Abstract
Purpose of Review
There is an urgent unmet clinical need for effective therapies for obstetric complications such as fetal growth restriction, pre-eclampsia and preterm birth. These global health conditions have long term consequences for the health of survivors and the families affected, as well as societal and economic implications. Despite decades of research, prevention and treatment options are limited and often demonstrate suboptimal efficacy. Thus, cutting-edge approaches, such as gene delivery, may offer a novel solution. With the advancement of prenatal gene therapy preclinical research for the treatment of inherited diseases, the safe delivery of these technologies in utero is becoming a reality.
Recent Findings
We will give insights into the current preclinical research specifically relating to maternal gene delivery to improve the outcomes of pregnancy-related conditions.
Summary
Obstetric disorders lack effective prevention and treatment options. Innovative approaches, such as gene transfer, may provide a promising alternative. This review summarises potential gene therapies in preclinical development for fetal growth restriction, pre-eclampsia and preterm birth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complications during pregnancy pose significant risks for both the mother and baby, potentially leading to long-term morbidity or even death. The management of these conditions frequently necessitates premature delivery, increasing the risk of enduring health issues, including cerebral palsy, respiratory problems and metabolic diseases [1]. Despite extensive research, predicting, preventing, and treating conditions like fetal growth restriction (FGR), pre-eclampsia, and preterm birth remains challenging. Limited treatment options exist for most obstetric disorders, and promising preclinical interventions often fall short of reaching patients. Ethical considerations surrounding the administration of experimental treatments that could affect both the mother and developing fetus further impede efforts in this field [2].
Gene therapy involves delivering genetic material to provide prolonged treatment for diseases. Positive results from various trials indicate the potential for long-term treatment or even cures for previously untreatable diseases [3]. In recent years, prenatal gene therapies have proved successful in preclinical animal models of a variety of conditions whose pathologies originate in utero, ranging from diseases of the nervous system to congenital deafness and hemoglobinopathies [4,5,6,7]. Over the last decade, the advantages of prenatal gene therapy have been widely discussed [8,9,10,11,12,13,14]. While clinical trials for in utero gene therapy are yet to be conducted, notable progress in the safety and efficacy of various gene and cell therapies, along with advancements in prenatal diagnostics, signals a new era in the treatment of conditions during pregnancy.
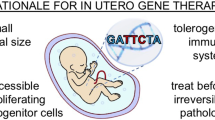
In addition to the direct targeting of genetic or irreversible diseases in utero, gene transfer technology has recently been utilised in preclinical models of obstetric conditions for which there are no effective treatments and that result in high rates of neonatal mortality and life-long morbidity. Here, we will review emerging preclinical maternal gene delivery research for FGR, pre-eclampsia and preterm birth (summarised in Fig. 1).
Summary of gene targets for the prevention and treatment of obstetric conditions. For FGR and pre-eclampsia, the rationale has been to improve the placental pathology experienced by both disorders; either to enhance angiogenesis (VEGF) or to improve nutrient transport across the placenta. For preterm birth, the antimicrobial peptide HBD3 was delivered to the cervix to enhance cervical immunity and prevent bacteria in the vagina from accessing the uterine cavity. VEGF: vascular endothelial growth factor; IGF-1: insulin-like growth factor 1; HO-1: heme oxygenase 1; pAR-1: Protease-activated receptor-1; HBD3: human beta defensin 3. Partially created using Biorender.com
FGR
FGR is a condition impacting approximately 8% of pregnancies where impaired materno-placental perfusion results in poor fetal growth, likely the result of insufficient nutrient and oxygen transfer. Consequently, these babies may have to be delivered prematurely and there is an increased risk of both short and long term health complications. Currently, no effective treatments to improve placental perfusion exist [15]. In recent years, two transgenes have demonstrated therapeutic potential for FGR; vascular endothelial growth factor (VEGF), which stimulates blood vessel formation, and insulin-like growth factor 1 (IGF-1), a hormone that promotes growth via the regulation of growth hormone (GH).
VEGF
In 2008, David et al. employed adenoviral (Ad) delivery of VEGF directly into the uterine arteries during a normal sheep pregnancy. The overexpression of VEGF-A, a member of the VEGF family, enhanced vasodilation, resulting in increased blood flow in the uterine arteries [16]. Ad.VEGF-A165, the predominant VEGF-A isoform, later promoted sustained increased uterine artery blood flow four weeks after treatment without impacting maternal and fetal blood pressure in normal sheep pregnancies [17].
In growth restricted sheep pregnancies, fetal growth was significantly increased in the weeks following bilateral uterine artery delivery of Ad.VEGF-A165. Transduced arteries exhibited signs of vasorelaxation but there was no significant impact on uterine blood flow or neovascularisation [18]. Perinatally, there were trends for increased birthweight and longer gestation length, as well as some evidence of increased umbilical girth. The extent of intervention needed to support neonatal survival was comparable between control and treatment groups and the therapy did improve the overall growth rate of neonates and the final weight measurement at postnatal day 83 [19].
This research was extended to include a guinea pig model of maternal nutrient restriction-induced FGR, as these animals have comparable placental physiology to humans. VEGF-A165 transgene delivery to the outside of the uterine artery demonstrated optimal transduction efficiency of these vessels, while absent from fetal tissues [20, 21]. Importantly, overall term fetal weight was significantly increased; specifically increased were the brain, lung and liver weights, as well as the crown-rump length. Once again, this therapy demonstrated increased uterine artery relaxation [21].
Preclinical studies using the construct intended for the first human trial, Ad.VEGF-DΔNΔC, reported increased transduction efficiency in human and animal endothelial cells compared to Ad.VEGF-A165 [22]. It was speculated that VEGF-DΔNΔC may promote endothelial cell proliferation and reduce vasocontraction to stimulate angiogenesis and vasodilation, without inducing vascular permeability, via the upregulation of eNOS and iNOS [23]. Importantly, a toxicology study, using human villus explants and performing ex vivo perfusion experiments, found limited transfer of Ad.VEGF-DΔNΔC across the placental barrier, no evidence of placental toxicity and no substantial impact on placental morphology or function. Although very low concentrations of vector were detected in a small number of fetal perfusates, ultimately, the risk of vector transfer to the fetal side of the placenta was determined to be low [24]. Further, no histological, haematological or biochemical abnormalities were identified in treated pregnancies [25].
The translation of new therapeutics for obstetric conditions has long been overlooked by the pharmaceutical industry. VEGF gene delivery for placental insufficiency was granted European Medicines Agency orphan status in 2015, which offers incentives and benefits to encourage the development of therapies for underrepresented and rare conditions [26]. Establishing clinical efficacy and, importantly, safety will be essential as well as ensuring informed patient consent as this treatment navigates the translational pathway. In order to proceed to a phase I/IIa clinical trial to evaluate the safety and efficacy of VEGF gene delivery, the multicentre EVERREST Prospective Study (NCT02097667) appraised various strategies to identify which FGR pregnancies could benefit most from this intervention [27]. At diagnosis of severe, early-onset FGR, ultrasound measurements, such as abnormal umbilical artery (UmA) Doppler velocimetry, and maternal serum placental growth factor (PlGF) concentration demonstrated robust prediction of fetal or neonatal death and delivery at or prior to 28 + 0 weeks [28•].
Other researchers have also highlighted the therapeutic benefit of VEGF gene supplementation. In baboons, microbubble VEGF DNA delivery to the placental basal plate prevented estradiol-induced suppression of uterine artery remodelling [29] and Swingle et al. recently investigated the optimal ionizable lipid nanoparticle for VEFG-A mRNA delivery, with the aim to target placental insufficiency, which increased placental vasodilation in mice [30]. These studies and the outcome of an EVERREST phase I/IIa clinical trial will likely be beneficial to the future development of maternal gene therapies for other obstetric conditions, such as pre-eclampsia, that have no effective treatments.
IGF-1
Adopting a different strategy to target the placental insufficiency associated with FGR, a comparison of the intraplacental adenoviral delivery of eight growth factors found that angiopoietin-2, IGF-1 and platelet-derived growth factor-B (PDGF-B) increased placental growth in normal mouse pregnancies [31]. Subsequently, several studies in mice, rabbits and guinea pigs have investigated IGF-1 gene delivery to target placental insufficiency; IGF-1 is essential for placental and fetal growth and is downregulated in cases of FGR.
Using a mouse model of FGR induced by uterine artery branch ligation, Jones et al. demonstrated that intraplacental adenoviral delivery of human (h)IGF-1 increased the expression and translocation of glucose transporters and amino acid isoform transporters to the placental membrane. This suggests a potential mechanism to correct placental insufficiency by increasing nutrient transport [32, 33]. Long-term outcomes were improved in mouse offspring, with Ad.hIGF-1 protecting against FGR-induced cardiac dysfunction in adults [34]. Using a rabbit model, whereby select fetuses within the litter are naturally growth restricted, Ad.hIGF-1 restored fetal weight without impacting placental weight, which remained significantly reduced compared to non-growth restricted pregnancies [35].
In order to improve the safety, efficacy and acceptability of gene delivery in pregnancy, researchers led by Dr Jones have been optimising a non-viral nanoparticle delivery system. The hIGF-1 transgene was delivered to mice using a non-immunogenic co-polymer (pHPMA-b-pDMAEMA) and placental specificity was enhanced by the use trophoblast-specific promoters. This approach increased the number of fetal capillaries in the placental labyrinth and maintained, but did not restore, normal fetal growth in mice [36]. However, these therapeutic benefits were not replicated in an endothelial nitric oxide synthase knockout (eNOS-/-) mouse model of FGR, suggesting that eNOS/NOS is required for IGF-1 signalling in the placenta [37].
In a maternal nutrient restriction guinea pig model of FGR, nanoparticle delivery of hIGF-1, under the control of the Cyp19a1 trophoblast-specific promotor, increased fetal circulating glucose levels. This was associated with the upregulation of glucose transporters in the placenta, suggesting improved nutrient transfer, but again fetal weight was not improved [38•, 39]. Additionally, sex specific effects of both maternal nutrient restriction and non-viral hIGF-1 therapy were identified [40]. Another important consideration is the impact of this intervention on normal placental development; while nanoparticle hIGF-1 delivery increased placental expression of growth factors and restored mediators associated with epithelial-mesenchymal transition (EMT), in normal placentas AKT/mTOR signalling and the expression of certain growth factors was reduced [41]. While IGF-1 gene delivery is making strides to improve nutrient transport, further evidence of improvements to fetal and neonatal outcomes is needed to demonstrate robust therapeutic benefit.
Pre-eclampsia
Pre-eclampsia is a systemic disorder characterised by sudden-onset maternal hypertension and proteinuria, typically after 20 weeks gestation. It is thought to be caused by abnormal development of the placenta, or placental malperfusion, associated with inappropriate spiral artery remodelling. Inflammatory factors are released into the maternal circulation, leading to tissue hypoxia, high blood pressure and organ damage [42]. In severe cases, the condition can escalate to eclampsia and multi-organ failure, which account for around 50,000 maternal deaths and 500,000 newborn or perinatal deaths per year [43]. Currently, clinical treatments for pre-eclampsia target maternal hypertension and other complications, but the only effective cure is delivery, which is often preterm [44].
In pre-eclampsia development, placental damage can cause soluble fms-like tyrosine kinase 1 (sFLT1), a circulating anti-angiogenic protein, to be abnormally overexpressed in trophoblasts and secreted into the serum, which contributes to maternal hypertension, proteinuria and fetal growth restriction [45]. In fact, adenoviral and non-viral delivery of sFlt1 have been used to model pre-eclampsia in rodents [46, 47]. Thus, inhibition of sFLT1 may have therapeutic potential.
VEGF
sFlt1 is a VEGF-antagonist and, subsequently, reduced serum VEGF concentration is also a hallmark of pre-eclampsia [48]. As discussed above, VEGF plays a role in the development of normal placental vascularisation and angiogenesis, and this is mediated by binding to VEGF receptor 1 (VEGFR)1 and 2 [49]. It has been hypothesised that targeting VEGF to reduce sFTL1 levels may rescue normal vascularisation in pre-eclampsia. In a normal mouse pregnancy, human VEGF165 complementary (c)DNA was delivered into the extraamniotic space of each uterine horn encapsulated in a replication-defective hemagglutinating virus of Japan‐envelope (HVJ‐E) vector derived from murine respirovirus. While VEGF concentration was not significantly increased, sFLT1 concentration was reduced in the days following gene transfer and dam blood pressure was reduced for 48 h. This therapy did not disrupt parturition or affect litter sizes [50]. Systemic adenoviral delivery of VEGF121 restored circulating VEGF levels in a pre-eclampsia high blood pressure mouse model (BPH/5) and improved angiogenic potential by increasing endothelial tube length. Consequently, blood pressure was normalised, proteinuria was prevented and fetal resorptions were reduced [51]. Overexpressing FLT1, the human gene for VEGFR1 protein, in human trophoblast cells using the RALA nanoparticle delivery system reduced the secretion of sFLT1, demonstrating potential therapeutic benefit of this strategy [47].
HO-1
Another target for pre-eclampsia gene delivery is heme oxygenase 1 (HO-1), a stress response protein, which plays a role in angiogenesis, as well as inhibiting the expression of sFLT1 [52]. Levels of HO-1 mRNA are reduced in pre-eclampsia compared to unaffected pregnancies, which may explain the excess of circulating sFLT1 [53]. The effect of overexpression of HO-1 has been explored using mesenchymal stem cells (MSC). Human placenta derived MSCs were isolated and the HO-1 gene was introduced via lentivirus. VEGF expression was increased and sFLT1 expression was decreased in transfected cells. In vitro assays demonstrated enhanced cell branching and the potential for vascular remodelling if used therapeutically [54]. Further, the transplantation of these cells in a pre-eclampsia-like rat model increased VEGF concentration, decreased sFLT1 secretion and improved placental perfusion; thus, demonstrating the potential to restore placental function during the development of pre-eclampsia [55]. HO-1 gene delivery may also be of benefit to other pregnancy disorders; adenoviral delivery of HO-1 increased fetal growth in rats [56] and reduced abortion rate in a mouse model of spontaneous miscarriage (CBA/J x DBA/2J) [56, 57].
PAR-1
Appropriate regulation of the thrombin cascade is critical for vascular remodelling and normal placental development. Protease-activated receptor-1 (PAR-1) is overexpressed by trophoblasts during pre-eclampsia and increases trophoblast sFLT1 levels through its high affinity for thrombin binding. A PAR-1 antagonist could potentially reduce pre-eclampsia symptoms by decreasing sFLT1 levels [58]. A human trophoblast cell line was transfected with PAR-1 short hairpin (sh)RNA. This inhibited PAR-1 expression, supressed sFLT1 expression and secretion and promoted vascular remodelling. In a pre-eclampsia rat model, placental delivery of PAR-1 shRNA normalised blood pressure, reduced proteinuria, and reduced sFLT1 levels which, ultimately, improved the placental microvasculature [59].
Gene delivery to suppress adrenergic beta 1 receptor (ADRB1) has also been suggested for pregnancies affected by hypertension or gestational diabetes mellitus [60]. Other pathways involved in the pathogenesis of pre-eclampsia, such as the FKBPL-CD44 pathway, could also be explored and targeted using these therapies, extrapolating better understanding of disease development as well as identifying novel targets for therapeutics [61].
Preterm Labour
Preterm birth is defined as delivery before 37 weeks of gestation. Complicating 11.1% of all pregnancies, it is the leading cause of neonatal morbidity and mortality. Approximately 40% of spontaneous cases of preterm labour are associated with microbes [1]. Recently, Suff et al. (2020) described the protective benefit of adeno-associated virus (AAV) delivery of the antimicrobial peptide human beta defensin 3 (HBD3) in a mouse model of infection-induced preterm birth.
Preterm labour was induced by intravaginal delivery of bioluminescent Escherichia coli, which allowed the spread of infection to be monitored by imaging. Following prophylactic intravaginal AAV8.HBD3 gene delivery, uterine bioluminescence was significantly reduced, suggesting that bacterial ascension from the vagina into the uterus was impeded, perhaps by enhancing cervical innate immunity. Although the treatment did not prolong gestation, the number of pups born alive was significantly increased, as was the overall pup survival up to 7 days [62, 63]. There was no evidence of transduction extending to the uterus or systemically to the maternal liver. Transient transgene expression up to 14 days was observed; ideal for pregnancy-related conditions, which require temporary intervention [62].
Gene transfer to promote an antimicrobial and anti-inflammatory environment warrants further investigation for the prevention and treatment of preterm labour. Other potential therapeutic strategies could include progesterone gene supplementation to maintain pregnancy or the transfer of genes that promote uterine quiescence or inhibit uterine contractions. However, improved neonatal outcome must remain central to the development of any maternal treatment, as prolonging gestation will be detrimental if the fetus is held in an adverse environment.
Ethical Considerations
For the translation of maternal gene therapy to be feasible for human application, it is essential that there are established methods to accurately predict and diagnose these conditions and their outcomes. Spencer et al. addressed this in the EVERREST Prospective Study for early onset FGR, but for conditions such as preterm labour that are often spontaneous and for which the underlying stimuli are multifactorial, robust predictive tests and reliable biomarkers continue to elude the medical community. Further to this, the conditions targeted by maternal gene therapy must result in severe maternal or neonatal morbidity or mortality to warrant experimental treatment and the efficacy must be superior to currently available treatments [64].
When considering in utero approaches, safety becomes particularly crucial. Not only are therapies delivered during critical points of fetal development but there is also the mother’s safety to consider. Any intervention during pregnancy carries further risk of infection, miscarriage or preterm labour. Viral vectors present many advantages, including efficient gene transfer, sustained gene expression, broad cellular tropism and limited toxicity. However, a barrier to their success is their potential to stimulate humoral and cellular immune responses. Extensive preclinical optimisation of gene delivery methods is, therefore, essential to safely employ these innovative techniques in pregnancy, where it is imperative that inflammation is tightly controlled. In addition, the long-term impact of altering gene expression, the potential of off-target effects and the potential effect on germ cells need to be thoroughly examined for both mother and child. Thus far, preclinical animal studies have not established negative side effects of prenatal gene therapy on fetal germ cells but the long-term effects on maternal organ systems is still unknown [9]. Sheppard et al. investigated the ethics of testing novel treatments during pregnancy and surveyed the acceptability of delivering experimental maternal treatments in order to improve fetal outcome in the context of FGR. No legal or ethical objections were identified and, overall, women seemed accepting of trialling a treatment that could improve the outcome of their baby [65].
Conclusion
There is a critical unmet need for novel and innovative therapies for the prevention and management of obstetric conditions. The promising targets we have reviewed here may offer wide-ranging application as they demonstrate potential overlap between gestational disorders. There is a substantial amount of research on VEGF gene delivery, predominantly for FGR but also for pre-eclampsia. The potential protective role of HO-1 could benefit FGR, pre-eclampsia and recurrent miscarriage. While gene delivery strategies for the prevention or treatment of preterm birth are still emerging, the multifactorial nature of the condition offers a wealth of potential therapeutic targets, which could address risk factors such as infection, inflammation, cervical injury or myometrial contraction. The safety and efficacy of maternal gene therapies are likely to be improved by the continued development of new technologies such as non-viral approaches and the targeting of specific cell populations, reducing the risk of off-target effects, with the ultimate goal of developing a therapeutic that will be acceptable for clinical testing in pregnancy. Healthy pregnancies are essential for humans to thrive; innovation and a drive towards personalised medicine may produce the long-awaited breakthroughs so urgently needed in the field of obstetrics.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Boyle AK, Rinaldi SF, Norman JE, Stock SJ. Preterm birth: inflammation, fetal injury and treatment strategies. J Reprod Immunol. 2017;119:62–6.
David AL, Ahmadzia H, Ashcroft R, Bucci-Rechtweg C, Spencer RN, Thornton S. Improving development of drug treatments for pregnant women and the Fetus. Ther Innov Regul Sci. 2022;56(6):976–90.
Papanikolaou E, Bosio A. The promise and the hope of gene therapy. Front Genome Ed. 2021;3:618346.
Massaro G, Mattar CNZ, Wong AMS, Sirka E, Buckley SMK, Herbert BR, et al. Fetal gene therapy for neurodegenerative disease of infants. Nat Med. 2018;24(9):1317–23.
Rashnonejad A, Amini Chermahini G, Gunduz C, Onay H, Aykut A, Durmaz B, et al. Fetal gene therapy using a single injection of recombinant AAV9 rescued SMA phenotype in mice. Mol Ther. 2019;27(12):2123–33.
Ma W, Wei X, Gu H, Liu D, Luo W, An D, et al. Therapeutic potential of adenovirus-encoding brain-derived neurotrophic factor for spina bifida aperta by intra-amniotic delivery in a rat model. Gene Ther. 2020;27(12):567–78.
Shangaris P, Loukogeorgakis SP, Subramaniam S, Flouri C, Jackson LH, Wang W, et al. In utero gene therapy (IUGT) using GLOBE lentiviral vector phenotypically corrects the heterozygous humanised mouse model and its progress can be monitored using MRI techniques. Sci Rep. 2019;9(1):11592.
Karda R, Buckley SM, Mattar CN, Ng J, Massaro G, Hughes MP, et al. Perinatal systemic gene delivery using adeno-associated viral vectors. Front Mol Neurosci. 2014;7:89.
Peranteau WH, Flake AW. The future of in utero gene therapy. Mol Diagn Ther. 2020;24(2):135–42.
David AL, Peebles D. Gene therapy for the fetus: is there a future? Best Pract Res Clin Obstet Gynaecol. 2008;22(1):203–18.
Buckley SM, Rahim AA, Chan JK, David AL, Peebles DM, Coutelle C, et al. Recent advances in fetal gene therapy. Ther Deliv. 2011;2(4):461–9.
Coutelle C, Waddington SN. The concept of prenatal gene therapy. Methods Mol Biol (Clifton, NJ). 2012;891:1–7.
Waddington SN, Kramer MG, Hernandez-Alcoceba R, Buckley SM, Themis M, Coutelle C, et al. In utero gene therapy: current challenges and perspectives. Mol Ther. 2005;11(5):661–76.
Waddington SN, Peranteau WH, Rahim AA, Boyle AK, Kurian MA, Gissen P, et al. Fetal gene therapy. J Inherit Metab Dis. 2024;47(1):192–210.
David AL. Maternal uterine artery VEGF gene therapy for treatment of intrauterine growth restriction. Placenta. 2017;59(Suppl 1):S44–50.
David AL, Torondel B, Zachary I, Wigley V, Abi-Nader K, Mehta V, et al. Local delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheep. Gene Ther. 2008;15(19):1344–50.
Mehta V, Abi-Nader KN, Peebles DM, Benjamin E, Wigley V, Torondel B, et al. Long-term increase in uterine blood flow is achieved by local overexpression of VEGF-A(165) in the uterine arteries of pregnant sheep. Gene Ther. 2012;19(9):925–35.
Carr DJ, Wallace JM, Aitken RP, Milne JS, Mehta V, Martin JF, et al. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies. Hum Gene Ther. 2014;25(4):375–84.
Carr DJ, Wallace JM, Aitken RP, Milne JS, Martin JF, Zachary IC, et al. Peri- and postnatal effects of prenatal adenoviral VEGF gene therapy in growth-restricted sheep. Biol Reprod. 2016;94(6):142.
Mehta V, Ofir K, Swanson A, Kloczko E, Boyd M, Barker H, et al. Gene targeting to the uteroplacental circulation of pregnant guinea pigs. Reprod Sci. 2016;23(8):1087–95.
Swanson AM, Rossi CA, Ofir K, Mehta V, Boyd M, Barker H, et al. Maternal therapy with Ad.VEGF-A165 increases fetal weight at term in a guinea-pig model of fetal growth restriction. Hum Gene Ther. 2016;27(12):997–1007.
Rossi C, Lees M, Mehta V, Heikura T, Martin J, Zachary I, et al. Comparison of efficiency and function of vascular endothelial growth factor adenovirus vectors in endothelial cells for gene therapy of placental insufficiency. Hum Gene Ther. 2020;31(21–22):1190–202.
Mehta V, Abi-Nader KN, Shangaris P, Shaw SW, Filippi E, Benjamin E, et al. Local over-expression of VEGF-DΔNΔC in the uterine arteries of pregnant sheep results in long-term changes in uterine artery contractility and angiogenesis. PLoS ONE. 2014;9(6):e100021.
Desforges M, Rogue A, Pearson N, Rossi C, Olearo E, Forster R, et al. In Vitro Human placental studies to support adenovirus-mediated VEGF-DΔNΔC maternal gene therapy for the treatment of severe early-onset fetal growth restriction. Hum Gene Ther Clin Dev. 2018;29(1):10–23.
Vaughan OR, Rossi CA, Ginsberg Y, White A, Hristova M, Sebire NJ, et al. Perinatal and long-term effects of maternal uterine artery adenoviral VEGF-A165 gene therapy in the growth-restricted guinea pig fetus. Am J Physiol Regul Integr Comp Physiol. 2018;315(2):R344–53.
Spencer R, Rossi C, Lees M, Peebles D, Brocklehurst P, Martin J, et al. Achieving orphan designation for placental insufficiency: annual incidence estimations in Europe. BJOG. 2019;126(9):1157–67.
Spencer R, Ambler G, Brodszki J, Diemert A, Figueras F, Gratacos E, et al. EVERREST prospective study: a 6-year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction. BMC Pregnancy Childbirth. 2017;17(1):43.
• Spencer R, Maksym K, Hecher K, Maršál K, Figueras F, Ambler G, et al. Maternal PlGF and umbilical Dopplers predict pregnancy outcomes at diagnosis of early-onset fetal growth restriction. J Clin Invest. 2023;133(18):e169199. This study has important implications for the progression of the first in human trial for maternal gene therapy. It highlights the necessity for robust methods of outcome prediction for advancing experimental therapies.
Babischkin JS, Aberdeen GW, Lindner JR, Bonagura TW, Pepe GJ, Albrecht ED. Vascular endothelial growth factor delivery to placental basal plate promotes uterine artery remodeling in the primate. Endocrinology. 2019;160(6):1492–505.
Swingle KL, Safford HC, Geisler HC, Hamilton AG, Thatte AS, Billingsley MM, et al. Ionizable lipid nanoparticles for in vivo mRNA delivery to the placenta during pregnancy. J Am Chem Soc. 2023;145(8):4691–706.
Katz AB, Keswani SG, Habli M, Lim FY, Zoltick PW, Midrio P, et al. Placental gene transfer: transgene screening in mice for trophic effects on the placenta. Am J Obstet Gynecol. 2009;201(5):e4991–8.
Jones H, Crombleholme T, Habli M. Regulation of amino acid transporters by adenoviral-mediated human insulin-like growth factor-1 in a mouse model of placental insufficiency in vivo and the human trophoblast line BeWo in vitro. Placenta. 2014;35(2):132–8.
Jones HN, Crombleholme T, Habli M. Adenoviral-mediated placental gene transfer of IGF-1 corrects placental insufficiency via enhanced placental glucose transport mechanisms. PLoS ONE. 2013;8(9):e74632.
Alsaied T, Omar K, James JF, Hinton RB, Crombleholme TM, Habli M. Fetal origins of adult cardiac disease: a novel approach to prevent fetal growth restriction induced cardiac dysfunction using insulin like growth factor. Pediatr Res. 2017;81(6):919–25.
Keswani SG, Balaji S, Katz AB, King A, Omar K, Habli M, et al. Intraplacental gene therapy with Ad-IGF-1 corrects naturally occurring rabbit model of intrauterine growth restriction. Hum Gene Ther. 2015;26(3):172–82.
Abd Ellah N, Taylor L, Troja W, Owens K, Ayres N, Pauletti G, et al. Development of non-viral, trophoblast-specific gene delivery for placental therapy. PLoS ONE. 2015;10(10):e0140879.
Wilson RL, Troja W, Sumser EK, Maupin A, Lampe K, Jones HN. Insulin-like growth factor 1 signaling in the placenta requires endothelial nitric oxide synthase to support trophoblast function and normal fetal growth. Am J Physiol Regul Integr Comp Physiol. 2021;320(5):R653–62.
• Wilson RL, Lampe K, Gupta MK, Duvall CL, Jones HN. Nanoparticle-mediated transgene expression of insulin-like growth factor 1 in the growth restricted guinea pig placenta increases placenta nutrient transporter expression and fetal glucose concentrations. Mol Reprod Dev. 2022;89(11):540–53. The development of non-viral gene delivery systems, such as the one described in this study, may offer increased safety and efficacy for gene transfer during pregnancy, when it is essential that immune regulation is tightly controlled.
Wilson RL, Stephens KK, Lampe K, Jones HN. Sexual dimorphisms in brain gene expression in the growth-restricted guinea pig can be modulated with intra-placental therapy. Pediatr Res. 2021;89(7):1673–80.
Wilson RL, Stephens KK, Jones HN. Placental nanoparticle gene therapy normalizes gene expression changes in the fetal liver associated with fetal growth restriction in a fetal sex-specific manner. J Dev Orig Health Dis. 2023;14(3):325–32.
Davenport BN, Jones HN, Wilson RL. Placental treatment with insulin-like growth factor 1 via nanoparticle differentially impacts vascular remodeling factors in guinea pig sub-placenta/decidua. Front Physiol. 2022;13:1055234.
Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398(10297):341–54.
Gestational Hypertension and Preeclampsia. ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237–60.
Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV, et al. Pre-eclampsia. Nat Rev Dis Primers. 2023;9(1):8.
Shibuya M, Matsui H, Sasagawa T, Nagamatsu T. A simple detection method for the serum sFLT1 protein in preeclampsia. Sci Rep. 2021;11(1):20613.
Karumanchi SA, Stillman IE. In vivo rat model of preeclampsia. Methods Mol Med. 2006;122:393–9.
McNally R, Alqudah A, McErlean EM, Rennie C, Morshed N, Short A, et al. Non-viral gene delivery utilizing RALA modulates sFlt-1 secretion, important for preeclampsia. Nanomed (Lond). 2021;16(22):1999–2012.
Bolatai A, He Y, Wu N. Vascular endothelial growth factor and its receptors regulation in gestational diabetes mellitus and eclampsia. J Transl Med. 2022;20(1):400.
Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92.
Koyama S, Kimura T, Ogita K, Nakamura H, Khan MA, Yoshida S, et al. Transient local overexpression of human vascular endothelial growth factor (VEGF) in mouse feto-maternal interface during mid-term pregnancy lowers systemic maternal blood pressure. Horm Metab Res. 2006;38(10):619–24.
Woods AK, Hoffmann DS, Weydert CJ, Butler SD, Zhou Y, Sharma RV, et al. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension. 2011;57(1):94–102.
Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115(13):1789–97.
Gallardo V, González M, Toledo F, Sobrevia L. Role of heme oxygenase 1 and human chorionic gonadotropin in pregnancy associated diseases. Biochim Biophys Acta Mol Basis Dis. 2020;1866(2):165522.
Wu D, Liu Y, Liu X, Liu W, Shi H, Zhang Y, et al. Heme oxygenase-1 gene modified human placental mesenchymal stem cells promote placental angiogenesis and spiral artery remodeling by improving the balance of angiogenic factors in vitro. Placenta. 2020;99:70–7.
Liu Y, Shi H, Wu D, Xu G, Ma R, Liu X, et al. The protective benefit of heme oxygenase-1 gene-modified human placenta-derived mesenchymal stem cells in a N-nitro-L-arginine methyl ester-induced preeclampsia-like rat model: possible implications for placental angiogenesis. Stem Cells Dev. 2021;30(19):991–1002.
Kreiser D, Nguyen X, Wong R, Seidman D, Stevenson D, Quan S, et al. Heme oxygenase-1 modulates fetal growth in the rat. Lab Invest. 2002;82(6):687–92.
Zenclussen ML, Anegon I, Bertoja AZ, Chauveau C, Vogt K, Gerlof K, et al. Over-expression of heme oxygenase-1 by adenoviral gene transfer improves pregnancy outcome in a murine model of abortion. J Reprod Immunol. 2006;69(1):35–52.
Zhao Y, Koga K, Osuga Y, Nagai M, Izumi G, Takamura M, et al. Thrombin enhances soluble fms-like tyrosine kinase 1 expression in trophoblasts; possible involvement in the pathogenesis of preeclampsia. Fertil Steril. 2012;98(4):917–21.
Zhao Y, Zheng Y, Liu X, Luo Q, Wu D, Zou L. Inhibiting trophoblast PAR-1 overexpression suppresses sflt-1-induced anti-angiogenesis and abnormal vascular remodeling: a possible therapeutic approach for preeclampsia. Mol Hum Reprod. 2018;24(3):158–69.
Wieclawek A, Slawska H, Mazurek U. ADRB1 as a potential target for gene therapy of pregnancy induced hypertension and gestational diabetes mellitus. Clin Exp Hypertens. 2011;33(6):422–6.
Todd N, McNally R, Alqudah A, Jerotic D, Suvakov S, Obradovic D, et al. Role of a novel angiogenesis FKBPL-CD44 pathway in preeclampsia risk stratification and mesenchymal stem cell treatment. J Clin Endocrinol Metab. 2021;106(1):26–41.
Suff N, Karda R, Diaz JA, Ng J, Baruteau J, Perocheau D, et al. Cervical gene delivery of the antimicrobial peptide, human beta-defensin (HBD)-3, in a mouse model of ascending infection-related preterm birth. Front Immunol. 2020;11:106.
Boyle AK, Tetorou K, Suff N, Beecroft L, Mazzaschi M, Hristova M, et al. Ascending vaginal infection in mice induces preterm birth and neonatal morbidity. bioRxiv. 2023. 2023.08.14.553220.
Almeida-Porada G, Waddington SN, Chan JKY, Peranteau WH, MacKenzie T, Porada CD. In utero gene therapy consensus statement from the IFeTIS. Mol Ther. 2019;27(4):705–7.
Sheppard M, Spencer RN, Ashcroft R, David AL. Ethics and social acceptability of a proposed clinical trial using maternal gene therapy to treat severe early-onset fetal growth restriction. Ultrasound Obstet Gynecol. 2016;47(4):484–91.
Author information
Authors and Affiliations
Contributions
S.D. contributed to the writing of this manuscript (original draft preparation; review and editing). A.B. contributed to and supervised the writing of this manuscript (original draft preparation; review and editing).
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
D’Alessandro, S.C., Boyle, A.K. Maternal Gene Delivery for the Prevention and Treatment of Obstetric Conditions. Curr Stem Cell Rep (2024). https://doi.org/10.1007/s40778-024-00238-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s40778-024-00238-7