Abstract
Coking coal dust is extremely hydrophobic; therefore, combination with droplets in the air is difficult and dust suppression is challenging. Here, a dust suppressant spray for coking coal dust was studied in order to improve of the combination of droplets and coking coal dust. Based on monomer optimization and compounding analysis, two surfactant monomers, fatty alcohol ether sodium sulfate (AES) and sodium dodecyl benzene sulfonate (SDBS) were selected as the surfactant components of the dust suppressant. The surfactant monomers were combined with four inorganic salts and the reverse osmosis moisture absorption of each solution was determined. By combining the reverse osmosis moisture absorption values with the water retention experimental results, CaCl2 was identified as the optimal inorganic salt additive for the dust suppressant. Finally, the optimal concentration of each component was obtained using orthogonal experimental design i.e., AES (0.03%), SDBS (0.05%), and CaCl2 (0.4%). The dust suppressant solution formulated using this method had a high moisture absorption capacity and excellent performance.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Different mining processes, e.g., coal mining and transportation, generate varying amounts of dust. Airborne dust poses a serious threat to the health and safety of mine workers (Ahmed et al. 2017; Zhou et al. 2018a; Xu et al. 2019a; Wang et al. 2019a; Hua et al. 2020). In recent years, according to the National Occupational Diseases Statistics Report by the National Health Commission of China, most new pneumoconiosis cases were reported to occur in the coal and non-ferrous metal mining industries. Specifically, 40% of new pneumoconiosis cases were reported to occur in the coal mining industry (Vedal 1997; Li et al. 2002; Yin et al. 2019; Bao et al. 2020; Zhang et al. 2020). Thus, adoption of effective dust suppression technologies to reduce the concentration of dust in the coal mining field is imperative (Nie et al. 2017a, b; Peng et al. 2019).
Currently, spray-based dust reduction is the main dust control measure in underground mines (Dey 2012; Wang et al. 2019b, e; Zhou et al. 2019a, b; Han et al. 2020a). However, sprayed water has a large surface tension and coal dust is extremely hydrophobic, which limits the efficiency of dust suppression sprays in coal mines (Wang et al. 2020a; Xu et al. 2018; Han et al. 2020b, a). Some studies report the addition of surfactants to water, which provide the resulting solution with a lower surface tension and improve the wetting performance of the spraying process (Yang et al. 2007; Tessum and Raynor 2017). Other studies have shown that surfactants significantly reduce the surface tension of water, and that the reduction effect is dependent on surfactant type and concentration (Omane et al. 2018; Wang et al. 2019d). Tessum et al. (2014) studied the ability of various surfactant droplets to trap dust particles with a surface charge. The analysis indicated that different types of surfactant performed differently in trapping dust particles with a surface charge; in particular, it was shown that non-ionic surfactants had the highest dust trapping efficiency. Yang et al. (2014) reported that surface active ions in anionic surfactants generally carry a negative charge, and thus repelled coal dust particles with a negatively charged surface. The anionic surfactant particles exhibited low adsorption on the surface of coal dust particles. Therefore, anionic surfactants have a weaker wetting ability compared to that of non-ionic surfactants.
In addition, some researchers have developed and studied novel dust suppressants (Fan et al. 2018; Hu et al. 2019; Zhou et al. 2019a, b; Yan et al. 2020). Tong (2013) and Jiang et al. (2013) conducted theoretical and experimental analyses and concluded that the application of suitable compounding significantly improved the wetting performance of a solution during coal dust trapping. Li et al. (2010) prepared a new dust suppressant using a surfactant compounding method and obtained the optimal concentration through orthogonal experimental design. Based on the experimental results, compared to clean water, the dust suppressant improved the dust reduction efficiency by 20%. Kilaua and Pahlman (1987) found that the wetting performance of the surfactant solution is further enhanced via the addition of metal inorganic salts. Subsequently, Du and Zeng (2002) and He et al. (2008) investigated the variation in surface tension of surfactant solutions under the action of metal salts. Wu and Gu (2001) investigated the impact of inorganic salt additives on the wetting performance of anionic surfactant solutions through experimental and theoretical analyses. The results showed that the wetting ability of anionic surfactant solutions is significantly enhanced following the addition of Na2SO4. Li et al. (2016) compared the wetting properties of a sodium dodecyl sulfate (SDS) solution with five different additives and assessed its suitability for coal dust trapping using surface tension, contact angle, and reverse osmosis experiments.
Coking coal is a type of bituminous coal and is more hydrophobic than other types of coal dust, meaning its combination with water droplets is challenging. Thus, the dust capture rate of traditional spray technologies is extremely poor. The development of dust suppressants specifically for coking coal is rarely reported in the literature. In addition, inorganic salts are rarely added as water-retention agents and additives during the formulation of dust suppressants. In this study, two surfactant monomers were used as surfactant components to prepare a dust suppressant for coking coal dust via monomer optimization and compounding analysis. At the same time, through compounding of the surfactant monomer and inorganic salt, the reverse osmosis moisture absorption of each solution was measured. Using the reverse osmosis water retention results, a specific inorganic salt was chosen as the dust suppressant additive. Finally, the optimal combination of concentrations of the three components was obtained via orthogonal experimental design to obtain the spray dust suppressant formulation for coke coal dust.
2 Materials and methods
2.1 Materials
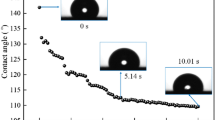
Coking coal samples were obtained from the Shanxi Wanfeng coal mine. The coking coal from the Shanxi Wanfeng coal mine had poor wetting properties (making wetting using water difficult), and a large contact angle (88.76°) (Liu 2006; Luo et al. 2016; Zhou et al. 2018b; Wang et al. 2019c). First, a pulverizer was used to crush the coal samples for one minute, and then the sample was passed through a sieve using a 150-mesh standard industrial sieve. Next, the obtained coal dust sample was dried in a vacuum dryer at 80 °C for 480 min. The coal dust sample was stored in a sealed pouch prior to analysis. The industrial evaluation indexes and characteristic particle diameters of the coal dust samples are shown in Table 1. Figure 1 shows the contact angle and size distribution of the coal dust samples. As the mining area uses tap water, tap water was used during the experiments.
If the suppressant spray is applied directly to the coal mine operation site, it will come into direct contact with coal mine workers. Thus, there are strict requirements for the selection of surfactants. When selecting dust suppressant sprays, they should be: non-toxic, harmless, non-corrosive, and easily soluble in water. In addition, the selected suppressants should be economical and easy to transport. Based on the above principles, through market research and field investigation, ten types of surfactant underwent preliminarily screening, as shown in Table 2.
During dust suppressant formulation as well as using multiple surfactants for compounding, the addition of hygroscopic inorganic salt additives to further improve the wetting and water retention properties of the solution should be considered (Tang et al. 2016; Kumar and Mandal 2016). The effect of hygroscopic inorganic salts and surfactants differ; some have synergistic effects and some antagonistic effects. In the experiments reported here, four types of hygroscopic inorganic salts (NaCl, CaCl2, Na2SO4, and Na2SiO3) were selected. The effect of each inorganic salt and surfactant was analyzed, and the inorganic salt with optimal performance was selected.
2.2 Experimental scheme
2.2.1 Preparation scheme
-
(1)
Surface tension experiments were used to investigate the ability of ten surfactant monomers to decrease the solution surface tension. At the same time, we investigated the performance of ten types of surfactant monomer for wetting coal dust using contact angle and reverse osmosis analyses. A total of six surfactant concentrations were tested during the experiments i.e., 0%, 0.00005%, 0.0005%, 0.005%, 0.05% and 0.5%. Based on the surface tension and wetting performance experimental results, six suitable surfactant monomers were selected.
-
(2)
The six surfactant monomers were tested using binary compounding analysis, and the performance of the compounded solutions was evaluated using reverse osmosis analysis. Based on the results of the performance evaluation, an optimal compounding scheme was selected. The concentration of the six monomer surfactants was set at 0.05% during the binary compounding analysis.
-
(3)
Four inorganic salts were used to prepare solutions with mass fractions of 1%. The preferred surfactant monomer selected during the compounding scheme in step (2) was used to prepare a 0.05% solution. Then, binary compounding of the surfactant monomer and inorganic salt solutions was performed, and the wetting performance of the compounded solution was investigated using reverse osmosis analysis. Solutions of the four different types of inorganic salt at equal mass concentrations were added to the coal dust samples and their water retention properties were investigated. One of the four inorganic salts was selected as the optimal additive for the dust suppressant, based on the water retention and reverse osmosis results.
-
(4)
A three-factor, three-level reverse osmosis orthogonal experimental design was performed using the two surfactant monomers and the inorganic salt selected in the binary compounding analysis described above. In addition, based on the ranges of the three factors and the comprehensive average of each level, the dust suppressant configuration scheme with optimal the dust suppression effect for coking coal was obtained. The manufacturing process for the dust suppressant is shown in Fig. 2.
2.2.2 Parameter measurement method
-
(1)
Surface tension analysis. A German Kruss K20 surface tension meter was used to detect the surface tension of the surfactant monomer and the compound solution. The surface tension of all solutions was measured at 25 °C using the hanging piece method. First, the platinum piece was placed and maintained in a vertical position on the experiment table, and then the instrument was rotated to allow the platinum piece to interact with the surface of the water. Then, the minimum tensile force required for the platinum piece to exit the aqueous solution was measured.
-
(2)
Contact angle analysis. In order to obtain the cylinder test piece of the coal samples, a mold containing 400 mg of pulverized coal was placed in a bench-top powder grinding machine for one minute at a pressure of 50 MPa. The obtained test pieces were 2 mm thick and had a smooth surface. A CA100B contact angle measurement instrument was used to measure the contact angle of the solution on a coal dust sample. Three test pieces were measured in triplicate for each solution to obtain the average value.
-
(3)
Reverse osmosis analysis. A custom-designed reverse osmosis device was used to evaluate the moisture absorption of the coal dust. First, a 10 mm-diameter glass tube containing 3 g of coal sample sealed with filter paper was weighed. Then, the glass test tube was submerged in a water tank for two hours. Next, the glass test tube was removed from the water tank and reweighed it. The difference between the two weights was used to calculate the moisture absorption of the coal dust samples.
-
(4)
Water retention analysis. The four inorganic salts i.e., NaCl, CaCl2, Na2SO4, and Na2SiO3, were used to prepare 1% solutions. Then, 10 mL of each solution was mixed with 10 g of dry coking coal dust in a petri dish and the petri dish was dried at room temperature. The petri dish was weighed at 12-h intervals to calculate the coking coal moisture content. The method used to calculate the moisture content is shown in Eq. (1).
$$\eta = \frac{{m_{2} - m_{1} }}{{m_{1} }} \times 100\%$$(1)where, η is the moisture content, m1 is the mass of the coal sample before wetting, and m2 is the mass of the coal sample after wetting.
3 Selection of the optimal surfactant
3.1 Surface tension analysis
The experimental results in Table 3 show that the surface tension was reduced by adding a surfactant into water. Due to the amphiphilic structure, the surfactant molecules were positively adsorbed onto the surface of the solution, causing a significant reduction in solution surface tension. The results showed that for most coal dust samples the critical surface tension was approximately 45 mN/m (Wu and Gu 2001). Table 3 also shows that the critical surface tension of some surfactants exceeded 45 mN/m at the highest concentration (0.5%). For example, the critical surface tension of SLS was 48.9 mN/m and that of 1227 was 45.6 mN/m. Therefore, coal dust wetting using these two surfactants was not optimal and they were eliminated. cm refers to the mass fraction of surfactant solution.
Table 3 also shows that as the surfactant mass concentration increased within the low range, the surface tension was significantly reduced. In addition, the surface tension remained stable after the mass concentration exceeded a certain value, which is defined as the critical micelle concentration (CMC) (Wang et al. 2020b; Jin et al. 2019). At high concentrations, the interface adsorption between amphiphilic molecules became saturated leading to the formation of colloidal aggregates of the amphiphilic molecules in solution and resulting in stable surface tension (Ma et al. 2004). In this study, the change in surface tension slope for the ten types of surfactant were measured in different concentration ranges to obtain the CMC of each surfactant (Fig. 3).
The surface tension rate of change for the ten surfactants in different concentration ranges: a a-SAA, b n-SAA, c z-SAA, d c-SAA. Note: ‘1–2’ refers to the surfactant concentration is between 0% and 0.00005%, ‘2–3’ refers to the surfactant concentration is between 0.00005% and 0.0005%, ‘3–4’ refers to the surfactant concentration is between 0.0005% and 0.005%. The rest can be deduced by analogy
Figure 3 indicates that when the concentration is between 0.05% and 0.5%, the surface tension rate of change for nine of the surfactants was below 5%; however, Tween-80 was an exception. The results showed that the CMC of Tween-80 was significantly higher than that of the other surfactants. At the highest concentration, the corresponding surface tension of Tween-80 was approximately 40 mN/m, which is close to the critical surface tension of coal dust. Thus, Tween-80 should not be selected as the dust suppressant. Based on the results in Fig. 3, the six surfactants i.e., AES, SDBS, Tween-80, AEO-9, Tween-20, CAT-97, and OB-2, have CMC values of approximately 0.005%, while SLS, CTAB, and 1227 have CMC values of approximately 0.05%. Based on these surface tension experimental results, three types of surfactant, SLS, 1227 and Tween-80, were eliminated.
3.2 Wetting performance analysis
The wetting performance of a surfactant solution is highly dependent on the type of surfactant and the mass concentration. The contact angle measurements and reverse osmosis moisture absorption values for the ten types of surfactant at various mass concentrations are shown in Fig. 4.
From Fig. 4a, it can be seen that the solution contact angle on the surface of the coal dust tablet continuously decreases with the increasing mass concentration of the surfactant. According to Young's equation, the relationship between the contact angle of a liquid on a solid surface and the interfacial tension is as follows:
where, θ is the contact angle (°); γsg, γsl, γlg are the tension (mN/m) at the solid–gas, solid–liquid, and liquid–gas interfaces, respectively. When a surfactant is present in the solution, γsl and γlg significantly decrease due to the adsorption of the surfactant at the solid–liquid and liquid–gas interfaces, which leads to a smaller contact angle value θ, as shown in Fig. 5.
The results of the reverse osmosis analysis in Fig. 4b show that for all ten surfactant solutions, the reverse osmosis moisture absorption increases with increasing mass concentration. By comparing Fig. 4a, b, we can see that both the reverse osmosis hygroscopicity and the contact angle essentially follow the same trend. Combining the contact angle and reverse osmosis results allowed us to select six surfactants based on wetting performance: anionic AES and SDBS, non-ionic AEO-9 and Tween-20, zwitterionic OB-2, and cationic CTAB.
Using Origin 9.0 software (Edwards 2002), curve fitting of the data for the reverse osmosis moisture absorption and contact angle analyses for the above six surfactant monomers was performed. The fitting results shown in Fig. 6 indicate that the amount of moisture absorption decreases as the contact angle increases. In addition, the moisture absorption and contact angle were negatively correlated, with a correlation coefficient of approximately 0.85. This indicates that it is feasible to utilize contact angle and reverse osmosis moisture absorption to assess the wetting performance of surfactant solutions. The relatively high error in the contact angle analysis and the difficulties encountered in producing the coke coal test piece meant the analysis was challenging (Huang et al. 2010). Therefore, in subsequent compounding analyses, only reverse osmosis absorption analysis was utilized to assess the wetting performance of the solution.
4 Compounding analysis
4.1 Surfactant binary compounding
A binary compound reverse osmosis experiment was conducted using the six selected monomer surfactants and the results are listed in Table 4.
From Table 4, it can be seen that most of the compound solutions have stronger wetting effects and a higher moisture absorption than the monomer solution (Zhang 2019; Xu et al. 2019b). By considering the moisture absorption growth rate, four compounding schemes i.e., Scheme 1 (AES + SDBS), Scheme 2 (AES + AEO-9), Scheme 10 (AEO-9 + 20), and Scheme 13 (AEO-9 + CTAB), were considered most effective. The wettability of the mixed solution was more than 20% higher than the monomer. Based on further comparisons, we found that the final moisture absorption value for Scheme 1 was the highest. In view of the properties of each surfactant and their usage habits in coal mines, the two surfactant monomers in Scheme 1 were chosen as the key components for the dust suppressant.
4.2 Inorganic salt additive analysis
Following the surfactant binary compounding analysis above, we selected AES and SDBS as the two basic components for the dust suppressant. Figure 7 shows the results of the reverse osmosis analysis using the two surfactant monomers (AES and SDBS) and inorganic salt additives. From Fig. 7, it can be seen that compounding of the surfactant AES with the four inorganic salts enhanced the moisture absorption of the coal dust to various degrees. This enhancement is because the surface of coal dust consists of hydrophilic and hydrophobic crystal lattices. The addition of a suitable surfactant creates an adsorption effect on the hydrophobic lattice, thus improving the wettability of the coal dust (Hapgood and Khanmohammadi 2009; Zhao et al. 2011; Hu 2014). However, adsorption of surfactant onto the coal dust surface was limited by electrostatic forces between the surfactant and the layers on the surface of the coal dust and the addition of inorganic salts resulted in the presence of ions in solution, which decreased the electrostatic force distance in the two-layer arrangement. As a result, the adsorption density of the surfactant ions was improved, and the wetting ability of the surfactant for coal dust was further enhanced. Therefore, the reverse osmosis moisture absorption capacity of the coal dust particles was enhanced by the addition of the salt additives (Zhan and Guo 2013; Wang et al. 2020b).
Figure 7 shows that for anionic SDBS, compounding with salts does not lead to significant improvement in the wetting performance of the solutions. Compared to the monomer solution, the addition of CaCl2 and NaCl increased the reverse osmosis moisture absorption up to a maximum of 10%. Further, Na2SiO3 and Na2SO4 produced an antagonistic effect on SDBS and resulted in a lower compound solution moisture absorption compared to that of the monomer; this indicates that these two inorganic salts are not suitable for use as dust suppressant additives to enhance the solution wettability.
Figure 8 shows the results of the moisture retention analysis for four inorganic salt solutions. It can be seen that the coal dust sample without inorganic salt additives has the fastest moisture evaporation rate; after 132 h, the moisture content was less than 5%. In contrast, the addition of inorganic salts increased the moisture content of the coal dust samples by more than 15%. This is because after the coal dusts meet water, lots of water molecules are adsorbed on the coal dust surface. The addition of an inorganic salt into this solution results in the formation of ions, which penetrate the crystal layers of the coal dust samples. As a result, the coal dust surface charge and the ions adsorbed on the crystal layers adsorb water molecules and a water film is formed. This water film absorbs and stores water, thereby enhancing evaporation resistance (Yao et al. 2017; Wang et al. 2020b).
Prior to 168 h, the moisture content of the coal dust samples containing NaCl was the highest. However, between 168 and 192 h, the moisture content of the coal dust samples containing NaCl decreases while evaporation for the other three coal dust samples reached equilibrium between 156 and 168 h. At 192 h, the moisture content of the CaCl2 coal dust samples was the highest (8.40%) and the moisture content of the coal dust with added NaCl, Na2SiO3, and Na2SO4 were 4.88%, 4.55%, and 3.38%, respectively. Based on the water retention analysis results, we found that the addition of CaCl2 led to optimal water retention. The addition of CaCl2 slows water evaporation in coking coal dust. Therefore, CaCl2 is recommended as a surfactant solution additive to further enhance the wettability of the surfactant solution and provide excellent water retention. Therefore, we recommended this inorganic salt is used as the additive for dust suppressants applied in engineering applications.
4.3 Orthogonal experimental design
Through the previous compounding experiments, we identified AES, SDBS and CaCl2 as the dust suppressant components. However, the optimal concentration of each component has not been determined. Orthogonal experimental design was used to determine the optimal concentration of the three compounds. A three-factor, three-level orthogonal experimental design was performed using the orthogonal table L9 (33). The results of the orthogonal experimental design are shown in Table 5.
Table 5 shows that, of the nine schemes analyzed, Scheme 1 is optimal. Under Scheme 1, the reverse osmosis hygroscopic mass was 120 mg. In order to determine the influence of the optimal concentration levels of the three compounds, the comprehensive average and reverse osmosis moisture absorption range for each level are listed in Table 6.
Range is a key indicator in evaluating the importance of each influencing factor. A larger range indicates that the factor has a greater influence on the result (Sun et al. 2019; Li et al. 2019). In contrast, a smaller range indicates that the factor has a smaller influence. Using the range analysis results in Table 6, the three factors were ordered based on influence of reverse osmosis moisture absorption as follows: AES > CaCl2 > SDBS. The comprehensive average calculation results in Table 6 show that K1 is the largest factor for AES, K2 is the largest factor for SDBS, and K1 is the largest factor for CaCl2. Therefore, the concentrations of AES, SDBS, and CaCl2 in the recommended formulation are 0.03%, 0.05%, and 0.4%, respectively. Using this formulation, the reverse osmosis hygroscopic mass of the solution was 128 mg, which indicates that the solution has improved wetting performance.
5 Conclusions
In this study, six surfactants were selected from a total of ten surfactant monomers as suitable dust suppressants for coking coal dust, using surface tension, contact angle, and reverse osmosis analyses. Next, the six selected surfactant monomers underwent binary compounding and the wettability of the solution was investigated by determining the reverse osmosis moisture absorption capacity. Based on the results, SDBS and AES were determined to be the optimal components of the dust suppressant. The wetting properties of SDBS and AES compounded with four inorganic salts were then investigated. By combining the wetting properties and the water retention properties of the four inorganic salt solutions, CaCl2 was selected as the optimal inorganic salt additive for the dust suppressant. Finally, three-factor, three-level orthogonal experimental design was performed to obtain the optimal combination of concentrations for the three components i.e., AES (0.03%), SBS (0.05%), and CaCl2 (0.4%). The dust suppressant solution prepared using the above scheme has a moisture absorption of up to 128 mg and excellent wetting performance. Using the methods described above, an improved surfactant formulation for the treatment of coking coal dust was obtained, which will provide direction for future research.
References
Ahmed M, Guo XX, Zhao XM (2017) Spectroscopic and microscopic characterization of atmospheric particulate matter. Instrum Sci Technol 45:659–682
Bao Q, Nie W, Liu CQ, Zhang HH, Wang HK, Jin H, Yan JY, Liu Q (2020) The preparation of a novel hydrogel based on cross linked polymers for suppressing coal dusts. J Clean Prod 249:119343
Dey S (2012) Enhancement in hydrophobicity of low rank coal by surfactants—a critical overview. Fuel Process Technol 94:151–158
Du J, Zeng XC (2002) An investigation on the variation of critical micelle concentrations of CTAB and SDS surfactant influenced by salts. J Sichuan Univ 39:733–736
Edwards PM (2002) Origin 7.0: Scientific Graphing and Data Analysis Software. J Chem Inf ComputSci 42(5):1270–1271
Fan T, Zhou G, Wang JY (2018) Preparation and characterization of a wetting-agglomeration-based hybrid coal dust suppressant. Process Saf Environ Prot 113:282–291
Han H, Wang PF, Li YJ, Liu RH, Tian C (2020a) Effect of water supply pressure on atomization characteristics and dust-reduction efficiency of internal mixing air atomizing nozzle. Adv Powder Technol 31:252–268
Han WB, Zhou G, Zhang QT, Pan HW, Liu D (2020b) Experimental study on modification of physicochemical characteristics of acidified coal by surfactants and ionic liquids. Fuel 266:116966
Hapgood KP, Khanmohammadi B (2009) Granulation of hydrophobic powders. Powder Technol 189:253–262
He GX, Sun HJ, Zan HT, Wu H (2008) Effect of inorganic salt on surface tension of SDS solution. Chem Ind Press Times 22:42–44
Hu F (2014) Study on influence of coal composition upon hydrophilicity of coal dust. Min Saf Environ Prot 41:19–22
Hu J, Nie W, Zhang YS, Wang HK, Zhang HH, Bao Q, Yan JY (2019) Development of environmental friendly dust suppressant based on the modification of soybean protein isolate. Processes 7(3):165
Hua Y, Nie W, Liu Q, Yin S, Peng HT (2020) Effect of wind curtain on dust extraction in rock tunnel working face: CFD and field measurement analysis. Energy 197:117214
Huang WG, Hu F, Liu NQ (2010) Study on the influence of surfactant on the wettability of coal dust. Min Saf Environ Prot 37:4–6
Jiang HB, Xiao YL, Zhao W (2013) Study on wetting property of coal dust by new surfactant solution. China Saf Sci Technol 9(06):11–15
Jin X, Wu LS, Li P, Ju Z, Xu ZQ, Liu J, Wang Z, Zhang SS (2019) Study on preparation and application effect of high-efficiency wetting type dust suppressant. Adv Environ Prot 9:64–70
Kilaua HW, Pahlman JE (1987) Coal wetting ability of surfactant solutions and the effect of multivalent anion additions. Colloid Surf 26:217–242
Kumar S, Mandal A (2016) Studies on interfacial behavior and wettability change phenomena by ionic and nonionic surfactants in presence of alkalis and salt for enhanced oil recovery. Appl Surf Sci 372:42–51
Li H, Zeng FG, Shao LY, Shi ZB (2002) Current status of study on the human health effects of inhalable particulates. J Environ Health 19:85–87
Li B, Wang N, Xue R, Yang YP (2010) Study on prescription of new coal dedusting agent. J China Coal Soc 37:10–16
Li JL, Zhou FB, Liu H (2016) The selection and application of a compound wetting agent to the coal seam water infusion for dust control. Int J Coal Prep Util 36(4):192–206
Li YJ, Wang PF, Liu RH, Gao RZ (2019) Optimization of structural parameters and installation position of the wall-mounted air cylinder in the fully mechanized excavation face based on CFD and orthogonal design. Process Saf Environ Prot 130:344–358
Liu MH (2006) Preparation and application of coal water slurry additives, 1st edn. Macmillan, Peking
Luo GH, Li B, Ding YY (2016) Study on influence of coal dust wettability by chemical composition and structure parameters. J Dalian Jiaotong Univ 37:64–67
Ma LJ, Zhou MX, Jing DK, Yu F (2004) Effect of temperature and inorganic salt on surface tension LMEE and SDS mixed aqueous solution. Fine Chem 21:42–44
National Standards of the People’s Republic of China GB/T 5751-2009, Chinese Classification of Coals, China Standard Press, Peking (2009)
Nie W, Wei WL, Hua Y, Liu HJ, Ma H, Gao M (2017a) Analysis on the dust pollution in fully mechanized rock headings Controlled by the Multi-Radial Vortex Wind. J Basic Sci Eng 25:65–77
Nie W, Wei WL, Ma X, Liu YH, Peng HT, Liu Q (2017b) The effects of ventilation parameters on the migration behaviors of head-on dusts in the heading face. Tunn Undergr Space Technol 70:400–408
Omane D, Liu WV, Pourrahimian Y (2018) Comparison of chemical suppressants under different atmospheric temperatures for the control of fugitive dust emission on mine hauls roads. Atmos Pollut Res 9:561–568
Peng HT, Nie W, Cai P, Liu Q, Liu ZQ, Yang SB (2019) Development of a novel wind-assisted centralized spraying dedusting device for dust suppression in a fully mechanized mining face. Environ Sci Pollut Res 26:3292–3307
Sun J, Wang YY, Gao DH (2019) Preparation and performance test of a condensed dust suppressant. Min R&D 39(04):111–115
Tang H, Zhao L, Sun W (2016) Surface characteristics and wettability enhancement of respirable sintering dust by nonionic surfactant. Colloids Surf A 509:323–333
Tessum MW, Raynor PC, Keating-Klika (2014) Factors influencing the airborne capture of respirable charged particles by surfactants in water sprays. J Occup Environ Hyg 11:571–582
Tessum MW, Raynor PC (2017) Effects of spray surfactant and particle charge on respirable coal dust capture. Saf Health Work 8:296–305
Tong B (2013) The compound theory of surfactant is briefly discussed. China Chem Trade 8:384
Vedal S (1997) Ambient particles and health. J Air Waste Manag Assoc 47(5):551–581
Wang HT, Du YH, Wei XB, He XX (2019a) An experimental comparison of the spray performance of typical water-based dust reduction media. Powder Technol 345:580–588
Wang HT, Wu JL, Du YH, Wang DM (2019b) Investigation on the atomization characteristics of a solid-cone spray for dust reduction at low and medium pressures. Adv Powder Technol 30:903–910
Wang PF, Tan XH, Zhang LY, Li YJ, Liu RH (2019c) Influence of particle diameter on the wettability of coal dust and the dust suppression efficiency via spraying. Process Saf Environ Prot 132:189–199
Wang X, Yuan S, Jiang B (2019d) Experimental investigation of the wetting ability of surfactants to coals dust based on physical chemistry characteristics of the different coal samples. Adv Powder Technol 30:1696–1708
Wang PF, Shi YJ, Zhang LY, Li YJ (2019e) Effect of structural parameters on atomization characteristics and dust reduction performance of internal-mixing air-assisted atomizer nozzle. Process Saf Environ Prot 128:316–328
Wang PF, Han H, Tian C, Liu RH, Jiang YD (2020a) Experimental study on dust reduction via spraying using surfactant solution. Atmos Pollut Res 11(6):32–42
Wang PF, Jiang YD, Liu RH, Liu LM, He YC (2020b) Experimental study on the improvement of wetting performance of OP-10 solution by inorganic salt additives. Atmos Pollut Res 11(6):153–161
Wu C, Gu DS (2001) Study on the improvement of wetting coal dust by anionic surfactant by Na2SO4. J Saf Environ 2:45–49
Xu G, Chen YP, Eksteen J, Xu JL (2018) Surfactant-aided coal dust suppression: a review of evaluation methods and influencing factors. Sci Total Environ 639:1060–1076
Xu CH, Wang DM, Wang HT, Ma LY, Zhu XL, Zhu YF, Zhang Y, Liu FM (2019a) Experimental investigation of coal dust wetting ability of anionic surfactants with different structures. Process Saf Environ Prot 121:69–76
Xu CW, Nie W, Liu ZQ (2019a) Multi-factor numerical simulation study on spray dust suppression device in coal mining process. Energy 182:544–558
Yan JY, Nie W, Zhang HH, Xiu ZH, Bao Q, Wang HK, Jin H, Zhou WJ (2020) Synthesis and performance measurement of a modified polymer dust suppressant. Adv Powder Technol 31(2):792–803
Yang J, Tan YZ, Wang ZH, Shang YD, Zhao WB (2007) Study on the surface characteristics and wetting mechanism of coal dust. J China Coal Soc 32:737–740
Yang J, Xu H, Gao JG (2014) Influence of particle size on surface characteristic and wetting mechanism of coal dust. J Coal Mine Saf 45:140–143
Yao QG, Xu CC, Zhang YS, Zhou G, Zhang SC, Wang D (2017) Micromechanism of coal dust wettability and its effect on the selection and development of dust suppressants. Process Saf Environ Prot 111:726–732
Yin S, Nie W, Liu Q, Hua Y (2019) Transient CFD modeling of space-time evolution of dust pollutants and air-curtain generator position during tunneling. J Clean Prod 239:117924
Zhan G, Guo ZC (2013) Basic properties of sintering dust from iron and steel plant and potassium recovery. J Environ Sci 25(6):1226–1234
Zhang BQ (2019) Study on preparation and properties of compound dust suppressant for construction site. J Guangzhou Chem Ind 47(07):74-76+92
Zhang HH, Nie W, Yan JY, Bao Q, Wang HK, Jin H, Peng HT, Chen DW, Liu ZQ, Liu Q (2020) Preparation and performance study of a novel polymeric spraying dust suppression agent with enhanced wetting and coagulation properties for coal mine. Powder Technol 364:901–914
Zhao ZB, Chen Y, Sun CY, Shu XQ (2011) Experimental study of coal dust wettability. J China Coal Soc 36:442–446
Zhou G, Ma YL, Fan T, Wang G (2018a) Preparation and characteristics of a multifunctional dust suppressant with agglomeration and wettability performance used in coal mine. Chem Eng Res Des 132:729–742
Zhou Q, Qin BT, Wang J, Wang HT, Wang F (2018b) Experimental investigation on the changes of the wettability and surface characteristics of coal dust with different fractal dimensions. Colloids Surf A 551:148–157
Zhou Q, Qin BT, Wang F, Wang HT, Hou J, Wang ZR (2019a) Effects of droplet formation patterns on the atomization characteristics of a dust removal spray in a coal cutter. Powder Technol 344:570–580
Zhou Q, Qin BT, Wang F, Wang HT (2019b) Experimental investigation on the performance of a novel magnetized apparatus used to improve the dust suppression ability of surfactant-magnetized water. Powder Technol 354:149–157
Zhou WD, Wang HT, Wang DM, Zhang K, Du YH, Yang HS (2020) The effect of geometries and cutting parameters of conical pick on the characteristics of dust generation: experimental investigation and theoretical exploration. Fuel Process Technol 198:106243
Acknowledgements
The project was supported by the National Natural Science Foundation of China (No. 51574123), and the Scientific Research Project of Hunan Province Office of Education (No. 18A185), which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, J., Wang, P., Pei, Y. et al. Preparation and performance analysis of a coking coal dust suppressant spray. Int J Coal Sci Technol 8, 1003–1014 (2021). https://doi.org/10.1007/s40789-021-00406-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-021-00406-8