Abstract
Purpose of Review
This concise review delves into the pivotal role of three-dimensional (3D) nanostructured scaffolds in fostering mesenchymal stromal cells (MSC) immunomodulatory capabilities, with a specific focus on orthopedic applications. In this ever-advancing research field, where inflammation and tissue repair are intricately linked, manipulation of the immunomodulatory properties of MSCs becomes crucial, especially for inflammatory-based diseases such as osteoarthritis (OA). The primary inquiries include the promise of nanoscale tools to revolutionize orthopedic regenerative medicine, the role of tailored design features in steering cellular immunomodulatory response, and the resulting beneficial impact on tissue regeneration.
Recent Findings
Recent studies demonstrate the crucial importance of precise control over 3D scaffold design at the nanoscale to maximize the efficacy of regenerative therapies. Compared to 2D, engineered 3D environments with specific chemical composition and finely tuned physical nano-features, heighten MSC secretion of immunosuppressive factors including transforming growth factor-β1 (TGF-β1), prostaglandin E2 (PGE2), indoleamine-pyrrole 2,3-dioxygenase (IDO), and interleukin-10 (IL-10), contributing to improve cartilage and osteo differentiation.
Summary
Nanostructured 3D scaffolds characterized by nano topography, roughness, high porosity, biomimetic stiffness and chemistry, offer a sophisticated means to optimize the immunosuppressive potential of MSCs by allowing the spatiotemporal control over signaling molecules at the nanoscale. Polymeric constructs, notably collagen-based ones, lead to heightened immunomodulatory response and superior cellular differentiation. This effect is because 3D constructs provide a biomimetic environment that enhances cell interaction, controls cell behavior, and modulates the secretion of anti-inflammatory cytokines. The integration of innovative 3D nanostructured approaches into MSC culture systems paves the way for significant strides in cell therapy, addressing current challenges in their clinical application and holding great promise for developing more effective and precise treatments for orthopedic inflammatory disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stromal cells (MSCs) from adult and gestational tissues have been proposed as promising therapeutics due to their safety [1] and plastic ability to create a pro-regenerative environment [2, 3]. The most characterized adult sources of MSCs are the bone marrow (BM) and adipose (fat) tissue (AD) as well as gestational tissues [4]. For orthopedic applications, BM represents the primary source although cartilage progenitor stem cells [5•], synovial fluid-derived stem cells [6], and fat pad-derived MSCs [7] have also been shown therapeutic potential, prompting the question of which source is the most effective. Due to their multipotency and immunomodulatory capabilities, MSCs provide a basis for safe regenerative therapies [8, 9] adapting to the needs of damaged tissues and contributing to the regeneration of various tissue types including bone, cartilage, and adipose [10]. MSC therapeutic potential is determined by their affinity for the tissue they have been isolated from [11,12,13,14], with BM-MSCs mainly eliciting osteogenic [15, 16] and chondrogenic [17, 18] differentiation, and being by nature engaged into bone remodeling and cartilage repair processes [19,20,21].
MSC immunomodulatory capacity is key for regenerative medicine, fostering a microenvironment conducive to optimal tissue repair by suppressing excessive inflammation and promoting successful regeneration [22,23,24]. This is an essential aspect to control especially in the orthopedic context, considering that joint fracture restoration starts with an instant inflammatory reaction [25] and an overly active or prolonged immune response can lead to tissue damage and impaired regeneration [26]. MSCs regulate the proliferation and function of crucial immune cells such as dendritic cells (DC), T-cells, regulatory T-cells (Tregs), natural killer (NK) cells, B-cells, monocytes, and macrophages [27]. These effects are achieved through both direct cell-to-cell contact [28,29,30] and paracrine signaling via the secretion of anti-inflammatory mediators in response to the surrounding environment [31,32,33], including transforming growth factor-β1 (TGF-β1), prostaglandin E2 (PGE2), indoleamine-pyrrole 2,3-dioxygenase (IDO), cyclooxygenase 2 (COX-2), and interleukin-10 (IL-10) [34,35,36]. Preclinical studies demonstrated that their ability to promote an anti-inflammatory environment leads to improved joint tissue regeneration [37,38,39], prompting the commencement of numerous clinical trials showcasing encouraging therapeutic outcomes across a spectrum of medical conditions [40, 41].
Despite the potential benefits, MSC efficacy has proven suboptimal in some cases, necessitating further evaluation [42, 43] to establish standardized protocols, optimize treatment regimens, reduce procedure costs, improve targeted delivery, ensure long-term efficacy and reduce potential safety issues [44]. The latter include uncontrolled cell growth, infection risks as a consequence of microbial contamination during manipulation phases, genomic instability and off-target effects [45]. Innovative technologies are essential to overcome these challenges and unlock MSC’s full potential in cell therapy.
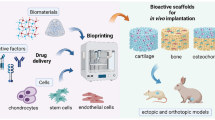
The use of engineered nanostructured 3D scaffolds stands as the groundbreaking stride due to their ability to exert precise control over the cellular microenvironment and ensure a balanced and favorable milieu for tissue repair [46, 47]. In essence, a scaffold is an artificial three-dimensional (3D) engineered nanostructure serving as architectural support that facilitates cell attachment and guides the development of functional and integrated tissues. 3D scaffolds are designed to mimic the native extracellular matrix (ECM), the milieu in which cells reside within tissues, with the purpose of offering a more physiologically relevant microenvironment for effective and clinically viable MSC-based therapies compared to traditional 2D cultures. As a matter of fact, in 2D cell cultures, lack the intricate spatial organization and cell-cell interactions that occur in vivo. In addition, thanks to their ability to enhance targeted drug delivery and release as well as modulate cellular response, 3D nanostructured scaffolds create a conducive microenvironment that promotes cell survival and therapeutic efficacy, making the combination more effective than standalone cell injections [49]. By tuning the architecture and the chemistry, this construct provides mechanical support and biochemical cues valuable for boosting cell attachment, proliferation, and differentiation [48]. Various engineered techniques and biomaterials are employed to produce scaffolds with specific nanoscale characteristics tailored to the desired tissue or application. The most widely employed methods for fabricating 3D nanostructured scaffolds include solvent casting combined with particulate leaching, freeze-drying, electrospinning, additive manufacturing methods such as stereolithography, fused deposition modeling, and 3D printing [50]. As for materials, a wide array is used ranging from natural polymers like collagen, fibrin, and chitosan, to synthetic polymers such as poly(lactic-co-glycolic acid) (PLGA), polyethylene glycol (PEG), and polycaprolactone (PCL) [51]. Each fabrication method and biomaterial offer unique advantages in terms of biocompatibility, degradation rate, porosity, topographies, and mechanical strength, allowing researchers to tailor scaffolds to specific applications and tissues. Therefore, scaffold design notably plays a key role in orchestrating immunomodulatory responses, especially observed in vitro [52]. While scaffold architecture and mechanical properties contribute to structural support and physical characteristics, the chemical composition actively and precisely tailors immunomodulatory upshot as influences cellular signaling pathways at the molecular and cellular levels [53, 54]. As shown by us and others [49, 55], the use of different nanotechnologies can further enhance the immunomodulation capability, opening the venue to the development of new MSC-based therapeutics for inflammatory-based conditions [56].
This concise review presents a brief overview of the current state-of-the-art methodologies in MSCs cell therapy for orthopedic inflamed-based disorders with emphasis on disorders characterized by inflammatory conditions such as osteoarthritis (OA). We will delve into the therapeutic enhancement of MSC immunosuppressive capacity through the utilization of 3D nanostructured scaffolds. The discussion will focus on the impact of scaffold features to achieve a therapeutic effect, with a primary focus on chemical composition.
MSCs for Musculoskeletal Disorders
Musculoskeletal disorders (MDs) encompass a broad range of conditions that affect the muscles, bones, joints, ligaments, tendons, and other compartments of the musculoskeletal system [57, 58]. Several MDs have an inflammatory component, either as a primary feature (traumas) or as a secondary response to injury or degeneration. Inflammation can hence be both, the cause and the consequence of various orthopedic conditions [59]. OA is the most prevalent MD, characterized by the degeneration of joint cartilage and the underlying bone [60]. Emerging research has highlighted the dysregulation of inflammatory processes in its pathogenesis [61] showing a high concentration of inflammatory cytokines within the joint (interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor- α (TNF-α)) [62, 63]. These mediators contribute to the breakdown of cartilage ECM and stimulate the production of matrix metalloproteinases, enzymes responsible for cartilage degradation [64, 65].
Numerous clinical trials explore MSCs in the management of OA [66,67,68,69,70,71,72], harnessing their regenerative and anti-inflammatory potential to mitigate symptoms, retard disease progression, and promote tissue repair [73, 74]. Several approaches are utilized for supplying cells to treat OA [75]. The most popular cell therapy procedure involves direct intra-articular administration of MSC suspension, cultured and expanded in the laboratory, into the affected joint. This minimally invasive approach aims to modulate the local inflammatory environment, promote tissue repair, and alleviate symptoms (i.e., pain and stiffness) [76,77,78]. Chahal and coworkers demonstrated both safety and efficacy of autologous BM-MSCs injected at different dosages (in the range of 1–50 × 10^6 cells) to reduce inflammation in patients with knee OA [79]. The higher injected dose determined higher expression levels of anti-inflammatory molecules and led to a notable reduction in the levels of cartilage catabolic biomarkers and inflamed synovitis. Nevertheless, as a solitary injection may not provide adequate treatment, the need for repetitive intra-articular injections at short intervals [80] or in combination with other surgical procedures arises.
MSCs are also used as adjuvants to surgical interventions for OA, such as arthroscopy or joint debridement. This strategy enhances the regenerative outcome combining surgical procedures with the immunosuppressive and regenerative properties of MSCs. Safety and efficiency of this treatment were confirmed by combining autologous AD-MSCs with arthroscopic abrasion arthroplasty for 27 patients with broad advanced OA [81]. Consistent and progressive improvement in cartilage quality and regrowth was observed via radiological monitoring over 36 months, confirming OA stabilization. Moreover, quantitative MRI analysis shows hyaline-like regenerative cartilage in all participants, indicating native-reproducible regeneration. An alternative method to directly use cells is known as microfracture. It consists of surgical damage of the subchondral layer to recruit endogenous MSCs from the BM to the injury site. This in situ recruitment enhances the natural regenerative capacity of the joint and promotes tissue healing [82,83,84] by stimulating the migration and activity of MSCs already present in the joint. Despite the impressive results achieved so far in vivo [85, 86], further attention is needed in this surgical field to investigate the effects of MSCs on inflammation since no clinical trial has yet reported this aspect.
Although MSCs’ adaptability enables adjustments, paving the way for personalized therapeutic applications, advancements in cell engineering are required as the translation of this technology is constrained by concerns [45]. One innovative and increasingly popular application of MSCs for inflamed-based orthopedic disorders involves combining them with biomaterials or scaffolds to create tissue-engineered constructs. These constructs can be directly implanted into the damaged joint to provide structural support and promote the formation of new functional tissues by the recruitment of surrounding MSCs, facilitating in turn tissue repair and regeneration.
Tuning MSC Properties with Nanostructured Materials
Cell therapy is effective in orthopedics [87] however, some orthopedic approaches require the use of biomaterials to provide structural support for endogenous cell growth and tissue regeneration. In this context, biomimicry offers a ground-breaking solution for the success of regenerative biomaterials [88,89,90,91]. By providing a physiologically relevant microenvironment, they contribute to the developing of effective and clinically viable MSC-based therapies compared to traditional 2D cultures known to lack the intricate spatial organization and cell-cell interactions that occur in vivo. Considering the crucial role of cell-cell interactions for the immunosuppressive effects of MSCs, the 3D nature of the scaffold also enables a more nuanced interplay and cross-talking between MSCs and immune cells, resulting in a more effective immunomodulatory reaction [92]. Hence, the potential benefits of utilizing 3D culture conditions to enhance MSC immunomodulation have been broadly reported in the literature [93,94,95]. Given the nanoscale hierarchical organization of the ECM, a transformative approach entails manipulating 3D biomimetic scaffolds at the nanoscale level. Nanostructured biomaterials, with their elevated surface area-to-volume ratio, facilitate stronger MSC-scaffold interactions and control cell behavior, including adhesion, migration, and signaling, fostering specific lineage commitment, and modulating the secretion of anti-inflammatory cytokines [96,97,98]. In this context, the latest frontier for clinical applications lies in the potential of acellularized 3D nanomaterials to emulate natural designs and processes and attract bioactive cells from other compartments. This is particularly important for the successful translation of MSC-based therapies to clinical settings, where scalability is a crucial consideration.
Design of Successful 3D Nanoscale Scaffolds
Scaffolds suitable for biomedical applications must be sterilizable, non-toxic and biocompatible without eliciting a clinically detectable foreign body reaction upon implantation or tampering with the biological function of the local microenvironment. Specific nano-design rules, including scaffold physical features, such as topography and mechanical properties alongside chemical composition (Fig. 1) may affect MSC plastic properties, especially their immunomodulation potential [99]. Moreover, through precise engineering of the biochemical stimuli within the scaffolds, it is possible to stimulate the cell paracrine secretion profile and, in turn, steer MSC immunosuppressive functions [87].
Cell-cell and cell-substrate connections allow cells to sense the physical properties of the underlying substrates and adjust their cytoskeletal organization, morphology, and functions accordingly. This mechanism, named mechanotransduction, is ruled by architectonic factors such as scaffold micro- and nano-topography, roughness, pore dimension and morphology, and stiffness [100,101,102].
Advances in scaffold production techniques for tissue engineering purposes have provided the possibility to create 3D scaffolds with defined micro- and nano-scale architectures that closely mimic the cell niche and thus improve tissue healing [52, 103]. This offers a spatial arrangement able to facilitate more natural cell-cell and cell-ECM interactions and regulates the signaling pathways involved in immunomodulation [104]. Moreover, nano-topologies affect cell growth, and MSC specification towards the osteogenic and chondrogenic lineage [105,106,107,108], and modulate their immunomodulatory phenotype by favoring the secretion of anti-inflammatory factors and other immunoregulatory molecules [109]. It was found that cells can respond to topographical cues down to 5 nm [110], suggesting it is essential to generate surface patterns at the nanoscale resolution. Based on the fabrication method, various nano-topographical surface templates can be obtained in a controllable and reproducible fashion to offer contact guidance to cells [111]. The topography of fibrous polymeric electrospun scaffolds creates a unique microenvironment to modulate the paracrine immunoregulatory function of AD-MSCs. Specifically, the mesh-like pattern showed the best immunoregulatory potential of rat AD-MSCs compared to the random and aligned ones, enhancing the secretion of PGE2, iNOS, and HGF anti-inflammatory cytokines, due to the activation of the NF-kB pathway by structural cues [112]. The conditioned medium from mesh-like topography promotes M2 phenotype in macrophages both in vitro and in vivo, indicating an anti-inflammatory effect. Olivares-Navarrete et al. also showed surface roughness impacts the immune response [113]. When a metallic spine implant material with micro- and nano-roughened surfaces was tested in vitro with human MSCs, the authors found that rough surfaces reduce the production of pro-inflammatory cytokines (IL-6 and IL-8) compared to flat or nanogroove surfaces. This led to an uninflamed environment that enhanced osteogenesis, as evidenced by high levels of alkaline phosphatase and osteocalcin. These results emphasize the influence of surface texture modulates the immune reaction towards a diminished inflammatory state, thereby safeguarding successful osseointegration.
Another crucial architectural feature to be fine-designed at the nanoscale to create a conducive 3D growth environment is porosity. Commonly, scaffolds with higher porosity, typically exceeding 70%, facilitate cell penetration, nutrient transport, and waste removal, thereby promoting tissue regeneration [114, 115]. Additionally, the pore size is critical for regenerating functional musculoskeletal tissues: when pores are too small (typically below 50 μm), cell penetration, migration, and metabolism are hindering, leading to limited tissue ingrowth, especially in dense or avascular environments such as cartilage [116]. Conversely, if the pores are too large (usually above 500 μm), the available surface area for cell attachment is reduced, compromising the mechanical strength of the scaffold due to increased void volume [117]. According to several studies, the ideal scaffold pore size falls between 100 and 350 μm [118,119,120,121,122,123]. A 3D highly porous polystyrene scaffold (90% porosity) strongly improves MSC immunoregulatory ability [124]. Cells in 3D cultures exhibit changes in their secretory profile and higher levels of the anti-inflammatory cytokine IL-4 were measured via ELISA assay when human umbilical cord-derived MSCs were seeded on nanostructured substrate compared to 2D culture. This creates a microenvironment conducive to tissue repair and modulates immune responses. Indeed, the in vivo, preclinical results showed that the percentage of the Treg population around the implant significantly increased after 4 weeks in a relevant mouse model. The influence of different type I-collagen scaffold microstructures on the immunomodulatory properties of allogeneic MSCs was also illustrated by Yuan et al., showing suppression in pro-inflammatory molecules (MHC-I and MHC-II) expression and ability to invoke a lower lymphocyte proliferation on 3D porous constructs compared 2D, even under simulated-inflammatory condition [125]. Hydrogels with smaller pores and highly biomimetic microstructure also improve MSC immunoregulatory properties compared to scaffolds and membranes by providing more anchor points and, in turn, mechano-sensing inputs.
Scaffold stiffness strongly affects MSC functions. Due to their ECM hierarchical structures, cartilage and bone present a unique combination of strength and toughness and are considered the stiffest tissues of the human body with Young’s modulus of 0.1-3 MPa and 10-30GPa, respectively [126, 127]. One of the widespread strategies to finely adjust the substrate stiffness involves changing the crosslinking density by physical or chemical methods [128]. Scaffold chemistry, topography, porosity, interconnectivity, pore size distribution, and morphology of the pores, deeply affect the mechanical properties of the scaffold as well [129]. While the biomimetic approach is preferred in orthopedic tissue engineering suggesting matching scaffold features to those of the tissue, divergent perspectives exist regarding the correlation between stiffness and augmented MSC immunomodulatory functionality. Increasing alginate scaffold stiffness from 3 to 18 kPa by using a larger amount of the crosslinker peptide stimulated IDO-1 and COX-2 expression in murine MSCs [130]. Conversely, substrate within the range of 0.3-2 kPa maximizes TNF-α-triggered MSC’s ability to generate paracrine factors compared to 100 kPa ECM [131]. Also, Zhang et al. demonstrated that soft methacrylate gelatin (2 kPa) favors macrophages toward anti-inflammatory phenotypes with a decreased capacity for spread and substantially enhanced the production of immunomodulatory factors, compared to stiff (29 kPa) and medium (10 kPa) scaffolds [132]. Importantly, they revealed that stiffness-mediated immunoregulatory impact on MSCs primarily stems from tumor necrosis factor-α-stimulated protein 6 (TSG-6) and exerts its influence on macrophages through the CD44 receptor, subsequently inhibiting the NF-κB pathway. This discovery is promising for enhancing MSC-based therapies within the realm of orthopedic regenerative medicine. Due to the contradictory outcomes, additional investigations are necessary to propose specific strength ranges that effectively enhance immunomodulation.
In addition to these physical features, the scaffold’s chemical composition dictates biocompatibility and interactions with cells by promoting adhesion, directing differentiation, and influencing paracrine signaling [133]. The selection of materials with bioactive properties influencing MSC behavior is crucial. Importantly, the nanoscale support can be exploited for targeted delivery and bolster the bioavailability of regulatory factors for intracellular signaling pathways and facilitating their integration into MSC-mediated pathways [134]. This spatiotemporal control over signaling molecules at the nanoscale provides a sophisticated means to optimize the therapeutic potential of MSCs in regenerative medicine. A deeper discussion about the impacts of scaffold chemical composition on MSC behavior is discussed in the following chapter.
Accurate tuning of design parameters is crucial to formulate a 3D scaffold characterized by well-suited mechanical and biological properties able to influence cellular fate in a targeted manner, paving the way for more effective and precisely tailored therapies for manifold medical conditions. By embracing the inherent abilities of MSCs with the unique properties of nanomaterials, the biomimetic paradigm, through meticulous research and innovation in scaffold architecture and composition, can elevate the precision and efficacy of MSC-mediated immunomodulation, bringing regenerative medicine one step closer to unlocking even greater potential.
3D Scaffold Composition: An Intricate Modulator of Cellular Responses
In the engineering design, the selection of the specific biomaterial for enhancing MSCs potentials is governed by matching the material properties with the clinical requirements. 3D scaffolds with various compositions can alter MSC immunophenotypic patterns more effectively than 2D cultures [135]. MSCs from human umbilical cord tissue tested on collagen, chitosan or PLGA scaffolds showed higher levels of pluripotent markers (Oct4, Nanog and Sox2). Substrates, especially collagen-based, up-regulate the expression of immunomodulatory genes (i.e., Il-1a, Il-1b, Il-1rn, Il-6st, hgf, and egf) and proteins [135]. In Arabiyat et al. work, the effects of 3D substrate chemistries were robustly scrutinized from a molecular point of view. They evaluated the impact of chemical functional groups on BM-MSC osteogenic and immunomodulatory behavior [136]. Poly(sophorolipids) (pLSL)-based 3D porous scaffolds obtained by salt-leaching method were functionalized with phosphate (PO4), amine (NH2), or carboxyl (COOH) groups. PO4-functionalization supported the superior degree of MSC osteogenesis, showing high secreted levels of osteoprotegerin, osteoactivin, and Bone Morphogenic Protein-2. Furthermore, due to the increased negative charge, PO4-ending substrate demonstrated the highest levels of immunomodulatory factor, overexpressing IDO and especially COX-2. Ammine-modified scaffolds were able to tune the immune response as well, as monocyte chemoattractant protein-1, IL-6 and PTGES-2 were overexpressed following 72 h of culture.
Most recent strategies consider alternative nanostructured types of biomaterials for tissue regeneration: ceramics and bioglasses [137,138,139], biodegradable metals [140, 141], decellularized tissues [142, 143], polymers, or combinations of them. Polymeric biomaterials are the most commonly employed to support MSCs due to their high biocompatibility and biodegradability [144]. For their versatile structure, they display a flexible design and chemical composition that can be fine-tailored during synthesis [145,146,147]. These features confer a wide range of properties that can fit custom requirements. Regarding their morphology and structure, different types of polymeric-based scaffolds can be distinguished. Polymers are often divided into two classes: synthetic and naturally derived.
Synthetic polymers are human-made materials with little to no interaction with the tissue produced from non-renewable resources, monomers derived from various feedstocks, or through industrial processes, with recent advancements focusing on biodegradable options from renewable resources. Polymeric scaffolds are particularly advantageous for applications that require well-defined mechanical features over time since their formulation and biological properties can be tailored ad-hoc [148]. A consequent benefit originating from their chemistry includes the possibility of obtaining highly controlled and consistent degradation properties [149], an essential aspect of achieving durability. Several synthetic polymers have been used for scaffolding, including poly(a-hydroxy acid) (PAHA), poly(orthoester) (POE), poly(anhydride) (PAH), poly(amino acid) (PAA), dextrin, poly(lactic acid) (PLA), poly(glycolic acid) (PGA) and their copolymers [149]. One of the most frequently used and promising synthetic polymers for orthopedic applications is PLGA, due to its proven in vivo biocompatibility and minimal systemic toxicity [150]. PLGA devices are biodegradable and approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) [151]. The potential of PLGA-based nanostructured scaffolds for strengthening MSC immunomodulatory potential was documented by Deng et al. who used a 3D printed technique for the generation of a porous construct and were able to initiate bone tissue regeneration through the accumulation of M2-macrophages [152]. The ability to regulate the expression of the anti-inflammatory cytokines IL-10 was ascertained after 1 day of incubation in mice. Furthermore, their polymeric scaffold promoted macrophages’ M1-to-M2 switch in animal models.
In contrast to synthetic biomaterials, natural polymers are derived from natural sources. They exhibit minimal toxicity as they often closely resemble the tissue they interact with [153]. The natural origin of these materials allows to design and engineer biomaterial systems that function at the molecular level. Controlling their physical properties, such as degradation rate and mechanical features, poses a challenge to their optimal usage. Examples of these proteins are collagen and gelatin; carbohydrates like cellulose, silk, keratin, fibrinogen, elastin, hyaluronic acid, starch, and chitosan: agarose and alginate, which are derived from seaweed [154, 155].
Collagen is among the most extensively researched natural polymers for biomedical applications. As one of the main structural proteins in several animal ECM, it offers several favorable binding sites for MSC adhesion, survival and proliferation and it provides the necessary mechanical strength and structural integrity to the tissues [156]. Recent studies have pointed out that biomimetic scaffolds made of collagen act as instructive microenvironment for MSC immunomodulatory function [157,158,159]. However, the specific mechanisms involved in this process are still ambiguous and might include the activation of specific intracellular signaling pathways liable for the expression of various anti-inflammatory genes, the release of cytokines and growth factors, and the crosstalk between MSCs and macrophages [160,161,162]. A revealing study by our group reports the development of a collagen-based scaffold to create a pro-regenerative environment at the molecular, cellular, and tissue levels by modulating the immune response at the site of the implant [163]. In this work, the micro-porous collagenous 3D substrate was obtained by freeze-drying technique and functionalized with an anti-inflammatory macromolecule, chondroitin sulfate, through carbodiimide chemistry. This biomaterial enhanced the MSC immunosuppressive potential (overexpression of Pges, iNOS, Cox-2 and Tgf-β) after 48 h of culture [164]. A thorough gene analysis revealed the up-regulation of genes associated with the early recruitment and retention of anti-inflammatory macrophages, which in turn led to the settling of the acute inflammatory phase following surgical implantation. The same scaffold was used by Bauza et al. to improve the immunosuppressive potential of human BM-MSCs for cartilage repair strategies in OA patients [5•]. Inflamed (IFN-γ and TNF-α) MSCs seeded on the micro-structured scaffold showed an increased release of immunosuppressive molecules which directly target cells of the innate and adaptive immune system, compared to 2D culture already after 24 h, leading to an incredible anti-inflammatory effect. Furthermore, a time-dependent increase of CD73 marker was found, suggesting that the selected nanoscale biomimetic tool can lead to the recruitment of anti-inflammatory macrophages and an immunological switch toward an anti-inflammatory state. In vivo experiments carried out in New Zealand rabbits confirmed the ability of this functionalized collagen microstructure to tune the post-traumatic inflammatory response resulting in significant improvements in cartilaginous tissue formation and suppression of tissue degeneration [165••].
Beyond that, a resourceful strategy for augmenting the potential of polymeric scaffolds relapses in the dispersion of biochemical cues such as anti-inflammatory biomolecules, growth factors, or inorganic bioactive phase within the biomaterials. This targeted approach leverages the chemical composition to fine-tune MSC behavior and function by providing sustained and localized delivery of the signals, thereby enhancing their efficacy in treating inflammatory and immune-related disorders. For example, the gradual release of TGF-β1 from a 3D printed scaffold not only promotes tissue-specific differentiation [166] but also introduces biodegradable Mg-doped hydroxyapatite that highly contributes to the bone regenerative process [167].
Future Directions in 3D Nanostructured Scaffolds Design
The use of 3D nanostructured scaffolds in orthopedic applications, emphasizing MSC immunomodulation, presents an exciting frontier underscored by precise inquiries. Table 1 summarizes the influence of the discussed scaffold design features on the immunomodulatory ability of MSCs.
Despite notable strides, several questions persist shaping the trajectory of ongoing research in this domain. Obtaining the right balance between porosity, mechanical properties, and bioactivity remains actively under investigation along with understanding the dynamic relationship between MSCs and scaffolds, crucial for optimizing cell behavior and function within the 3D environment. Persistent inflammatory microenvironments in orthopedic conditions further complicate scaffold design. Combination therapies to explore synergistic effects are anticipated to raise performances and researchers grapple with intricately incorporating growth factors and other therapeutic modalities into 3D scaffolds. Achieving targeted immunomodulation is complex, demanding fine-tuning of design and the release of factors to avoid under- or over-stimulation.
In parallel, addressing the heterogeneity of patient responses and achieving long-term stability amidst the dynamic and load-bearing nature of the orthopedic tissue are essential steps in pushing the boundaries of orthopedic regenerative medicine. In the coming years, precision engineering and medicine approaches may take center stage to optimize the therapeutic efficacy of MSC-based immunomodulation, with 3D scaffolds designed based on individual patient characteristics, including genetic profiles and immune responses. Upcoming designs may integrate multifunctional aspects as well as smart materials and nanotechnology into scaffolds, providing a dynamic platform for real-time monitoring and responsiveness to adjustments to the local environment during tissue repair. This could include sensors for detecting inflammatory signals and stimuli-responsive biomaterials [168] releasing anti-inflammatory factors, enhancing the precision and efficacy of immunomodulation. Furthermore, moving from preclinical success to clinical translation faces also regulatory hurdles, manufacturing scalability and affordability challenges.
The future of 3D nanostructured scaffolds hinges on deciphering these unanswered questions nowadays, ushering in a new era of targeted and personalized regenerative therapies.
Conclusion
3D scaffolds with nanoscale features play a pivotal role in enhancing the differentiation and immunomodulatory inherent capabilities of MSCs. The transition from 2D to 3D cultures provides a more physiologically relevant microenvironment, promoting intricate cell interactions, tissue-specific differentiation, and a robust immunomodulatory response. As the field of regenerative medicine continues to advance, the integration of nanoscale strategies into MSC culture systems stands as a key strategy for unlocking the full therapeutic potential of these remarkable cells and holds great promise for developing more effective and precise treatments for inflammatory disorders and other conditions where immunomodulation is key to successful therapeutic outcomes.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hoang DM, et al. Stem cell-based therapy for human diseases. 2022;7(1):272.
Blanpain C, Fuchs E. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344(6189):1242281.
Galderisi U, et al. Clinical trials based on mesenchymal stromal cells are exponentially increasing: where are we in recent years? Stem Cell Rev Rep. 2022;18(1):23–36.
Costela-Ruiz VJ, et al. Different Sources of mesenchymal stem cells for tissue regeneration: a guide to identifying the most favorable one in orthopedics and dentistry applications. Int J Mol Sci. 2022;23(11):6356. https://doi.org/10.3390/ijms23116356.
• Bauza G, et al. Improving the immunosuppressive potential of articular chondroprogenitors in a three-dimensional culture setting. Sci Rep. 2020;10(1):16610. This study offers a promising approach for cartilage repair strategies suggesting that BM-MSC enhanced immunomodulatory capability when cultured in a 3D biomimetic porous collageneous scaffolds compared to 2D, providing evidence for their potential in treating focal chondral defects in OA by enhancing immune tuning and regenerative outcomes.
Paradiso F, et al. Immunosuppressive potential evaluation of synovial fluid mesenchymal stem cells grown on 3D scaffolds as an alternative source of MSCs for osteoarthritis cartilage studies. Front Biomater Sci, 2022. 1. https://doi.org/10.3389/fbiom.2022.989708
Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902–7.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
Levy O, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):eaba6884.
Horwitz EM, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–5.
Qin Y, Guan J, Zhang C. Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad Med J. 2014;90(1069):643–7.
Oryan A, et al. Role of mesenchymal stem cells in bone regenerative medicine: What is the evidence? Cells Tissues Organs. 2017;204(2):59–83.
Fernandez-Moure JS, et al. Enhanced osteogenic potential of mesenchymal stem cells from cortical bone: a comparative analysis. Stem Cell Res Ther. 2015;6(1):203.
Corradetti B, et al. Osteoprogenitor cells from bone marrow and cortical bone: understanding how the environment affects their fate. Stem Cells Dev. 2015;24(9):1112–23.
Grotheer V, et al. Osteogenic differentiation of human mesenchymal stromal cells and fibroblasts differs depending on tissue origin and replicative senescence. Scientific Reports. 2021;11(1):11968.
Chen Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death & Differentiation. 2016;23(7):1128–39.
Tan AR, Hung CT. Concise review: mesenchymal stem cells for functional cartilage tissue engineering: taking cues from chondrocyte-based constructs. Stem Cells Transl Med. 2017;6(4):1295–303.
Somoza RA, et al. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20(6):596–608.
Gugjoo MB, et al. Cartilage tissue engineering: Role of mesenchymal stem cells along with growth factors & scaffolds. Indian J Med Res. 2016;144(3):339–47.
Marzona L, Pavolini B. Play and players in bone fracture healing match. Clin Cases Miner Bone Metab. 2009;6(2):159–62.
Zha K, et al. Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application npj. Regen Med. 2021;6(1):14.
Wang Z, et al. Global scientific trends on the immunomodulation of mesenchymal stem cells in the 21st century: A bibliometric and visualized analysis. 2022;13:984984. https://doi.org/10.3389/fimmu.2022.984984.
Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41(9):653–64.
Ayala-Cuellar AP, et al. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol Ther (Seoul). 2019;27(1):25–33.
Cooke JP. Inflammation and its role in regeneration and repair. Circ Res. 2019;124(8):1166–8.
Zhang B, et al. Toward a better regeneration through implant-mediated immunomodulation: Harnessing the immune responses. Adv Sci (Weinh). 2021;8(16):e2100446.
Joel MDM, et al. MSC: immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am J Transl Res. 2019;11(6):3890–904.
Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43.
Rasmusson I, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208–13.
Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72.
Han KH, et al. Immunosuppressive mechanisms of embryonic stem cells and mesenchymal stem cells in alloimmune response. Transpl Immunol. 2011;25(1):7–15.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–96.
Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402.
Zhao Q, Ren H, Han Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J Cell Immunotherapy. 2016;2(1):3–20.
Fu QL, et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy. 2012;67(10):1215–22.
Yagi H, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667–79.
Kwon DG, et al. State of the art: the immunomodulatory role of mscs for osteoarthritis. Int J Mol Sci. 2022;23(3):1618. https://doi.org/10.3390/ijms23031618.
Mehler VJ, Burns C, Moore ML. Concise review: exploring immunomodulatory features of mesenchymal stromal cells in humanized mouse models. Stem Cells. 2018;37(3):298–305.
Dabrowska S, et al. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front Immunol. 2020;11:591065.
Noronha NC, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10(1):131.
Kabat M, et al. Trends in mesenchymal stem cell clinical trials 2004–2018: Is efficacy optimal in a narrow dose range? 2020;9(1):17–27. https://doi.org/10.1002/sctm.19-0202.
Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. 2019;37(7):855–64.
Rendra E, Scaccia E, Bieback K. Recent advances in understanding mesenchymal stromal cells. F1000Res. 2020;9:156. https://doi.org/10.12688/f1000research.21862.1.
Barry F. MSC therapy for osteoarthritis: an unfinished story. J Orthop Res. 2019;37(6):1229–35.
Ocansey DKW, et al. Improved therapeutics of modified mesenchymal stem cells: an update. 2020;18(1):1–14. https://doi.org/10.1186/s12967-020-02234-x.
Efraim Y, et al. 3D structure and processing methods direct the biological attributes of ecm-based cardiac scaffolds. Sci Rep. 2019;9(1):5578.
Unnikrishnan K, Thomas Lv, Ram Kumar RM. Advancement of scaffold-based 3D cellular models in cancer tissue engineering: an update. Front Oncol. 2021;11:733652.
Sharma R, et al. An Insight of Nanomaterials in Tissue Engineering from Fabrication to Applications. Tissue Eng Regen Med. 2022;19(5):927–60.
Levy O, et al. Micro/Nano-Engineering of Cells for Delivery of Therapeutics, in Micro-and Nanoengineering of the Cell Surface. Elsevier; 2014. p. 253–79.
Bhushan S, et al. Scaffold fabrication techniques of biomaterials for bone tissue engineering: a critical review. Bioengineering (Basel). 2022;9(12):728. https://doi.org/10.3390/bioengineering9120728.
Polo-Corrales L, Latorre-Esteves M, Ramirez-Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14(1):15–56.
Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: A review. Bioact Mater. 2019;4:271–92.
Castilla-Casadiego DA, et al. Effects of physical, chemical, and biological stimulus on h-msc expansion and their functional characteristics. Ann Biomed Eng. 2020;48(2):519–35.
Cheng J, et al. Improving the immunomodulatory function of mesenchymal stem cells by defined chemical approach. Front Immunol. 2022;13:1005426.
Levy O, et al. A cell-based drug delivery platform for treating central nervous system inflammation. 2021;99:663–71. https://doi.org/10.1007/s00109-020-02003-9.
Corradetti B, et al. Hyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammation. 2017;7(1):7991. https://doi.org/10.1038/s41598-017-08687-3.
Iolascon G, Gimenez S. Mogyorosi DJJopr. A review of aceclofenac: analgesic and anti-inflammatory effects on musculoskeletal disorders. 2021;14:3651–63. https://doi.org/10.2147/JPR.S326101.
Loi F, et al. Inflammation, fracture and bone repair. 2016;86:119–130. https://doi.org/10.1016/j.bone.2016.02.020.
Szekanecz Z, et al. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. 2021;17(10):585–95. https://doi.org/10.1038/s41584-021-00652-9.
Collaborators GBDO. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508–22.
Woodell-May JE, Sommerfeld SD. Role of inflammation and the immune system in the progression of osteoarthritis. J Orthop Res. 2020;38(2):253–7.
Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–8.
Zhao X, et al. Immunomodulation of MSCs and MSC-Derived Extracellular Vesicles in Osteoarthritis. Front Bioeng Biotechnol. 2020;8:575057.
Kwon DG, et al. State of the art: the immunomodulatory role of MSCs for osteoarthritis. Int J Mol Sci. 2022;23(3):1618.
Pers YM, et al. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027–35.
Lamo-Espinosa JM, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14(1):246.
Gupta PK, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18(1):301.
Orozco L, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results. Transplantation. 2014;97(11):e66-8.
Emadedin M, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422–8.
Centeno CJ, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–53.
Soler R, et al. Final results of a phase I-II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23(4):647–54.
Davatchi F, et al. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19(3):219–25.
Malekpour K, et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. 2022;18(3):933–51.
Wi H, et al. Immunosuppression-enhancing effect of the administration of allogeneic canine adipose-derived mesenchymal stem cells (cA-MSCs) compared with autologous cA-MSCs in vitro. J Vet Sci. 2021;22(5):e63. https://doi.org/10.4142/jvs.2021.22.e63.
Dyrna F, et al. Stem cell procedures in arthroscopic surgery. Eur J Med Res. 2016;21(1):29.
Wei P, Bao R. Intra-articular mesenchymal stem cell injection for knee osteoarthritis: mechanisms and clinical evidence. Int J Mol Sci. 2022;24(1):59. https://doi.org/10.3390/ijms24010059.
Jo CH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45(12):2774–83.
Vega A, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
Chahal J, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med. 2019;8(8):746–57.
Matas J, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2018;8(3):215–24.
Freitag J, et al. Mesenchymal stem cell therapy combined with arthroscopic abrasion arthroplasty regenerates cartilage in patients with severe knee osteoarthritis: a case series. Regen Med. 2020;15(8):1957–77.
Fernandes TL, et al. Editorial: tissue engineering and cell therapy for cartilage restoration. Front Cell Dev Biol. 2022;10:947588.
Bark S, et al. Enhanced microfracture techniques in cartilage knee surgery: Fact or fiction? World J Orthop. 2014;5(4):444–9.
Peñalver JM, et al. All-arthroscopic nanofractured autologous matrix-induced chondrogenesis (A-NAMIC) technique for the treatment of focal chondral lesions of the knee. Arthrosc Tech. 2020;9(6):e755–9.
Strauss EJ, et al. Augmentation strategies following the microfracture technique for repair of focal chondral defects. Cartilage. 2010;1(2):145–52.
Mustapich T, et al. A novel strategy to enhance microfracture treatment with stromal cell-derived factor-1 in a rat model. Front Cell Dev Biol. 2020;8:595932.
Taraballi F, et al. Immunomodulatory potential of mesenchymal stem cell role in diseases and therapies: a bioengineering prospective. 2019;4:100017. https://doi.org/10.1016/j.regen.2019.100017.
Stafin K, Śliwa P, Piątkowski M. Towards polycaprolactone-based scaffolds for alveolar bone tissue engineering: a biomimetic approach in a 3D printing technique. Int J Mol Sci. 2023;24(22):16180. https://doi.org/10.3390/ijms24221618.
Gu Y, et al. 3D-printed biomimetic scaffolds with precisely controlled and tunable structures guide cell migration and promote regeneration of osteochondral defect. Biofabrication. 2023;16(1). https://doi.org/10.1088/1758-5090/ad0071.
Chung JJ, et al. Toward biomimetic scaffolds for tissue engineering: 3d printing techniques in regenerative medicine. Front Bioeng Biotechnol. 2020;8:586406.
Unnithan AR, et al. Strategic design and fabrication of biomimetic 3D scaffolds: unique architectures of extracellular matrices for enhanced adipogenesis and soft tissue reconstruction. Sci Rep. 2018;8(1):5696.
Saleh LS, Bryant SJ. The host response in tissue engineering: crosstalk between immune cells and cell-laden scaffolds. Curr Opin Biomed Eng. 2018;6:58–65.
Sarugaser R, et al. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4(8):e6498.
Tsimbouri P, et al. Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J Cell Biochem. 2014;115(2):380–90.
Harvey A, et al. Proteomic analysis of the extracellular matrix produced by mesenchymal stromal cells: implications for cell therapy mechanism. PLoS One. 2013;8(11):e79283.
Paschos NK, et al. Advances in tissue engineering through stem cell-based co-culture. J Tissue Eng Regen Med. 2015;9(5):488–503.
Kyurkchiev D, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6(5):552–70.
Taraballi F, et al. Biomimetic collagenous scaffold to tune inflammation by targeting macrophages. J Tissue Eng. 2016;7:2041731415624667.
Lee E, et al. Bone marrow-derived mesenchymal stem cell implants for the treatment of focal chondral defects of the knee in animal models: a systematic review and meta-analysis. Int J Mole Sci. 2023;24(4):3227.
Wang S, et al. The effect of physical cues of biomaterial scaffolds on stem cell behavior. Adv Healthc Mater. 2021;10(3):e2001244.
Teo BK, et al. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano. 2013;7(6):4785–98.
Akhmanova M, et al. Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int. 2015;2015:167025.
Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168.
Declercq HA, et al. The role of scaffold architecture and composition on the bone formation by adipose-derived stem cells. Tissue Eng Part A. 2014;20(1–2):434–44.
Salmasi S, et al. Role of nanotopography in the development of tissue engineered 3D organs and tissues using mesenchymal stem cells. World J Stem Cells. 2015;7(2):266–80.
Barlian A, Vanya K. Nanotopography in directing osteogenic differentiation of mesenchymal stem cells: potency and future perspective. Future Sci OA. 2022;8(1):Fso765.
Pedrosa CR, et al. Controlled nanoscale topographies for osteogenic differentiation of mesenchymal stem cells. ACS Appl Mater Interfaces. 2019;11(9):8858–66.
Wu YN, et al. Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomedicine. 2014;10(7):1507–16.
Ibrahim R, et al. Cell secretome strategies for Controlled Drug Delivery and Wound-Healing applications. Volume 14. Polymers (Basel); 2022. 14.
Curtis A, Wilkinson C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19(3):97–101.
Chen W, et al. Nanotopographical surfaces for stem cell fate control: engineering mechanobiology from the bottom. Nano Today. 2014;9(6):759–84.
Su N, et al. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials. 2017;141:74–85.
Olivares-Navarrete R, et al. Implant materials generate different peri-implant inflammatory factors: poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine (Phila Pa 1976). 2015;40(6):399–404.
Shimko DA, et al. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J Biomed Mater Res B Appl Biomater. 2005;73(2):315–24.
Danilevicius P, et al. The effect of porosity on cell ingrowth into accurately defined, laser-made, polylactide-based 3D scaffolds. Appl Surf Sci. 2015;336:2–10.
Chen X, et al. Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Nanomaterials. 2018;8(11):960.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91.
Murphy CM, Haugh MG, O’brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31(3):461–6.
Oh SH, et al. Fabrication and characterization of hydrophilic poly (lactic-co-glycolic acid)/poly (vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials. 2003;24(22):4011–21.
Salem AK, et al. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2002;61(2):212–7. https://doi.org/10.1002/jbm.10195.
Whang K, et al. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999;5(1):35–51.
Griffon DJ, et al. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313–20.
Karande TS, Ong JL, Agrawal CM. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann Biomed Eng. 2004;32(12):1728–43.
Isildar B, et al. 2D and 3D cultured human umbilical cord-derived mesenchymal stem cell-conditioned medium has a dual effect in type 1 diabetes model in rats: immunomodulation and beta-cell regeneration. Inflamm Regen. 2022;42(1):55.
Yuan T, et al. Modulation of immunological properties of allogeneic mesenchymal stem cells by collagen scaffolds in cartilage tissue engineering. J Biomed Mater Res A. 2011;98(3):332–41.
Guimarães CF, et al. The stiffness of living tissues and its implications for tissue engineering. Nat Rev Mater. 2020;5(5):351–70.
Handorf AM, et al. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis. 2015;11(1):1–15.
Zafar S, et al. Role of crosslinkers for synthesizing biocompatible, biodegradable and mechanically strong hydrogels with desired release profile. Polym Bull. 2022;79(11):9199–219.
Al-Maharma A, Patil S, Markert B. Effects of porosity on the mechanical properties of additively manufactured components: a critical review. Mater Res Express. 2020;7:122001.
Darnell M, Gu L, Mooney D. RNA-seq reveals diverse effects of substrate stiffness on mesenchymal stem cells. Biomaterials. 2018;181:182–8.
Wong SW, et al. Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking. Sci Adv. 2020;6(15):eaaw0158.
Zhuang Z, et al. Matrix stiffness regulates the immunomodulatory effects of mesenchymal stem cells on macrophages via AP1/TSG-6 signaling pathways. Acta Biomater. 2022;149:69–81.
Wang F, et al. Cell-scaffold interactions in tissue engineering for oral and craniofacial reconstruction. Bioact Mater. 2023;23:16–44.
Garg T, et al. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 2012;29(1):1–63.
Li J, et al. Substrate-independent immunomodulatory characteristics of mesenchymal stem cells in three-dimensional culture. PLoS One. 2018;13(11):e0206811.
Arabiyat AS, et al. Effect of poly(sophorolipid) functionalization on human mesenchymal stem cell osteogenesis and immunomodulation. ACS Appl Bio Mater. 2019;2(1):118–26.
Leppik L, et al. A new perspective for bone tissue engineering: human mesenchymal stromal cells well-survive cryopreservation on beta-TCP scaffold and show increased ability for osteogenic differentiation. Int J Mol Sci. 2022;23(3):1425. https://doi.org/10.3390/ijms23031425.
Toosi S, et al. Bioactive glass-collagen/poly (glycolic acid) scaffold nanoparticles exhibit improved biological properties and enhance osteogenic lineage differentiation of mesenchymal stem cells. Front Bioeng Biotechnol. 2022;10:963996.
Detsch R, et al. Osteogenic differentiation of umbilical cord and adipose derived stem cells onto highly porous 45S5 Bioglass(R)-based scaffolds. J Biomed Mater Res A. 2015;103(3):1029–37.
Park JW, Hanawa T, Chung JH. The relative effects of Ca and Mg ions on MSC osteogenesis in the surface modification of microrough Ti implants. Int J Nanomedicine. 2019;14:5697–711.
Hohenbild F, et al. An in vitro evaluation of the biological and osteogenic properties of magnesium-doped bioactive glasses for application in bone tissue engineering. Int J Mol Sci. 2021;22(23):12703. https://doi.org/10.3390/ijms222312703.
Liu S, et al. Immune characterization of mesenchymal stem cells in human umbilical cord Wharton’s jelly and derived cartilage cells. Cell Immunol. 2012;278(1–2):35–44.
Papalamprou A, et al. Xenogeneic cardiac extracellular matrix scaffolds with or without seeded mesenchymal stem cells exhibit distinct in vivo immunosuppressive and regenerative properties. Acta Biomater. 2016;45:155–68.
Shoichet MS. Polymer scaffolds for biomaterials applications. Macromolecules. 2009;43(2):581–91.
Shi C, et al. Polymeric biomaterials for bone regeneration. Ann Jt. 2016;1:27–27.
Simmons CA, et al. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35(2):562–9.
Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–6.
Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16 discussion 16.
Dhandayuthapani B, et al. Polymeric scaffolds in tissue engineering application: a review. Int JPolym Sci. 2011;2011:1–19. https://doi.org/10.1155/2011/290602.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3(3):1377–97.
Wu XS. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers: Part III Drug delivery application. Artif Cells Blood Substit Immobil Biotechnol. 2004;32(4):575–91.
Deng M, et al. Modification of PLGA scaffold by MSC-derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF-beta-induced protein. Adv Healthc Mater. 2020;9(13):e2000353.
Muhamad II, Selvakumaran S, Lazim NAM. Designing polymeric nanoparticles for targeted drug delivery system. One Central Press. 2014;37:287–312.
Chen M, et al. Natural polymer-based scaffolds for soft tissue repair. Front Bioeng Biotechnol. 2022;10:954699.
Fan J, et al. A review of recent advances in natural polymer-based scaffolds for musculoskeletal tissue engineering. Polymers (Basel). 2022;14(10):2097. https://doi.org/10.3390/polym14102097.
Somaiah C, et al. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS One. 2015;10(12):e0145068.
Glass KA, et al. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials. 2014;35(22):5921–31.
Yang J, et al. Regulation of the secretion of immunoregulatory factors of mesenchymal stem cells (MSCs) by collagen-based scaffolds during chondrogenesis. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 2):983–91.
Corradetti B, et al. Heparan sulfate: a potential candidate for the development of biomimetic immunomodulatory membranes. Front Bioeng Biotechnol. 2017;5:54.
Burdick JA, Mauck RL, Gerecht S. To serve and protect: hydrogels to improve stem cell-based therapies. Cell Stem Cell. 2016;18(1):13–5.
Rashedi I, et al. Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS One. 2017;12(10):e0187348.
Liu Y, et al. Enhanced therapeutic effects of MSC-derived extracellular vesicles with an injectable collagen matrix for experimental acute kidney injury treatment. Stem Cell Res Ther. 2020;11(1):161.
Corradetti B, et al. Immune tuning scaffold for the local induction of a pro-regenerative environment. Sci Rep. 2017;7(1):17030.
Corradetti B, et al. Chondroitin sulfate immobilized on a biomimetic scaffold modulates inflammation while driving chondrogenesis. Stem Cells Transl Med. 2016;5(5):670–82.
•• Bauza-Mayol G, et al. Biomimetic scaffolds modulate the posttraumatic inflammatory response in articular cartilage contributing to enhanced neoformation of cartilaginous tissue in vivo. Adv Healthc Mater. 2022;11(1):e2101127. Findings from this study suggest that biomimetic collagen-chondroitin sulfate scaffolds have the potential to increase the immunomodulatory capacity of MSC post-cartilage damage, promoting tissue regeneration over inflammation in vivo.
Banche-Niclot F, et al. 3D printed scaffold based on type I collagen/PLGA_TGF-β1 nanoparticles mimicking the growth factor footprint of human bone tissue. Polymers (Basel). 2022;14(5):857. https://doi.org/10.3390/polym14050857.
Brozovich A, et al. Osteogenesis in the presence of chemotherapy: A biomimetic approach. J Tissue Eng. 2022;13:1–20.
Banche-Niclot F, et al. PEG-coated large mesoporous silicas as smart platform for protein delivery and their use in a collagen-based formulation for 3D printing. Int J Mol Sci. 2021;22(4):1718. https://doi.org/10.3390/ijms22041718.
Author information
Authors and Affiliations
Contributions
“F.B. wrote the main manuscript text and prepared Fig. 1. J.L. helped in the main draft writing, PM, CB and FT conceived the idea and wrote the manuscript as well.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banche-Niclot, F., Lim, J., McCulloch, P. et al. Mesenchymal Stromal Cell Immunomodulatory Potential for Orthopedic Applications can be fine-tuned via 3D nano-engineered Scaffolds. Curr Stem Cell Rep (2024). https://doi.org/10.1007/s40778-024-00239-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s40778-024-00239-6