shamanskiy VA (2020) Thermochemical interaction of wood and polyethylene during co-oxidation in the conditions of thermogravimetric analysis. React Kinet Mech Catal 131:845–857. https://doi.org/10.1007/s11144-020-01880-y
Donskoy I, Kozlov A, Svishchev D, Penzik M (2022) Experimental study on fixed-bed combustion and agglomeration of sawdust–polyethylene mixtures. Energy Sources A. https://doi.org/10.1080/15567036.2022.2030440
Du S, Yuan S, Zhou Q (2021) Numerical investigation of co-gasification of coal and PET in a fluidized bed reactor. Renew Energy 172:424–439. https://doi.org/10.1016/j.renene.2021.03.035
Glushkov D, Kuznetsov G, Paushkina K (2020) Switching coal-fired thermal power plant to composite fuel for recovering industrial and municipal waste: combustion characteristics, emissions, and economic effect. Energies 13:259. https://doi.org/10.3390/en13010259
Gulmine JV, Janissek PR, Heise HM, Akcelrud L (2003) Degradation profile of polyethylene after artificial accelerated weathering. Polym Degrad Stab 79:385–397. https://doi.org/10.1016/S0141-3910(02)00338-5
Hakkarainen M, Albertsson AC (2004) Environmental degradation of polyethylene. In long term properties of polyolefins. Adv Polym Sci 169:177–200. https://doi.org/10.1007/b13523
Hasanzadeh R, Mojaver P, Azdast T, Chitsaz A, Park CB (2022) Low-emission and energetically efficient co-gasification of coal by incorporating plastic waste: a modeling study. Chemosphere 299:134408. https://doi.org/10.1016/j.chemosphere.2022.134408
Havelcova M, Bicakova O, Sykorova I, Weishauptova Z, Melegy A (2016) Characterization of products from pyrolysis of coal with the addition of polyethylene terephthalate. Fuel Proc Tech 154:123–131. https://doi.org/10.1016/j.fuproc.2016.08.022
Hong D, Li P, Si T, Guo X (2021) ReaxFF simulations of the synergistic effect mechanisms during co-pyrolysis of coal and polyethylene/polystyrene. Energy 218:119553. https://doi.org/10.1016/j.energy.2020.119553
Janajreh I, Raza SS, Valmundsson AS (2013) Plasma gasification process: Modeling, simulation and comparison with conventional air gasification. Energy Convers Manag 65:801–809. https://doi.org/10.1016/j.enconman.2012.03.010
Kozlov A, Svishchev D, Donskoy I, Shamansky V (2015) Impact of gas-phase chemistry on the composition of biomass pyrolysis products. J Therm Anal Calorim 122:1089–1098. https://doi.org/10.1007/s10973-015-4951-z
Kriz V, Bicakova O (2011) Hydrogen from the two-stage pyrolysis of bituminous coal/waste plastics mixtures. Int J Hydrogen Energy 36:9014–9022. https://doi.org/10.1016/j.ijhydene.2011.03.136
Lopez G, Artetxe M, Amutio M, Alvarez J, Bilbao J, Olazar M (2018) Recent advances in the gasification of waste plastics. A critical overview. Renew Sustain Energy Rev 82:576–596. https://doi.org/10.1016/j.rser.2017.09.032
Mastellone ML (2010) Co-gasification of coal, plastic waste and wood in a bubbling fluidized bed reactor. Fuel 89:2991–3000. https://doi.org/10.1016/j.fuel.2010.05.019
Mastellone ML, Arena U (2004) Bed defluidisation during the fluidised bed pyrolysis of plastic waste mixtures. Polym Degrad Stab 85:1051–1058. https://doi.org/10.1016/j.polymdegradstab.2003.04.002
Mastelone ML, Zaccariello L, Santoro D, Arena U (2012) The O2-enriched air gasification of coal, plastics and wood in a fluidized bed reactor. Waste Manage 32:733–742. https://doi.org/10.1016/j.wasman.2011.09.005
McIlveen-Wright DR, Rinto F, Armesto L, Caballero MA, Aznar MP, Cabanillas A, Huang Y, Franco C, Gulyutlu I, McMullan JT (2006) A comparison of circulating fluidised bed combustion and gasification power plant technologies for processing mixtures of coal, biomass and plastic waste. Fuel Proc Tech 87:793–801. https://doi.org/10.1016/j.fuproc.2006.04.002
Melendi-Espina S, Alvarez R, Diez MA, Casal MD (2015) Coal and plastic waste co-pyrolysis by thermal analysis-mass spectrometry. Fuel Proc Tech 137:351–358. https://doi.org/10.1016/j.fuproc.2015.03.024
Nicholls P (1934) Underfeed combustion, effect of preheat, and distribution of ash in fuel beds. Bureau of Mines, Bulletin 378. Washington: Government printing office
Onay O, Koca H (2015) Determination of synergetic effect in co-pyrolysis of lignite and waste tyre. Fuel 150:169–174. https://doi.org/10.1016/j.fuel.2015.02.041
Piatkowski N, Wieckert C, Weimer AW, Steinfeld A (2011) Solar-driven gasification of carbonaceous feedstock—a review. Energy Environ Sci 4:3–82. https://doi.org/10.1039/C0EE00312C
Predvoditelev AS et al (1949) Combustion of carbon. AN USSR Publ, Moscow
Qin J, Zhao R, Chen T, Zi Z, Wu J (2019) Co-combustion of municipal solid waste and coal gangue in a circulating fluidized bed combustor. Int J Coal Sci Technol 6:218–224. https://doi.org/10.1007/s40789-018-0231-4
Ranzi E, Faravelli T, Manenti F (2016) Pyrolysis, gasification, and combustion of solid fuels. Adv Chem Eng 49:1–94. https://doi.org/10.1016/bs.ache.2016.09.001
Sahu P, Vairakannu P (2022) CO2 based synergistic reaction effects with energy and exergy (2E) analysis of high density polyethylene with high ash bituminous coal for syngas production. Fuel 311:122500. https://doi.org/10.1016/j.fuel.2021.122500
Salganskaya MV, Glazov SV, Salganskii EA, Zholudev AF (2010) Filtration combustion of charcoal-polyethylene systems. Russ J Phys Chem B 4:928–933. https://doi.org/10.1134/S1990793110060096
Salganskaya MV, Glazov SV, Salganskii EA, Zholudev AF, Stesik LN (2013) Filtration combustion of systems with polymer materials. Khim Fiz 32(3):57–61. https://doi.org/10.7868/S0207401X13030072
Sharypov VI, Beregovtsova NG, Kuznetsov BN, Cebolla VL, Collura S, Finqueneisel G, Zimny T, Weber JV (2007) Influence of reaction parameters on brown coal–polyolefinic plastic co-pyrolysis behavior. J Anal Appl Pyrolysis 78:257–264. https://doi.org/10.1016/j.jaap.2006.08.004
Shi L, Cheng X, Liu Q, Liu Z (2018) Reaction of volatiles from a coal and various organic compounds during co-pyrolysis in a TG-MS system. Part 2. Reaction of volatiles in the free gas phase in crucibles. Fuel 213:22–36. https://doi.org/10.1016/j.fuel.2017.10.086
Singh DK, Tirkey JV (2022) Performance optimization through response surface methodology of an integrated coal gasification and CI engine fuelled with diesel and low-grade coal-based producer gas. Energy 238C:121982. https://doi.org/10.1016/j.energy.2021.121982
Straka P, Bicakova O (2014) Hydrogen-rich gas as a product of two-stage co-gasification of lignite/waste plastics mixtures. Int J Hydrogen Energy 39:10987–10995. https://doi.org/10.1016/j.ijhydene.2014.05.054
Sugiyama S, Suzuki N, Kato Y, Yoshikawa K, Omino A, Ishii T, Yoshikawa K, Kiga T (2005) Gasification performance of coals using high temperature air. Energy 30:399–413. https://doi.org/10.1016/j.energy.2004.06.001
Tanoue K, Nagao M, Yoshida A, Nishimura T (2014) Heat transfer and phase change in a polystyrene packed bed during melting. Int J Heat Mass Transfer 79:324–331. https://doi.org/10.1016/j.ijheatmasstransfer.2014.08.019
Uwaoma RC, Henning CN, Bunt JR, Leokaoke NT, Neomagus HWJP (2022) Comparison of industrial wastes as a binder in the agglomeration of coal fines. Results Eng 16:100729. https://doi.org/10.1016/j.rineng.2022.100729
Vershinina K, Nyashina G, Strizhak P (2022) Combustion, pyrolysis, and gasification of waste-derived fuel slurries, low-grade liquids, and high-moisture waste: review. Appl Sci 12:1039. https://doi.org/10.3390/app12031039
Wen Y, Liu S, Fu S, Wang Z, Hu H, Jin L (2023) Insight into influence of process parameters on co-pyrolysis interaction between Yulin coal and waste tire via rapid infrared heating. Fuel 337:127161. https://doi.org/10.1016/j.fuel.2022.127161
Wong SL, Ngadi N, Abdullah TAT, Inuwa IM (2015) Current state and future prospects of plastic waste as source of fuel: a review. Renew Sustain Energy Rev 50:1167–1180. https://doi.org/10.1016/j.rser.2015.04.063
Wu Y, Zhu J, Zhao S, Wang D, Jin L, Hu H (2021a) Co-pyrolysis behaviors of low-rank coal and polystyrene with in-situ pyrolysis time-of-flight mass spectrometry. Fuel 286(2):119461. https://doi.org/10.1016/j.fuel.2020.119461
Wu Y, Zhu J, Wang Y, Yang H, Jin L, Hu H (2021b) Insight into co-pyrolysis interactions of Pingshuo coal and high-density polyethylene via in-situ Py-TOF-MS and EPR. Fuel 303:121199. https://doi.org/10.1016/j.fuel.2021.121199
Wu Y, Wang G, Zhu J, Wang Y, Yang H, Jin L, Hu H (2022) Insight into synergistic effect of co-pyrolysis of low-rank coal and waste polyethylene with or without additives using rapid infrared heating. J Energy Inst 102:384–394. https://doi.org/10.1016/j.joei.2022.05.005
Xinjie L, Singh S, Yang H, Wu C, Zhang S (2021) A thermogravimetric assessment of the tri-combustion process for coal, biomass and polyethylene. Fuel 287:119355. https://doi.org/10.1016/j.fuel.2020.119355
Zaichenko AY, Zhirnov AA, Manelis GB, Polinachik EV, Zholudev AF (2010) Filtration combustion of carbon in a non-one dimensional solid flow. Theor Found Chem Eng 44:30–35. https://doi.org/10.1134/S0040579510010045
Zaichenko AY, Glazov SV, Salgansky EA, Kislov VM, Podlesniy DN, Zhavoronkov AI, Salganskaya MV (2017) Filtration combustion of viscous hydrocarbon liquids. Theor Found Chem Eng 51:673–679. https://doi.org/10.1134/S0040579517050396
Zakharov V, Kozlov A, Donskoy I (2018) Modeling of changes in the heating value of coal transported to Russias far north regions on the example of the republic of Sakha (Yakutia). Izv RAS Energetika 6:132–141. https://doi.org/10.31857/S000233100003526-2
Zakharyan EM, Petrukhina NN, Maksimov AL (2020) Pathways of chemical recycling of polyvinyl chloride: part 1. Russ J Appl Chem 93:1271–1313. https://doi.org/10.1134/S1070427220090013
Zhang H, Wang G, Wang J, Xue Q (2021) Low-temperature treatment of polyethylene plastics and semi-coke mixture and CO2 gasification of finely ground products. Fuel 285:119215. https://doi.org/10.1016/j.fuel.2020.119215
Zhang T, Yuchi W, Bai Z, Hou R, Feng Z, Guo Z, Kong L, Bai J, Meyer B, Li W (2022) Insight into the charging methods effects during clean recycling of plastic by co-pyrolysis with low-rank coal. J Clean Prod 333:130168. https://doi.org/10.1016/j.jclepro.2021.130168
Zhou L, Luo T, Huang Q (2009) Co-pyrolysis characteristics and kinetics of coal and plastic blends. Energy Convers Manag 50:705–710. https://doi.org/10.1016/j.enconman.2008.10.007
Acknowledgements
The work was carried out within the Project No. FWEU-2021-0005 of the RF Basic Research Program for 2021-2030 using the resources of the High-Temperature Circuit Central Collective Use Center (Ministry of Education and Science of Russia, Project No. 13.CKP.21.0038).
Author information
Authors and Affiliations
Contributions
Conceptualization: I.G., A.K., M.P.; Supervision: L.D.; Investigation: M.P., A.K.; Formal analysis: I.G., D.S.; Writing (original draft): I.G., A.K.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donskoy, I.G., Kozlov, A.N., Penzik, M.V. et al. Agglomeration of coal and polyethylene mixtures during fixed-bed co-gasification. Int J Coal Sci Technol 11, 21 (2024). https://doi.org/10.1007/s40789-024-00670-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-024-00670-4