Abstract
This work evaluated the effects of inherent alkali and alkaline earth metals on nitrogen transformation during steam gasification of Shengli lignite at the temperature of 873–1173 K in a fluidized-bed/fixed-bed quartz reactor. The results indicated that the alkali metal Na and alkaline earth metals Ca, Mg in coal have different effects on inherent nitrogen transformation to NH3, HCN and char-N during the lignite steam gasification. Specifically during the steam gasification of Shengli lignite, Na and Ca, Mg not only catalyze the inherent nitrogen conversions to NH3, but also promote the secondary reactions of the nascent char-N as well as the generation of NH3 from the generated HCN, meanwhile they also inhibited the inherent nitrogen conversion to HCN and char-N. The presence of Na, Ca and Mg hindered the formation of oxidized nitrogen (N-X) functional groups, but enhanced pyridinic nitrogen (N-6) and quaternary nitrogen’s (N-Q) formation in char.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gasification is an important route for efficient and clean utilization of the coal resources. It is not only the foundation for synthesizing chemical products and developing poly-generation technologies, such as the Integrated Gasification Combined Cycle (IGCC), but also the key to the development of other high-tech energy technologies (Xu et al. 2015a, b; Tian et al. 2017a, b). Nitrogen-containing compounds found in gasified product gas include NH3, HCN, etc. When the gasified product gas is used in gas turbine combustion, fuel cell power generation and synthesis process of some value-added chemical products, the N-containing compounds, like NH3 and HCN, will be converted into NOx and arouse environmental problems and catalyst deactivation (Chang et al. 2003), consequently applications of gasified product gas are still limited. Therefore, in order to realize clean coal utilization, control NOx release and protect environment, it is of great significance to study the nitrogen conversion rule in gasification.
Alkali and alkaline earth metals are important mineral components in coal and play essential roles in catalysis during gasification. Alkali and alkaline earth metals considerably change the characteristics of gasification of coal char and volatiles reforming behavior over the char (Bai et al. 2014). Specifically, alkali and alkaline earth metals change the kinetics of coal gasification, enhance the gasification reactivity of char, increase the rate of gaseous products formation and change the composition of the produced tar (Kitsuka et al. 2007; Zhang et al. 2017; Zubek et al. 2018; Tian et al. 2017a, b; Li et al. 2000). Meanwhile, the nitrogen transformation is affected by the reactivity of coal char and the composition of tar (Park et al. 2005). Therefore, it is necessary to study the effects of alkali and alkaline earth metals on nitrogen transformation behaviors in coal gasification. For examples, Tian et al. (2006a, b) studied the effects of coal ash and externally loaded-Na on fuel-N conversion during the reforming of coal and biomass in steam, and showed that the presence of ash increased the yields of NH3 while decreased the yields of HCN. However, the increased yields of NH3 did not match exactly to the reduced amount of HCN yields and loading of NaCl or Na2CO3 into the lignite also affected HCN and NH3 yields during gasification. Zhao et al. (2003, 2004a, b), Zhao (2003) studied the effect of minerals on nitrogen transformation in coal gasification. On one hand, it was concluded that minerals had a prominent promotion effect on the NH3 release both in the steam and CO2 gasification, and reduced the NH3 release was due to removal of minerals. On the other hand, the amount of HCN released from acid washed coal was greater than that of raw coal during the steam gasification. Compare with steam gasification of raw coal, the nitrogen in the char was greatly reduced for the acid-washed coal. Mckenzie et al. (2008) studied NH3 and HCN formation mechanisms during gasification of three rank-ordered coals in steam and oxygen. It was pointed out that the alkali and alkaline earth metallic species in lignite tended to favour the release of coal-N as tar-N but limited char-N conversion during gasification. In the study of coal gasification properties and nitrogen migration pattern, Xu (2012) found that Ca promoted the formations of N2 and NH3 while suppressed the generations of tar-N and char-N. Alkali metals Na and K promoted the formation of NH3, although showed no obvious effect on the HCN release. By studying the pyrolysis and combustion of model chars, Zhao et al. (2003) found that the presence of Na was in favor of the transformation of char-nitrogen to volatile-nitrogen at high temperature, but the influence of Ca was negligible.
However, there are few studies about the effects of alkali and alkaline earth metals on the nitrogen transformation in coal gasification process. Besides, some above-mentioned work have been devoted to study of nitrogen migration and transformation to gas phases, but few work have been focusing on nitrogen transformation to liquid phases and solid phases. Meanwhile, it should be pointed out that previous studies still showed inconsistent results and the conclusions could not come to an agreement. Therefore, further study is still in immediate need in order to unveil the effects of alkali and alkaline earth metals on nitrogen migration and transformation during coal gasification. Accordingly, this research is designed to make progress in this area.
Our previous work (Zhang et al. 2013; Zheng et al. 2017; Wang et al. 2014a, b, c, d) studied the nitrogen migration and transformation in the Shengli lignite pyrolysis by employing Na-loaded coal samples. It was concluded that rapid temperature ramping was beneficial to converting coal-N to NH3 and HCN. With different pyrolysis temperatures and Na loading amounts,Na showed the different effects on the conversions of coal-N to NH3 and char-N. In contrary, HCN conversion was not affected by the temperatures and Na loadings. Meanwhile, Na and Ca in coal minerals enhanced the nitrogen conversion to NH3 while effectively inhibited its conversion to HCN. Based on the previous researches and the knowledge that tar yield could be reduced by steam in gasification process (Wang et al. 2014a, b, c, d), this study further investigated effects of inherent alkali and alkaline earth metals on the nitrogen transformation in the steam gasification of Shengli lignite. In particular, this study is focusing on the nitrogen transformations to gaseous products (e.g. NH3, HCN) as well as solid products (e.g. char-N), together with the effects on N-containing functional groups in char.
2 Experimental
2.1 Coal samples
Shengli lignite was crushed and sieved to the size of 60–96 μm. The hydrochloric acid-washing method was adopted to remove alkali and alkaline earth metals from Shengli lignite. The acid washing employed the following conditions: 5 mol/L HCl aqueous; washing time and temperature, 12 h and room temperature, respectively. The acid-washed coal was washed with deionized water exhaustively until the absence of chlorine ion and vacuum-dried at 343 K for 24 h. Removal of alkali and alkaline earth metals of Shengli lignite was as acid-washed coal,which marked as SLD. While the raw Shengli lignite referred to as SLR. The proximate and ultimate analysis of SLR and SLD are shown in Table 1.
2.2 Gasification
The apparatus flow chart of steam gasification experiment is shown in Fig. 1. A quartz tube reactor used in this study was the fixed-bed/fluidized-bed reactor described previously (Zhang et al. 2013).There was a quartz frit at the top and bottom of the quartz tube reactor. The bottom quartz frit was to distribute fluidized gas and the top quartz frit was to prevent coal char escaping from the reactor. In the experiment, UHP argon was firstly introduced to the system for 10 min with the flow rate 0.53 L/min to remove the air; then reactor was filled with sand that particle size was 0.25–0.3 mm as the fluidizing agent and heated up to the reaction temperature. Argon was used as carrier gas (1.0 L/min) to entrain coal particles into the reactor. At the same time, 15 vol% steam was generated and pumped into the reactor. After 15 min argon purging, about 1 g coal sample was introduced into the reactor at a feed rate of 66.67 mg/min. The HCN in the gas products was absorbed by 0.1 mol/L NaOH aqueous solution while the NH3 was absorbed by 0.02 mol/L H2SO4 aqueous solution. The absorption of NH3 and HCN were carried out in two independent tests. The residual solid remained in the reactor was transferred and weighed as char.
2.3 Products analysis
The ash component analysis of SLR and SLD were measured using X-ray fluorescence spectrometer (XRF-1800) with an accuracy of 1 kHz/s. NH3 and HCN absorbed in the solution were quantified through Nessler’s reagent spectra photometric method (HJ535-2009) and isonicotinic acid pyrazolone spectra photometric method (HJ 484-2009), respectively, using the ultraviolet spectrophotometer (TU-1901 type). The nitrogen content in char was quantified by Elemental analyzer (Elemental Vario MICRO cube). The nitrogen functional groups in SLR, SLD and their gasification chars were all determined using an AXIS ULTRADLD X-ray photoelectron spectrometer. The monochromatic AlK-α X-ray source was used, and the energy was 40 eV, the step length was 50 meV. The binding energy correction arising from charging was made by assign a binding energy of 284.6 eV to the C1s component. Deconvolution analysis of N 1s spectra was also carried out according to the least-squares curve-fitting technique using XPS PEAK 4.1 software. The nitrogen contents in samples were used to calculate the nitrogen distribution. The calculation methods were as follows:
3 Results and discussion
3.1 Removal of inherent alkali and alkaline earth metals and its effect on coal-N
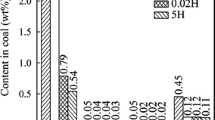
The hydrochloric acid-washing method is used to remove the ash from the Shengli lignite and the ash compositions are shown in Table 2. It is obvious that alkali and alkaline earth metals are removed by acid-washing. There are four types of alkali and alkaline earth metals found in coal: water-soluble, ion-exchangeable, hydrochloric soluble and stable forms (Lin et al. 2016; Bai et al. 2017). Among these four types,hydrochloric-soluble alkali metals and alkaline earth metals is organic salt presenting as coordination form in coal structure. Hydrochloric acid-washing effectively removed water-soluble, ion-exchanged and hydrochloric-soluble alkali metals and alkaline earth metals, and the removal rate was above 81%. After the acid-washing, only a small amount of alkali and alkaline earth metals remained in the ash in stable forms. Besides the alkali and alkaline earth metals, transition metal such as Fe also affects the nitrogen transformation in the coal. However, the Fe2O3 only occupy 1.83% in raw coal sample, and the removal rate of Fe are relatively low (60%) compared to those from alkali and alkaline earth metals, especially Ca (84.7%), Mg (81.1%), Na (88.5%) and Sr (92.3%). Therefore, we concentrate on the difference in the nitrogen transformation between SLR and SLD during the steam gasification, which is mainly resulted from the variations of alkali and alkaline earth metals. As mentioned, the hydrochloric acid-washing mainly affected the contents of alkali metal Na and alkaline earth metals Ca, Mg, while K content did not change obviously after the acid-washing. Moreover, Sr content was considerably very low (0.13 wt% in SLR and 0.01 wt% in SLD), thus Sr should have negligible affection to the nitrogen transformation behavior during gasification.
X-ray photoelectron spectroscopy (XPS) was adopted to determine the N-containing functional groups in SLR and SLD. Different N-containing functional groups and their corresponding electron binding energies were as follows: pyridinic nitrogen (N-6) at 398.7 ± 0.4 eV, pyrrolic nitrogen (N-5) at 400.5 ± 0.3 eV, quaternary nitrogen (N-Q) at 401.1 ± 0.3 eV and oxidized nitrogen (N-X) at 403.5 ± 0.5 eV, respectively (Kapteijn et al. 1999). The N1s XPS spectra of SLR and SLD are shown in Fig. 2 and the relative content of N-containing functional groups are shown in Table 3. The results show that the N-containing functional groups in SLR and SLD mainly are N-6, N-5 and N-Q, and the content of each N-containing functional group is almost the same, which indicates that hydrochloric acid-washing does not alter the N-containing functional groups in Shengli lignite.
3.2 Effects of inherent alkali and alkaline earth metals on the content of char-N and nitrogen functional groups of char
The influence of inherent alkali and alkaline earth metals on the contents of char-N in SLR and SLD steam gasification at different temperatures is shown in Fig. 3. The char-N yields after SLR and SLD steam gasification are both decreased with the increasing of gasification temperature. At the same gasification temperature, the content of char-N in SLD is greater than that in SLR. Increased steam gasification temperature results in not only promoting gasification reaction but also contributing to release more gaseous N-containing species, which are derived from the interaction between nitrogen radicals from N-containing heterocyclic rings and hydrogen radicals from steam. Therefore, the nitrogen amount in solid char is reduced. In addition, although removal of volatiles and ordered char structure reduce the char reactivity, alkali and alkaline earth metals promote the secondary reaction of the nascent char and finally contribute to an acceleration of the reaction rate. Reactions between Na, Ca and char lead to the surface charge migration, for example, some intermediates (i.e. O−Na+ and O−Ca2+O−) were formed when Na and Ca bond with oxygen in the char matrix during metal–carbon bond formation. Thus the Na and Ca promote the secondary reaction of nascent char by changing the electron-cloud distribution of the surface carbon atoms, increasing the active sites on char and decreasing the char reaction energy barrier (Xu et al. 2015a, b; Li et al. 2015; Liu et al. 2018). Moreover, Mg promotes the gasification reactivity by forming strong active centers on the char surface (Ohtsuks et al. 1997). Further, the enhanced secondary reaction of nascent char are helpful to release more nitrogen radicals from the N-containing heterocyclic ring opening reaction to react with hydrogen radicals (from H2O) to produce NH3. Finally, the nitrogen content in the char decreases. For SLD with little Na, Ca and Mg, both gasification reaction and nascent char secondary reaction are suppressed, thus more nitrogen is remained as char-N. From this discussion, it is understandable that the alkali metal Na and alkaline earth metals Ca and Mg in coal inhibit the nitrogen conversion to char-N during the steam gasification.
The N1s XPS spectra of char produced by SLR and SLD gasification at 973 K are shown in Fig. 4. The relative contents of N-containing functional groups in each char are shown in Table 4. As for the components in Fig. 4 and Table 4, N-6 refers to the nitrogen on the boundary of aromatic molecular structure, N-5 describes the nitrogen in the five-membered ring on the boundary of coal molecular structure, N-Q represents the protonated pyridine nitrogen incorporated into the multiple aromatic structural unit and N-X refers to a structure where a nitrogen atom in a pyridine is directly linked to an oxygen atom (Zhang et al. 2011; Meng et al. 2017; Wang et al. 2017). From Fig. 4, the N-containing functional groups found in both chars were N-6, N-5, N-Q and N-X, among which N-Q is the major constituent. According to Table 4, both SLR and SLD char show similar N-5 content. Compared to SLD char, more N-6 and N-Q with less N-X are found in SLR char. Combined with Fig. 2 and Table 3, N-5 is diminished from 61 wt% to about 26 wt% and N-X is absent in coal samples which only be detected in char samples, indicating that the introduction of H2O in gasification converts N-5 to N-X, N-6 and N-Q. In thermal conversion, with the increase of temperature, the N-5 is converted to N-6 during condensation of the carbon matrix, and the five-membered ring nitrogen at coal molecular boundary will be clogged into grapheme layers and converted to N-Q (Pels et al. 1995; Xu et al. 2016). That is to say, with the increase of temperature, the pyrrolic nitrogen (N-5) in coal char is converted into pyridinic nitrogen (N-6) and quaternary nitrogen (N-Q). The N-Q is the most stable form of nitrogen structure (Zhang et al. 2011; Stańczyk et al. 1995). The introduce of oxygen molecule breaks one of C–N bonds in N-containing aromatic ring on char surfaces and then reacts with the nitrogen to form a –CNO intermediate, finally these intermediates convent into N-X (Gong et al. 1999). In the steam gasification process, the catalytic Na, Ca and Mg increase the release of free radicals to react with five-membered ring nitrogen to convert to N-6. Some nitrogen on the opened five-membered ring are clogged into the interior of the aromatic structure and converted to N-Q during polycondensation of the aromatic radicals. In addition, the introduced H2O generates oxygen in the reaction, which is adsorbed on the nascent char surface. The adsorbed oxygen reacts with the C–N bond to generate –CNO and finally converts to N-X in the nascent char secondary reaction. The acid-washing treatment removed most active Na, Ca, and Mg in the coal, therefore decreased the reactivity of coal and char, and the amount of free radicals generated in the gasification was reduced. So more N-containing free radicals formed by N-5 cracking react with H2O to produce N-X instead of N-6 and N-Q. Therefore, the reduction of Na, Ca, and Mg are beneficial to the N-X formation and not contributive to the formations of N-6 and N-Q in char.
3.3 Effects of inherent alkali and alkaline earth metals on NH3 formation
The influence of inherent alkali and alkaline earth metals on the NH3 release during the gasification of SLR and SLD at different temperatures is shown in Fig. 5. It can be seen from the figure that the NH3 released from SLR is higher than that from SLD at the same temperature. The NH3 released from both SLR and SLD increased with temperature, releasing 27.34% and 32.63% at 873 K, and gradually increasing to 87.103% and 75.58% at 1173 K. In the coal thermal conversion process, there are three major routes of NH3 formation: the thermally stable N-containing structures in the coal are hydrogenated to NH3 during the primary pyrolysis; thermal cracking/gasification of nascent char-N as well as the thermal cracking/reforming of volatile-N (HCN and tar-N) (Tian et al. 2005; Li and Tan 2000). In the steam gasification, Na and Ca combined with carboxyl functional groups in the lignite and decreased the activation energy consequently increased the coal gasification rate (Sha et al. 1986; Wang et al. 2013, 2014a, b, c, d). So that, SLR has a deeper thermally cracking than SLD at the same gasification temperature, and resulting in generating more free radicals attack the N-containing heterocyclic in coal. More stable N-containing structures in the coal, like N-Q, were cracked and then converted into N-containing small molecules to form NH3 and released into the gas phase. In addition, the NH3 also produced via the hydrolysis of the generated HCN. Since Na, Ca and Mg in lignite promoted the hydrolysis of HCN by accelerating the dissociation rate of H2O into H+ and OH− (Wang et al. 2014a, b, c, d), the content of NH3 was increased at the reaction system. The secondary reaction of char was also promoted by the alkali and alkaline earth metals. Specifically, Na and Ca increased the reactivity of char, and Mg formed active sites on char surfaces thus promoted the reaction (Ohtsuks et al. 1997; Huang et al. 2009). Numerous H radicals from steam were adsorbed on the nascent char surface and reacted with N-containing heteroaromatic rings in the char to generate gaseous NH3 (Chang et al. 2006). After acid-washing, the great reduction of Na, Ca, and Mg in coal results in the dramatic decreased of NH3 release. Removal of catalytic Na, Ca and Mg decreased the conversion of nitrogen to NH3. At the same time, increased temperature led to more severe thermal cracking, thus more free radicals were generated from aromatic ring opening reactions and attacked the N-containing heterocyclic rings to subsequently produce NH3 in small molecule form. Therefore, the amount of released NH3 increased with the increasing of the gasification temperature.
3.4 Effects of inherent alkali and alkaline earth metals on HCN formation
Effects of inherent alkali and alkaline earth metals on HCN production in the steam gasification of SLR and SLD at different temperatures is shown in Fig. 6. For both SLR and SLD, as gasification temperature increased, HCN release firstly increased from 1.6%, 9.7% to 2.3%, 15.9% when the temperature rises from 873 to 973 K, and then decreased to 0.43%, 9.9% at temperature 1173 K. According to Fig. 6, the HCN production of SLR is lower than that of SLD at the same temperature. In the coal thermal conversion process, HCN was mainly generated from the thermally less stable N-containing structures in coal and the thermal cracking of volatile-N (Tian et al. 2005; Li et al. 1996).With the intensified thermal cracking of the macromolecular structure at 873–973 K, the unstable N-containing structure, like N-6 and part of N-5, are cracked and converted to HCN. Moreover, the breakdown of the carbon network structure in the macromolecular structure of coal is enhanced to the activation of the nitrogen-containing surface species, and further convert to HCN. The nitrogen-containing surface species is an important intermediate product for HCN formation (Orikasa and Tomita 2003). At higher temperatures 1073 K and 1173 K, plenty of volatiles vigorously escape from lignite and are trapped in the reactor in a short time. Consequently, the generated volatiles-N is cracked to form an intermediate product HCNO, and HNCO reacts with H2O in system to form NH3, lead to small amount of volatile-N are cracked to form HCN (Ledesma et al. 1998; Nelson et al. 1996). Moreover, the residence time of HCN produced from volatiles-N was lengthened and more contact opportunities between HCN and H2O were created, leading to the consumption of HCN as well as production of NH3 through HCN hydrolysis reaction. In addition, the char or reactor surface promotes further reaction of HCN that in the gas phases, resulting in HCN release further reduction (Park et al. 2008). The presence of alkali metal Na and alkaline earth metals Ca and Mg increase the generation of volatiles and thus promote the HCN hydrolysis reaction, resulting in decreased HCN release. Besides, the generated HCN reacts with Na, Ca to produce intermediates NaCN and CaCN2, which will subsequently interact with H2O to form NH3 (Tian et al. 2006a, b; Schäfer and Bonn 2002). Moreover, Na in ash catalyzes the volatile-N conversion into soot-N (Tian et al. 2006a, b) during coal gasification, which also contributes to the reduced HCN release for SLR sample. In the contrary, acid-washing limits the HCN secondary reactions by diminishing Na, Ca and Mg amounts, and prevents the suppression of HCN release.
The influence of inherent alkali and alkaline earth metals on the total release of NH3 and HCN in steam gasification of SLR and SLD at different temperatures is shown in Fig. 7. According to Fig. 7, at each temperature, the total amount of NH3 and HCN produced by SLR is greater than that of the SLD during the steam gasification except for at low temperature of 873 K. At 873 K, the total amount of NH3 and HCN produced of SLR is lower than that of the SLD due to the significant small amount of HCN release. Based on Figs. 5 and 6, NH3 emissions from the SLR and SLD gasification are almost the same at 873 K. However, the amount of HCN release of SLR is significantly less than that of SLD due to the removal of most Na, Ca, and Mg through acid-washing. However, it should be mentioned that at higher temperature, for example 973 K, compared to SLD, the NH3 release is greater than the HCN decrease of SLR at the same gasification temperature, which indicates there are other generation sources of NH3 during the steam gasification.
3.5 Effects of inherent alkali and alkaline earth metals on the distribution of nitrogen
Figure 8 shows the distribution of nitrogen during the steam gasification process of SLR and SLD at different temperatures, respectively. In the figure, other-N represents nitrogenous species except for char-N, NH3 and HCN, and its content is obtained by difference. From Fig. 8a, for SLR steam gasification, at temperature 873 K, char-N is the most abundant among the nitrogen products, occupying 48% of the total nitrogen content. Nitrogen content for other products decrease in the order: NH3-N > other-N > HCN-N, with corresponding percentage of 27.34%, 23.09%, 1.61%, respectively. With the temperature increase to 973–1073 K, NH3-N is the dominant component in nitrogen products, which account for 78.68%–84.92%, followed by char-N(10%–14.84%), other-N(4.23%–4.39%) and HCN-N(0.69%–2.25%). For SLD steam gasification in Fig. 8b, when temperatures are 873 K and 973 K, char-N is the main component in nitrogen products, which account for 51.76% and 56.24% of the nitrogen content, respectively. NH3-N contains 28.27% and 32.63%, HCN-N includes 9.68%–15.94% and other-N possesses 1.45%–4.03% of the nitrogen content at 873 K and 973 K, respectively. When the temperature increases to 1073 K, NH3-N turns to be responsible for the most nitrogen content 75.58% among products containing nitrogen, which much more than those of char-N (10.25%), HCN-N (9.92%) and other-N (4.25%). In summary, Char-N, NH3-N, HCN-N and other-N has different distributions in the nitrogen products from steam gasification of SLR and SLD. According to Sects. 3.2–3.4, the inherent alkali and alkaline earth metals play essential role in the nitrogen migration and transformation when forming char-N, NH3-N and HCN-N, consequently affect the distribution of nitrogen in the N-containing products of Shengli lignite steam gasification.
3.6 Effects of inherent alkali and alkaline earth metals on nitrogen transformation route
The conversion route of nitrogen in the steam gasification of Shengli lignite is shown in Fig. 9. The nitrogen structure in the Shengli lignite and gasified char in Fig. 9 is determined by X-ray photoelectron spectroscopy (XPS). The nitrogen structure in the tar is determined according to the literature (Li et al. 1998; Kelemen et al. 1998). In all of the following processes, alkali metal Na and alkaline earth metals Ca and Mg work as the catalysts. During gasification, the inherent nitrogen is converted into tar-N, char-N, NH3 and HCN during primary gasification. As the reaction proceeds, the nascent char-N undergoes a secondary reaction with H radicals provided by H2O to generate NH3, thus causing the decrease of residual nitrogen in the char. The generated HCN reacts with H2O and is hydrolyzed to NH3, results in the decreasing of HCN release and the increasing in the NH3 release during the steam gasification. By affecting these mutual transformation pathways of nitrogen, the alkali metal Na and alkaline earth metals Ca and Mg in coal alter the final distribution of nitrogen in gas and solid phases after the Shengli lignite steam gasification.
4 Conclusions
This paper mainly studied effects of inherent alkali and alkaline earth metals on the transformation of nitrogen during steam gasification of Shengli lignite at different temperatures. The following conclusions can be drawn based on the experimental data:
-
(1)
Alkali and alkaline earth metals in the Shengli lignite minerals, especially Na, Ca and Mg, affect the nitrogen transformation to both gas phases (NH3, HCN) and solid phase (Char-N) during the steam gasification.
-
(2)
In the steam gasification of Shengli lignite, inherent Na, Ca and Mg catalyze the nitrogen conversion in the coal to NH3, promote the secondary reactions of the nascent char-N and the generated HCN converting to NH3, together with inhibiting the nitrogen conversions to HCN and char-N.
-
(3)
The N-X functional groups in the char is produced by the steam gasification, and the presence of Na, Ca and Mg is not conducive to the formation of N-X, but beneficial to the formations of N-Q and N-6.
References
Bai L, Karnowo Kudo S, Norinaga K, Wang YG, Hayashi JI (2014) Kinetics and mechanism of steam gasification of char from hydrothermally treated woody biomass. Energy Fuels 28(11):7133–7139
Bai YH, Zhu SH, Luo K, Gao MQ, Yan LJ, Li F (2017) Coal char gasification in H2O/CO2: release of alkali and alkaline earth metallic species and their effects on reactivity. Appl Therm Eng 112:156–163
Chang LP, Xie ZL, Xie KC, Pratt KC, Hayashi JI, Chiba T, Li CZ (2003) Formation of NOx precursors during the pyrolysis of coal and biomass. Part VI. Effects of gas atmosphere on the formation of NH3 and HCN. Fuel 82(10):1159–1166
Chang LP, Xie ZL, Xie KC (2006) Study on the formation of NH3 and HCN during the gasification of brown coal in steam. Process Saf Environ Prot 84(6):446–452
Gong B, Buckley AN, Lamb RN, Nelson PF (1999) XPS determination of the forms of nitrogen in coal pyrolysis. Surfac Interface Anal 28(1):126–130
Huang YQ, Yin XL, Wu CZ, Wang CW, Xie JJ, Zhou ZQ, Mall LL, Li HB (2009) Effects of metal catalysts on CO2 gasification reactivity of biomass char. Biotechnol Adv 27(5):568–572
Kapteijn F, Moulijn JA, Matzner S, Boehm HP (1999) The development of nitrogen functionality in model chars during gasification in CO2 and O2. Carbon 37(7):1143–1150
Kelemen SR, Gorbaty ML, Kwiatek PJ, Fletcher TH, Watt M, Solum MS, Pugmire RJ (1998) Nitrogen transformations in coal during pyrolysis. Energy Fuels 12(1):159–173
Kitsuka T, Bayarsaikhan B, Sonoyama N, Hosokai S, Li CZ, Norinaga K, Hayashi JI (2007) Behavior of inherent metallic species as a crucial factor for kinetics of steam gasification of char from coal pyrolysis. Energy Fuels 21(2):387–394
Ledesma EB, Li CZ, Nelson PF, Mackie JC (1998) Release of HCN, NH3 and HNCO from the thermal gas-phase cracking of coal pyrolysis tars. Energy Fuels 12(3):536–541
Li CZ, Tan LL (2000) Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part III: Further discussion on the formation of HCN and NH3 during pyrolysis. Fuel 79(15):1899–1906
Li CZ, Nelson PF, Ledesma EB, Mackiej C (1996) An experimental study of the release of nitrogen from coals pyrolyzed in fluidized-bed reactors. Symp (Int) Combust 26(2):3205–3211
Li CZ, Buckley AN, Nelson PF (1998) Effects of temperature and molecular mass on the nitrogen functionality of tars produced under high heating rate conditions. Fuel 77(3):157–164
Li CZ, Sathe C, Kershaw JR, Pang Y (2000) Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal. Fuel 79(3):427–438
Li Y, Zhou CL, Li N, Zhi KD, Song YM, He RX, Teng YY, Liu QS (2015) Production of high H2/CO syngas by steam gasification of Shengli lignite: catalytic effect of inherent minerals. Energy Fuels 29(8):4738–4746
Lin XC, Yang YP, Yang SS, Li SY, Tian YJ, Wang YG (2016) Initial deposition feature during high-temperature pressurized pyrolysis of a typical Zhundong coal. Energy Fuels 30(8):6340–6341
Liu Y, Guan YJ, Zhang K (2018) CO2 gasification performance and alkali/alkaline earth metals catalytic mechanism of Zhundong coal char. Korean J Chem Eng 6:1–8
Mckenzie LJ, Tian FJ, Guo X, Li CZ (2008) NH3 and HCN formation during the gasification of three rank-ordered coals in steam and oxygen. Fuel 82(7):1102–1107
Meng XL, Gao MQ, Chu RZ, Miao ZY, Wu GG, Bai L, Liu P, Yan YF, Zhang PC (2017) Construction of a macromolecular structural model Chinese lignite and analysis of its low-temperature oxidation behavior. Chin J Chem Eng 25(9):1314–1321
Nelson PF, Li CZ, Ledesma E (1996) Formation of HNCO from the rapid pyrolysis of coals. Energy Fuels 10(1):264–265
Ohtsuks Y, Wu XH, Furimaky YE (1997) Effect of alkali and alkaline earth metals on nitrogen release during temperature programmed pyrolysis of coal. Fuel 76(14):1361–1367
Orikasa H, Tomita A (2003) A study of the HCN formation mechanism during the coal char gasification by O2. Energy Fuels 17(6):1536–1540
Park DC, Day SJ, Nelson PF (2005) Nitrogen release during reaction of coal char with O2, CO2, and H2O. Proc Combust Inst 30(2):2169–2175
Park DC, Day SJ, Nelson PF (2008) Formation of N-containing gas-phase species from char gasification in steam. Fuel 87(6):807–814
Pels JR, Kapteijn F, Moulijn JA, Zhu Q, Thomas KM (1995) Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 33(11):1641–1653
Schäfer S, Bonn B (2002) Hydrolysis of HCN as an important step in nitrogen oxide formation in fluidized combustion. Part II: Heterogeneous reactions involving limestone. Fuel 81(13):1641–1646
Sha XZ, Huang YH, Cao JQ, Ren DQ (1986) The correlation of rank of coals and their mineral constituents with gasification reactivity of coal chars. J Fuel Chem Technol 14(2):108–116
Stańczyk K, Dziembaj R, Piwowarska Z, Witkowski S (1995) Transformation of nitrogen structures in carbonization of model compounds determined by XPS. Carbon 33(10):1383–1392
Tian FJ, Yu JL, Mckenzie LJ, Hayashi JI, Chiba T, Li CZ (2005) Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part VII: Pyrolysis and gasification of cane trash with steam. Fuel 84(4):371–376
Tian FJ, Yu JL, Mckenzie LJ, Hayashi JL, Li CZ (2006a) Formation of NOx precursors during the pyrolysis of coal and biomass. Part IX: Effects of coal ash and externally loaded-Na on fuel-N conversion during the reforming of coal and biomass in steam. Fuel 85(10):1411–1417
Tian FJ, Yu JL, Mckenzie LJ, Hayashi JI, Li CZ (2006b) Formation of HCN andNH3 during the reforming of Quinoline with steam in a fluidized-bed reactor. Energy Fuels 20(1):159–163
Tian B, Qiao YY, Bai L, Feng W, Jiang Y, Tian YY (2017a) Pyrolysis behavior and kinetics of the trapped small molecular phase in a lignite. Energy Convers Manag 140:109–120
Tian B, Qiao YY, Fan JF, Bai L, Tian YY (2017b) Coupling pyrolysis and gasification process for Methane-rich syngas production: fundamental studies on pyrolysis behavior and kinetics of a calcium-rich high volatile bituminous coal. Energy Fuels 31(10):10665–10673
Wang YF, Jin WL, Huang TH, Zhu LZ, Wu CQ, Yu GS (2013) Characteristics of alkali and alkaline-earth metals for the catalytic gasification of coal char in a fixed-bed reactor. Energy Technol 1(9):544–550
Wang FJ, Zhang S, Chen ZD, Liu C, Wang YG (2014a) Tar reforming using char as catalyst during pyrolysis and gasification of Shengli brown coal. J Anal Appl Pyrolysis 105(5):269–275
Wang SR, Zhang F, Cai QJ, Li XB, Zhu LJ, Wang Q, Luo ZY (2014b) Catalytic steam reforming of bio-oil model compounds for hydrogen production over coal ash supported Ni catalyst. Int J Hydrog Energy 39(5):2018–2025
Wang YG, Zheng PP, Yang SS, Zhang S, Bai YP, Jia XL (2014c) Influence of demineralization using acid wash on N migration and transformation during pyrolysis of Shengli brown coal. J Fuel Chem Technol 42(5):519–526
Wang YG, Zhou JL, Bai L, Chen YJ, Zhang S, Lin XC (2014d) Impacts of inherent o-containing functional groups on the surface properties of Shengli lignite. Energy Fuels 28(2):862–867
Wang YG, Niu ZS, Shen J, Bai L, Niu YX, Wei XY, Li RF, Zhang J, Zhou WY (2017) Extraction of direct coal liquefaction residue using dipropylamine as a CO2-triffered switchable solvent. Fuel Process Technol 159:27–30
Xu J (2012) Experiment study on properties of coal gasification and the migration pattern of nitrogen. Huazhong University of Science and Technology, Wuhan
Xu XQ, Wang YG, Chen ZD, Bai L, Zhang KJ, Yang SS, Zhang S (2015a) Influence of cooling treatments on char microstructure and reactivity of Shengli brown coal. J Fuel Chem Technol 43(1):1–8
Xu XQ, Wang YG, Chen ZD, Chen XJ, Zhang HY, Bai L, Zhang S (2015b) Variation in char structure and reactivity due to the pyrolysis and in situ gasification using Shengli brown coal. J Anal Appl Pyrolysis 115:233–241
Xu MX, Li SY, Wu YH, Jia LF, Lu QG (2016) Effects of CO2 on the fuel nitrogen conversion during coal rapid pyrolysis. Fuel 184:430–439
Zhang YC, Zhang J, Sheng CD, Chen J, Liu YX, Zhao L, Xie F (2011) X-ray photoelectron spectroscopy(XPS) investigation of nitrogen functionalities during coal char combustion in O2/CO2 and O2/Ar atmospheres. Energy Fuels 25(1):240–245
Zhang S, Bai YP, Mi L, Zheng PP, Chen XJ, Xu DP, Wang YG (2013) Effect of heating rate on the migration and transformation of N during pyrolysis of Shengli brown coal. J Fuel Chem Technol 41(10):1153–1159
Zhang L, Li TT, Wang S, Li D, Li CZ (2017) Effects of alkali and alkaline earth metallic species and chemical structure on nascent char-O2 reactivity. Energy Fuels 31(12):13578–13584
Zhao YH (2003) Effect of minerals on transformation of nitrogen during coal pyrolysis/gasification. Taiyuan University of Technology, Taiyuan
Zhao ZB, Li W, Qiu JS, Li BQ (2003) Effect of Na, Ca and Fe on the evolution of nitrogen species during pyrolysis and combustion of model chars. Fuel 82(15):1839–1844
Zhao YH, Chang LP, Xie KC, Li LZ (2004a) Effect of mineral matter on transformation of nitrogen during coal conversion. Mod Chem Ind 24(1):11–15
Zhao YH, Lin JY, Chang LP, Zhao W, Xie KC (2004b) The effect of mineral matter on the NH3 formation during coal pyrolysis and gasification. Environ Chem 23(1):26–30
Zheng PP, Wang YG, Wu X, Liu C, Bai YP, Lin XC (2017) Transformation of nitrogen during pyrolysis of N-loaded Shengli brown coal. J Fuel Chem Technol 45(4):418–426
Zubek K, Czerskai G, Porada S (2018) Comparison of catalysts based on individual alkali and alkaline earth metals with their composites used for steam gasification of coal. Energy Fuels 32(5):5684–5692
Acknowledgements
The authors gratefully acknowledge the financial support provided by the 12th Five-Year Plan of National Science and Technology Support (Grant 2012BAA04B02) and the National Natural Science Foundation of China (No. 21406261).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zheng, P., Wang, Y., Liu, C. et al. Effects of inherent alkali and alkaline earth metals on nitrogen transformation during steam gasification of Shengli lignite. Int J Coal Sci Technol 6, 197–206 (2019). https://doi.org/10.1007/s40789-019-0248-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-019-0248-3